Abstract
Major histocompatibility complex (MHC) class I presentation of exogenous antigens is the mechanism enabling professional antigen-presenting cells (APCs) to induce CD8+ T-cell responses against viruses and tumors that do not have access to the classical MHC class I pathway. We have characterized the uptake, processing, and MHC class I cross-presentation by human dendritic cells (DCs) of cell-associated antigens derived from physiologically relevant sources, namely, vaccinia virus-infected apoptotic and necrotic cells. We show that cross-presentation is a rapid process, detectable within 2 to 4 hours after uptake of dead cells, and that proteolysis by cathepsin D in an acidic endosomal compartment is essential for cross-presentation. The presentation is abolished when the phagocytic or macropinocytic functions of the cells are inhibited and is dependent on transporter associated with antigen processing, sensitive to brefeldin A, and requires functional proteasomes. Altogether, these data suggest that antigens derived from apoptotic and necrotic cells require access to the cytosol to intersect with the conventional MHC class I pathway for presentation of cytosolic proteins. (Blood. 2003;102:4448-4455)
Introduction
In vivo and in vitro, professional antigen-presenting cells (APCs), in particular dendritic cells (DCs), are capable of processing cell-associated antigens for presentation on major histocompatibility complex (MHC) class I molecules. This phenomenon was first described in vivo in a mouse model and termed cross-priming.1 Cross-priming has physiologic importance for the initiation of immune responses against tumors and viruses that do not have access to the classical pathway for MHC class I presentation in APCs.2 Furthermore, it is an essential element in the maintenance of peripheral tolerance to self-antigens, that is, “cross-tolerance.”3
In vivo in mice, the DC responsible for cross-priming CD8+ T cells is primarily the CD11C+/CD8α+ DC, at least for cell-associated antigens.4,5 This has been confirmed by in vivo depletion of CD11c+ DCs, which abrogated cross-priming of cell-associated and bacterial antigens in mice. In vitro, several studies showed that human myeloid DCs efficiently cross-present antigens, in particular cell-associated antigens.6-9 Presentation by human DCs of antigens derived from virus-infected apoptotic and necrotic cells can efficiently activate antigen-specific T cells.6-9
MHC class I presentation of exogenous antigens by APCs can occur via at least 2 distinct mechanisms.10-14 Antigens derived from many sources, for example, soluble proteins, immune complexes, and protein-coated beads, can be conveyed from the endocytic compartment into the cytosol in APCs.10-13,15,16 In the cytosol, antigens are degraded into oligopeptides and transported via transporter associated with antigen processing (TAP) into the endoplasmic reticulum (ER) for loading onto newly synthesized MHC class I molecules.10-13 In addition, cell-associated viral antigens appear to require TAP.17 Alternatively, antigens can be processed in endosomal compartments where peptides are generated, acquired by recycling MHC class I molecules, and transported to the cell surface for presentation.15,16 Soluble protein, multibranched lysine with attached peptides, and proteins chaperoned by heat shock proteins (HSPs) have been shown to use this route for presentation.11,14,18 Nearly all studies of cross-presentation pathways, however, used manipulated antigens or artificial particles, for example, protein-coated beads that do not naturally occur as antigen sources in vivo.12,16,19-21 In vivo, immune complexes, opsonized antigens, and apoptotic and necrotic cells should be the major sources of exogenous antigens for cross-presentation.
The pathways used by DCs for MHC class I cross-presentation have not been well characterized. Rodriquez et al showed that uptake via Fc-receptors (FcRs) of immune-complexed antigens by the murine DC line D1 delivered the antigen into the endosomes followed by an active and selective transport of free antigen out to the cytosol.22 Moreover, this presentation of ovalbumin (OVA) was dependent on cleavage by proteasomes.22 The same was true for antigen derived from human cytomegalovirus (CMV)-infected fibroblasts by human DCs9 and vaccinia-infected fibroblasts by mouse macrophages and DCs.23
Therefore, the aim of this study was to characterize the cross-presentation pathways used by human DCs for antigens derived from apoptotic and necrotic cells.6 We used apoptotic or necrotic monocytes infected with recombinant vaccinia virus expressing influenza A virus matrix protein 1 (MP). We found that dead cells are internalized by DCs via mechanisms dependent on cytoskeletal actin rearrangements or functional ion channels, which are required for phagocytosis and macropinocