Abstract
We conducted a randomized trial to compare the intensive conventional chemotherapy regimen ACVBP (doxorubicin, cyclophosphamide, vindesine, bleomycin, prednisone) with standard CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) in previously untreated patients with poor-risk aggressive lymphoma. Patients aged 61 to 69 years who had aggressive non-Hodgkin lymphoma with at least one prognostic factor of the age-adjusted international prognostic index (IPI) were included. ACVBP consisted of an induction phase of intensified chemotherapy and central nervous system (CNS) prophylaxis followed by a sequential consolidation phase. Of the 708 patients registered for the study, 635 were eligible. The rate of complete response was 58% in the ACVBP group and 56% in the CHOP group (P = .5). Treatment-related death occurred in 13% of the ACVBP group and 7% of the CHOP group (P = .014). At 5 years, the event-free survival was 39% in the ACVBP group and 29% in the CHOP group (P = .005). The overall survival was significantly longer for patients treated with ACVBP, at 5 years it was 46% compared with 38% for patients treated with CHOP (P = .036). CNS progressions or relapses were more frequent in the CHOP group (P = .004). Despite higher toxicity, the ACVBP regimen, used as first-line treatment for patients with poor-risk aggressive lymphoma, is superior to standard CHOP with regard to both event-free survival and overall survival. (Blood. 2003;102:4284-4289)
Introduction
The incidence of non-Hodgkin lymphoma, especially that of aggressive histology, is steadily increasing.1 However, during the last 30 years, improvement in treatment outcome remained modest2 and less than 50% of the patients with aggressive lymphoma are cured.
The standard treatment used since the 1970s is the CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy regimen,3 delivering 8 cycles at a 3-week interval in advanced disease. Subsequently, new combinations including additional non-cross-reacting drugs such as methotrexate, bleomycin, or cytarabine have been proposed.4-6 However, multicenter randomized trials failed to demonstrate any survival advantage of these second- and third-generation regimens over the standard CHOP.7,8
Since 1980, the Groupe d'Etudes des Lymphomes (GELA) has developed a chemotherapy schedule, the ACVBP (doxorubicin, cyclophosphamide, vindesine, bleomycin, prednisone) regimen, which consisted of an induction phase of 4 cycles of intensified CHOP with central nervous system (CNS) prophylaxis followed by a sequential consolidation phase.9,10 In a previous study, the comparison of this regimen with m-BACOD (cyclophosphamide, doxorubicin, vincristine, bleomycin, methotrexate, and dexamethasone), a third-generation regimen, in patients with low-risk aggressive lymphoma, showed that ACVBP could be beneficial for patients with more advanced disease.11
Therefore, in 1993, the GELA initiated a phase 3 study comparing ACVBP to CHOP in patients with poor-prognosis aggressive lymphoma. We restricted this trial to patients aged between 61 and 69 years because another study from our group had shown that patients younger than 60 years of age with poor-risk aggressive lymphoma could benefit from consolidative high-dose therapy with stem cell rescue in the front-line regimen.12
Patients, materials, and methods
Patients
Newly diagnosed patients between 61 and 69 years of age with diffuse mixed, diffuse large-cell, immunoblastic, lymphoblastic, or Burkitt lymphoma were eligible for the present study providing that they had at least one adverse prognostic factor as defined by the age-adjusted international prognostic index (IPI) for non-Hodgkin lymphoma (namely stage III or IV disease, elevated lactate dehydrogenase [LDH] level and Eastern Cooperative Oncology Group [ECOG] performance status of 2 to 4).13
Patients were not included if they had any of the following conditions: lymphoblastic or Burkitt lymphoma with bone marrow or CNS involvement; primary cerebral lymphoma; history of low-grade lymphoma; positive serology to human immunodeficiency virus; previous treatment with chemotherapy, radiotherapy, or organ transplantation; concomitant or previous cancer (except in situ cervix carcinoma or skin epithelioma); congestive heart failure; recent myocardial infarction or conduction abnormalities; uncontrolled diabetes mellitus; and liver or kidney failure. This study was approved by the Hôpital Saint Louis (Paris, France) institutional review board. Informed consent was provided according to the Declaration of Helsinki.
Histologic analysis
A review of histologic material by 2 independent pathologists from the GELA was planned in the protocol and carried out in 92% of the enrolled patients. Lymphomas were initially classified according to the Working Formulation.14 Results were subsequently converted by the committee pathologists according to the new World Health Organization (WHO) classification.15
Staging
The extent of the disease was studied by physical examination, computerized tomographic scan of chest and abdomen, cerebrospinal fluid examination, bone marrow biopsy, and other investigational procedures according to clinical symptoms. The number of extranodal sites and the diameter of the largest tumor mass were determined. Patients were staged according to the Ann Arbor classification. Performance status was assessed according to the ECOG scale (0 to 4).16 Serum LDH level was expressed as the ratio over the maximum normal value.
Treatments
After giving written informed consent, patients were randomly assigned to receive either ACVBP or CHOP regimen. The ACVBP regimen consisted of 4 induction courses given every 3 weeks of doxorubicin (75 mg/m2) on day 1, cyclophosphamide (1200 mg/m2) intravenously on day 1, vindesine (2 mg/m2) on days 1 and 5, bleomycin (10 mg) on days 1 and 5, prednisone (60 mg/m2) orally from day 1 to day 5, and intrathecal methotrexate (15 mg) on day 2. Granulocyte-macrophage or granulocyte colony-stimulating factor was administered subcutaneously on days 6 through 13 of each cycle.17 Patients then received a sequential consolidation therapy with 2 courses of intravenous methotrexate (3 g/m2) plus leucovorin rescue, 4 courses of etoposide (300 mg/m2) and ifosfamide (1500 mg/m2) with mesna protection, and 2 courses of cytosine-arabinoside (100 mg/m2) subcutaneously for 4 days, each consolidation course being administered at a 14-day interval. The CHOP regimen was administered as described by Coltman et al,3 namely 8 cycles with a 3-week interval of doxorubicin (50 mg/m2) intravenously on day 1, cyclophosphamide (750 mg/m2) intravenously on day 1, vincristine (1.4 mg/m2) intravenously on day 1 (with the dose capped at 2.0 mg), and prednisone (40 mg/m2) orally from day 1 to day 5. No growth factor support and no CNS prophylaxis were planned in this group. In each treatment arm no dose adjustment was planned according to hematologic toxicity, but courses were postponed until leukocyte count rose above 2.0 × 109/L and platelet count rose above 100 × 109/L. The ECOG criteria were used to evaluate toxicity.16
Dose intensity of drugs could be compared only for doxorubicin and cyclophosphamide during the 4 first cycles of each treatment regimen. The received dose intensity was calculated as previously described.18
Response
In each treatment arm, response was assessed by repeat staging 4 weeks after the therapy ended. Disappearance of all lesions and laboratory abnormalities related to the lymphoma-defined complete remission (CR). A decrease of more than 75% of the measurable lesions with disappearance of laboratory abnormalities was considered as unconfirmed CR (CRu).19 Partial response (PR) was defined by a 50% to 75% regression of the tumor size. Stable disease was defined by regression by less than 50% of any measurable lesion. Patients who had progressive disease at any time were considered as failure and were given other treatment at the discretion of the treating physician.
Statistical methods
Study design. This study was a prospective, randomized study. Randomization was stratified according to the participating centers by sets of 4 successive patients. The randomization was generated by the GELA program coordinating center, which issued treatment allocation by telephone after confirmation of the patient eligibility. Case report forms collected at participating centers were sent to the coordinating center and keyed-in twice for verification. Outliers and erroneous values were checked routinely.
The main objective of the trial was to detect a 10% difference between ACVBP and CHOP regimens on the assumption of a 25% 2-year event-free survival rate in the CHOP arm (2-sided test: type I error of 0.05, type II error of 0.10). Secondary end points were response to treatment, toxicity, disease-free survival, number of CNS relapse, and overall survival. This design required the randomization of 600 eligible patients.
Statistical analyses. The stopping date was August 1, 2001. Patient characteristics, complete remission rates, and frequencies of adverse reactions were compared by the chi-square and Fisher exact tests.20 Event-free survival was measured from the date of randomization to disease progression, relapse, or death from any cause or the stopping date. Disease-free survival was measured from the date of remission to either relapse or death from any cause or the stopping date. Overall survival was measured from the date of randomization to either death from any cause or the stopping date. When the stopping date was not reached, data were censored at the date of the last follow-up evaluation. The survival functions were estimated by the Kaplan-Meier method21 and compared by log-rank test.22 Cox proportional hazards model23 was used to assess treatment effect controlling for the values of the age-adjusted IPI.13 Tests for comparison were regarded as significant if the 2-sided P value was less than .05.
Results
Patient characteristics
Between March 1993 and June 1998, 708 patients were registered for the study by the 84 participating centers. Seventy-three patients (ACVBP group, 36; CHOP group, 37) were considered ineligible for the following reasons: incorrect histology as determined by central review, 50 patients (carcinoma, 7; Hodgkin disease, 5; lymphocytic lymphoma, 7; follicular lymphoma, 22; mantle cell lymphoma, 8; other lymphoma, 1); Burkitt lymphoma with bone marrow involvement, 1 patient; positive serology for HIV, 4 patients; age-adjusted IPI equal to 0, 3 patients; younger than 61 years of age, 11 patients; and previous cancer, 4 patients. Thus, 635 patients were eligible for analysis; 323 patients were allocated to ACVBP treatment and 312 to CHOP. As shown in Table 1, the 2 groups were well balanced with respect to major prognostic factors.
Initial characteristics of the patients according to the treatment group
. | ACVBP n = 323 (%) . | CHOP n = 312 (%) . | P . |
|---|---|---|---|
| Median age, y | 65 | 65 | |
| Sex | 0.9 | ||
| Male | 182 (56) | 177 (57) | |
| Female | 141 (44) | 135 (43) | |
| Histology | 0.8 | ||
| Diffuse large B cell | 257 (80) | 244 (78) | |
| Burkitt | 5 (1) | 6 (2) | |
| Lymphoblastic | 3 (1) | 3 (1) | |
| Peripheral T-cell NOS | 29 (9) | 28 (9) | |
| Angio-immunoblastic T cell | 8 (2) | 11 (4) | |
| Anaplastic large-cell T/NK | 12 (4) | 10 (3) | |
| Aggressive unclassifiable | 9 (3) | 10 (3) | |
| Immunophenotype* | 0.5 | ||
| B | 263 (85) | 253 (84) | |
| T | 47 (15) | 49 (16) | |
| B symptoms* | 0.2 | ||
| Absent | 163 (51) | 142 (46) | |
| Present | 155 (49) | 168 (54) | |
| Performance status (ECOG) | 1 | ||
| 0-1 | 220 (68) | 212 (68) | |
| Greater than 1 | 103 (32) | 100 (32) | |
| Ann Arbor stage | 0.6 | ||
| I-II | 56 (17) | 59 (19) | |
| III-IV | 267 (83) | 253 (81) | |
| Number of extranodal sites* | 0.8 | ||
| 0-1 | 163 (51) | 156 (50) | |
| More than 1 | 155 (49) | 156 (50) | |
| Sites of extranodal involvement* | |||
| Bone marrow | 78 (24) | 96 (31) | 0.08 |
| Liver | 34 (10) | 44 (14) | 0.2 |
| Spleen | 78 (24) | 75 (24) | 0.9 |
| Skin | 24 (7) | 14 (4) | 0.1 |
| Lungs | 27 (8) | 39 (12) | 0.09 |
| Head and Neck | 34 (11) | 28 (9) | 0.5 |
| Epidural involvement | 9 (3) | 10 (3) | 0.8 |
| Serum LDH level | 0.1 | ||
| N or less | 92 (28) | 71 (23) | |
| Greater than N | 231 (72) | 241 (77) | |
| Serum albumin level* | 0.4 | ||
| Less than 35 g/L | 169 (56) | 151 (52) | |
| 35 g/L or greater | 133 (44) | 138 (48) | |
| Number of age-adjusted IPI factors | 0.6 | ||
| 1 | 113 (35) | 105 (34) | |
| 2 | 142 (44) | 130 (42) | |
| 3 | 68 (21) | 77 (24) |
. | ACVBP n = 323 (%) . | CHOP n = 312 (%) . | P . |
|---|---|---|---|
| Median age, y | 65 | 65 | |
| Sex | 0.9 | ||
| Male | 182 (56) | 177 (57) | |
| Female | 141 (44) | 135 (43) | |
| Histology | 0.8 | ||
| Diffuse large B cell | 257 (80) | 244 (78) | |
| Burkitt | 5 (1) | 6 (2) | |
| Lymphoblastic | 3 (1) | 3 (1) | |
| Peripheral T-cell NOS | 29 (9) | 28 (9) | |
| Angio-immunoblastic T cell | 8 (2) | 11 (4) | |
| Anaplastic large-cell T/NK | 12 (4) | 10 (3) | |
| Aggressive unclassifiable | 9 (3) | 10 (3) | |
| Immunophenotype* | 0.5 | ||
| B | 263 (85) | 253 (84) | |
| T | 47 (15) | 49 (16) | |
| B symptoms* | 0.2 | ||
| Absent | 163 (51) | 142 (46) | |
| Present | 155 (49) | 168 (54) | |
| Performance status (ECOG) | 1 | ||
| 0-1 | 220 (68) | 212 (68) | |
| Greater than 1 | 103 (32) | 100 (32) | |
| Ann Arbor stage | 0.6 | ||
| I-II | 56 (17) | 59 (19) | |
| III-IV | 267 (83) | 253 (81) | |
| Number of extranodal sites* | 0.8 | ||
| 0-1 | 163 (51) | 156 (50) | |
| More than 1 | 155 (49) | 156 (50) | |
| Sites of extranodal involvement* | |||
| Bone marrow | 78 (24) | 96 (31) | 0.08 |
| Liver | 34 (10) | 44 (14) | 0.2 |
| Spleen | 78 (24) | 75 (24) | 0.9 |
| Skin | 24 (7) | 14 (4) | 0.1 |
| Lungs | 27 (8) | 39 (12) | 0.09 |
| Head and Neck | 34 (11) | 28 (9) | 0.5 |
| Epidural involvement | 9 (3) | 10 (3) | 0.8 |
| Serum LDH level | 0.1 | ||
| N or less | 92 (28) | 71 (23) | |
| Greater than N | 231 (72) | 241 (77) | |
| Serum albumin level* | 0.4 | ||
| Less than 35 g/L | 169 (56) | 151 (52) | |
| 35 g/L or greater | 133 (44) | 138 (48) | |
| Number of age-adjusted IPI factors | 0.6 | ||
| 1 | 113 (35) | 105 (34) | |
| 2 | 142 (44) | 130 (42) | |
| 3 | 68 (21) | 77 (24) |
NOS indicates not otherwise specified; NK, natural killer; N, normal value; and IPI, international prognostic index for aggressive lymphoma.13
Data were unavailable for some patients.
Response to treatment
Response to treatment could be assessed in 612 patients (96%). The rate of complete response (CR and CRu) was 58% in the ACVBP group and 56% in the CHOP group (P = .5). The mean received dose intensity of doxorubicin during the first 4 cycles of treatment was 23.0 mg per square meter per week (92% of the designed dose intensity) in the ACVBP group and 15.7 mg per square meter per week (94% of the designed dose intensity) in the CHOP group, respectively. The mean received dose intensity of cyclophosphamide was 372 mg per square meter per week (93% of the designed dose intensity) in the ACVBP group and 238 mg per square meter per week (95% of the designed dose intensity) in the CHOP group, respectively.
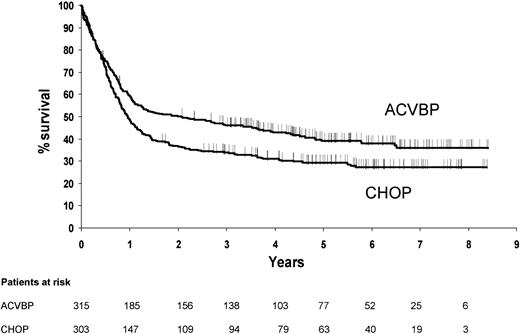
Event-free survival
The median follow-up was 68 months. As shown on Figure 1, the event-free survival was significantly longer for patients treated with ACVBP (P = .005). The 5-year estimated event-free survival rates were 39% in the ACVBP group (95% confidence interval, 0.34 to 0.45) and 29% in the CHOP group (95% confidence interval, 0.24 to 0.34). This difference remained statistically significant in an intent-to-treat analysis of the 708 patients registered for the study (P = .007), in an analysis of the 649 patients who had a central pathology review (P = .004) and in an analysis of the 618 eligible patients remaining after exclusion of the Burkitt and lymphoblastic histologies (P = .005) as shown on Figure 2.
Estimated event-free survival according to treatment group (P = .005).
Estimated event-free survival after exclusion of patients with Burkitt and lymphoblastic lymphoma according to treatment group (P = .005).
Estimated event-free survival after exclusion of patients with Burkitt and lymphoblastic lymphoma according to treatment group (P = .005).
In a multivariate analysis, the treatment group was found to affect event-free survival independently of the age-adjusted IPI (P = .003; risk ratio, 1.34; 95% confidence interval, 1.09 to 1.61). Nine CNS progressions or relapses occurred in the ACVBP group compared with 26 in the CHOP group (P = .002; risk ratio, 2.99; 95% confidence interval, 1.48 to 6.03). As shown in Table 2, most of these progressions occurred during treatment and were restricted to CNS.
Characteristics of the central nervous system recurrences according to the treatment group
. | ACVBP . | CHOP . |
|---|---|---|
| Total | 9 | 26 |
| Histology | ||
| Diffuse large B cell | 7 | 21 |
| Lymphoblastic | 0 | 1 |
| Peripheral T cell | 1 | 2 |
| Aggressive unclassifiable | 1 | 2 |
| Occurrence | ||
| On therapy | 6 | 21 |
| Off therapy | 3 | 5 |
| Isolated CNS recurrence | 7 | 18 |
| With systemic recurrence | 2 | 8 |
| Localization | ||
| Meningeal | 4 | 17 |
| Cerebral | 3 | 5 |
| Both | 2 | 4 |
. | ACVBP . | CHOP . |
|---|---|---|
| Total | 9 | 26 |
| Histology | ||
| Diffuse large B cell | 7 | 21 |
| Lymphoblastic | 0 | 1 |
| Peripheral T cell | 1 | 2 |
| Aggressive unclassifiable | 1 | 2 |
| Occurrence | ||
| On therapy | 6 | 21 |
| Off therapy | 3 | 5 |
| Isolated CNS recurrence | 7 | 18 |
| With systemic recurrence | 2 | 8 |
| Localization | ||
| Meningeal | 4 | 17 |
| Cerebral | 3 | 5 |
| Both | 2 | 4 |
CNS indicates central nervous system.
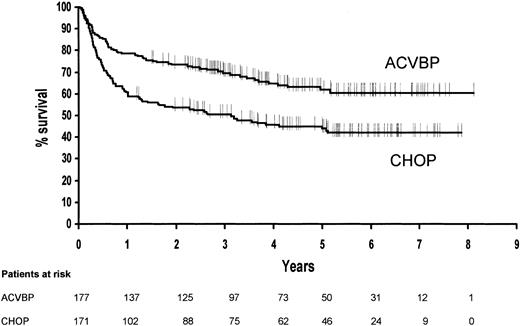
Disease-free survival
Sixty-three of the 177 patients in complete remission in the ACVBP group relapsed compared with 95 of the 171 patients in the CHOP group. At 5 years, the estimated disease-free survival rates were 62% (95% confidence interval, 0.54 to 0.70) in the ACVBP group and 44% (95% confidence interval, 0.36 to 0.52) in the CHOP group (P = .0002) (Figure 3).
Estimated disease-free survival for patients in complete remission according to treatment group (P = .0002).
Estimated disease-free survival for patients in complete remission according to treatment group (P = .0002).
Overall survival
There were 175 deaths among the patients treated with ACVBP and 200 among those treated with CHOP (Figure 4). The 5-year estimated survival rates were 46% in the ACVBP group (95% confidence interval, 0.40 to 0.52) and 38% in the CHOP group (95% confidence interval, 0.32 to 0.43). The difference in overall survival between the 2 treatment groups was statistically significant (P = .036). This difference remained significant in an intent-to-treat analysis of the 708 patients registered (P = .039), in an analysis of the 649 patients who had a central pathology review (P = .023), and in an analysis of the 618 eligible patients remaining after exclusion of the Burkitt and lymphoblastic histologies (P = .039) (Figure 5). In a multivariate analysis, this advantage in overall survival appeared to be independent of the age-adjusted IPI (P = .028; risk ratio, 1.26; 95% confidence interval, 1.01 to 1.53).
Estimated overall survival after exclusion of patients with Burkitt and lymphoblastic lymphoma according to treatment group (P = .039).
Estimated overall survival after exclusion of patients with Burkitt and lymphoblastic lymphoma according to treatment group (P = .039).
Toxicity
As shown in Table 3, the patients treated with ACVBP had a higher rate of leukopenia, thrombocytopenia as well as infection, and mucositis than those treated with CHOP. The incidence of grade 3 and 4 leukopenia and thrombocytopenia was significantly higher in the ACVBP group (P < .001) leading to a higher incidence of severe or life-threatening infections (P < .001). Treatment-related deaths were significantly more frequent in the ACVBP group with 43 deaths (infectious complication, 28; methotrexate overdose or allergy, 4; cerebral vascular accident, 3; hepatic failure, 2; cardiac failure, 2; pulmonary toxicity, 1; bowel occlusion, 1; unexplained death, 2) compared with 23 deaths among patients treated with CHOP (infectious complication, 14; cerebral vascular accident, 3; hepatic failure, 1; pulmonary toxicity, 1; tumor lysis syndrome, 1; unexplained death, 3) (P = .014). In an explanatory multivariate analysis, performance status greater than 2 at the time of diagnosis was found to be the most important factor influencing the risk of treatment-related death in both groups (ACVBP; P = .0004, CHOP; P = .0003, respectively). In addition, an age older than 65 years was also correlated with this risk in the ACVBP group (ACVBP; P = .004, CHOP; P = .98, respectively).
Percent of worst toxicity according to the treatment group
. | Percent of group . | . | . | . | . | . | . | . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | ACVBP . | . | . | . | . | CHOP . | . | . | . | . | . | |||||||||
| Toxicity grade . | 1 . | 2 . | 3 . | 4 . | 5 . | 1 . | 2 . | 3 . | 4 . | 5 . | P . | |||||||||
| Leukopenia | 0.3 | 1.7 | 5.1 | 91.5 | 0 | 3.6 | 8.6 | 19.0 | 53.8 | 0 | .001 | |||||||||
| Thrombocytopenia | 5.9 | 13.4 | 19.3 | 48.3 | 0 | 9.8 | 6.9 | 4.7 | 10.6 | 0 | .001 | |||||||||
| Infection | 13.9 | 33.7 | 18.1 | 8.1 | 8.7 | 13.9 | 21.0 | 8.1 | 2.0 | 4.5 | .001 | |||||||||
| Mucositis | 10.3 | 16.0 | 20.7 | 10.0 | 0 | 8.7 | 11.9 | 3.8 | 0.7 | 0 | .001 | |||||||||
| Cardiac | 3.0 | 5.0 | 2.7 | 2.0 | 0.6 | 3.8 | 3.1 | 3.1 | 0.3 | 0.6 | .2 | |||||||||
| Hepatic | 8.8 | 5.4 | 1.4 | 0.6 | 0.6 | 3.8 | 3.1 | 3.1 | 0.3 | 0.3 | .5 | |||||||||
| Neurologic | 8.5 | 7.8 | 2.7 | 0.9 | 0.9 | 14.6 | 9.0 | 3.8 | 2.1 | 0.9 | .1 | |||||||||
. | Percent of group . | . | . | . | . | . | . | . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | ACVBP . | . | . | . | . | CHOP . | . | . | . | . | . | |||||||||
| Toxicity grade . | 1 . | 2 . | 3 . | 4 . | 5 . | 1 . | 2 . | 3 . | 4 . | 5 . | P . | |||||||||
| Leukopenia | 0.3 | 1.7 | 5.1 | 91.5 | 0 | 3.6 | 8.6 | 19.0 | 53.8 | 0 | .001 | |||||||||
| Thrombocytopenia | 5.9 | 13.4 | 19.3 | 48.3 | 0 | 9.8 | 6.9 | 4.7 | 10.6 | 0 | .001 | |||||||||
| Infection | 13.9 | 33.7 | 18.1 | 8.1 | 8.7 | 13.9 | 21.0 | 8.1 | 2.0 | 4.5 | .001 | |||||||||
| Mucositis | 10.3 | 16.0 | 20.7 | 10.0 | 0 | 8.7 | 11.9 | 3.8 | 0.7 | 0 | .001 | |||||||||
| Cardiac | 3.0 | 5.0 | 2.7 | 2.0 | 0.6 | 3.8 | 3.1 | 3.1 | 0.3 | 0.6 | .2 | |||||||||
| Hepatic | 8.8 | 5.4 | 1.4 | 0.6 | 0.6 | 3.8 | 3.1 | 3.1 | 0.3 | 0.3 | .5 | |||||||||
| Neurologic | 8.5 | 7.8 | 2.7 | 0.9 | 0.9 | 14.6 | 9.0 | 3.8 | 2.1 | 0.9 | .1 | |||||||||
Discussion
In this randomized trial, we found a significant survival benefit for patients with poor-prognosis aggressive non-Hodgkin lymphoma who were treated with the ACVBP regimen compared with those treated with standard CHOP. As the complete remission rates were similar in the 2 treatment groups, the advantage in the ACVBP appeared to be related to a longer disease-free survival, with an 18% difference at 5 years. Several randomized studies have compared CHOP with modified CHOP-like regimens24-26 or other chemotherapy combinations as m-BACOD, MACOP-B (methotrexate, doxorubicin, cyclophosphamide, vincristine, bleomycin, prednisone), or PROMACECytaBOM (prednisone, doxorubicin, cyclophosphamide, etoposide, cytarabine, bleomycin, vincristine).7,8,27-30 With the exception of the study from the Australian and New Zealand Lymphoma Group,30 all of the studies failed to show any advantage of the new regimen.
A small proportion of patients included in this trial had a lymphoblastic or a Burkitt lymphoma without marrow or CNS involvement. These patients are now treated in specific programs but, at the time of trial activation, previous GELA studies had shown that the outcome of such patients with lymphoblastic lymphoma treated with either CHOP, ACVBP, or a leukemia-derived scheme did not differ31 and that the outcome of patients with Burkitt lymphoma treated with ACVBP was similar to those of patients with other aggressive lymphomas.32 The exclusion of patients with lymphoblastic or Burkitt histology from survival studies did not modify the results of the present study. Major prognostic factors were well balanced between the treatment groups. A nonsignificant excess of bone marrow and lung involvement was noted in the CHOP group but this did not translate into unbalanced numbers of extranodal sites or repartition of IPI factors.
It is not yet clear which part of the ACVBP regimen is the most important in reducing the risk of relapse. Influence of dose intensity on treatment outcome in aggressive non-Hodgkin lymphoma is debatable. Several retrospective studies have shown that a decrease in dose intensity, compared with the planned schedule, was associated with a decreased response rate and a shorter survival.18,33,34 However, a prospective study failed to demonstrate that increasing dose intensity might positively influence outcome.35 Only recently, the German Lymphoma Study Group has shown that shortening intervals between CHOP cycles could improve treatment results.36 Our trial was not designed as a dose intensity study but the received dose intensity of doxorubicin and cyclophosphamide during the first 4 cycles was respectively 46% and 56% higher in the patients treated with ACVBP.
The sequential consolidation phase of ACVBP regimen introduces drugs not administered during the induction phase of treatment, such as high-dose methotrexate, etoposide, ifosfamide, and cytosine-arabinoside. Except for the usual risks related to high-dose methotrexate,37 toxicity of this phase is reduced and it is delivered in an outpatient setting. Although high-dose therapy with stem cell rescue has been shown to be superior to this sequential consolidation phase in younger responding patients with poor-risk lymphoma,12 2 prospective studies from our group failed to demonstrate a benefit of a conventional intensification of this phase.10,38
CNS recurrence is an almost uniformly fatal complication in aggressive lymphoma. Its incidence in patients treated with CHOP or CHOP-like regimens ranges from 5% to 11%.39 Risk factors for CNS relapse are advanced stage, involvement of more than one extranodal site, elevated LDH level, and poor performance status, all factors identified in the IPI.13 In the present study, these risk factors were equally balanced between the treatment groups. By contrast, the incidence of isolated CNS relapse in patients with aggressive lymphoma treated with ACVBP regimen has been estimated to be 1.6%.40 In this regimen, a CNS prophylaxis is administered, consisting of 4 intrathecal injections of methotrexate during induction followed by 2 consolidative courses of high-dose intravenous methotrexate. Results of the present trial confirm that such a prophylaxis is necessary in patients with poor-risk aggressive lymphoma. As only one patient with lymphoblastic lymphoma experienced a CNS relapse after CHOP therapy, it is unlikely that inclusion of such patients in this trial could have influenced this risk.
The first cycles of ACVBP have a regular hematologic toxicity, a grade 4 leukopenia being the rule in this population of patients older than 60 years of age. Growth factors reduce the duration of leukopenia and decrease the risk of severe and life threatening infection.17 However, in this trial, severe or fatal infections occurred in 36% of the patients treated with ACVBP compared with 15% of those treated with CHOP. This justifies a close monitoring of patients treated with ACVBP in the early phase of the treatment, especially those with an initial poor performance status or those older than 65 years of age. As toxicity is strongly reduced in patients younger than 60 years of age,9-12 it is reasonable to assume that the advantage of ACVBP over standard CHOP should be confirmed in this population.
We have recently shown that the addition of rituximab, a monoclonal anti-CD20 antibody, to CHOP prolongs survival of elderly patients with diffuse large B-cell lymphoma.41 These results have prompted us to investigate the association of rituximab and ACVBP in patients younger than 65 years of age.
We conclude that, in patients with poor-risk aggressive lymphoma, the ACVBP regimen significantly improves event-free and overall survival compared with CHOP, despite its higher toxicity, especially in patients older than 65 years of age. A prophylaxis of CNS relapse is mandatory in this population.
Appendix
The following investigators participated in the study: N. Albin, C. Allard, M. Aoudjane, D. Assouline, B. Audhuy, M. Azagury, J. C. Barats, C. Beaumont, E. Baumelou, P. Biron, D. Bordessoulle, R. Bouabdallah, C. Bouleuc, P. Bourquard, P. Bourquelot, F. Boué, D. Boulat, P. Brice, J. Brière, G. Brun, D. Caillot, O. Casasnovas, P. Carde, S. Castaigne, S. Chèze, B. Christian, P. Colin, C. Collet, T. Conroy, T. Cosnard, B. Corront, H. Curé, A. Delmer, V. Delwail, L. Detourmignies, A. Devidas, H. Dombret, J. F. Dor, C. Doyen, F. Dreyfus, S. Dront, G. Dupont, B. Dupriez, J. C. Eisenmann, M. Fabbro, G. Fillet, M. Flesch, C. Fruchart, J. Gabarre, C. Haioun, O. Hermine, F. Huguet, M. Janvier, E. Jourdan, J. M. Karsenti, Y. Kerneis, F. Kohser, V. Leblond, P. Lederlin, S. Lefort, P. Lenain, G. Lepeu, S. Lepretre, X. Levaltier, A. Le Rol, G. Marit, C. Martin, F. Mayer, C. Nouvel, P. Morel, M. Moriceau, J. N. Munck, G. Nedelec, F. Offner, J. M. Pavlovitch, I. Plantier, P. Y. Péaud, A. M. Peny, J. Pico, C. Platini, J. P. Pollet, B. Quesnel, O. Reman, B. Richard, R. Riou, P. Rodon, J. F. Rossi, B. Salles, G. Salles, C. Sarazin, D. Schlaifer, C. Sebban, M. Simon, P. Solal-Celigny, J. J. Sotto, A. Stamatoullas, G. Tertian, A. Thyss, J. D. Tigaud, G. Tobelem, C. Traullé, P. Travade, A. Van Hoof, A. Zaniboni, and J. M. Zini. The following members of the Groupe d'Etude des Lymphomes de l'Adulte performed the pathologic review: T. Molina, C. Guettier, J. Brière, J. Diebold, B. Fabiani, P. Gaulard, and T. Petrella.
A complete list of the members of the Groupe d'Etude des Lymphomes de l'Adulte appears in the “Appendix.”
Prepublished online as Blood First Edition Paper, August 14, 2003; DOI 10.1182/blood-2003-02-0542.
Supported by grants from the Programme Hospitalier de Recherche Clinique (AOM95061) from the Ministère de la Santé and by grants from Amgen, Roche, Schering-Plough, and Asta Medica.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Isabelle Gaillard, Denis Collet, Laure Di Tullio, and Nicolas Nio for their skillful assistance in data management.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal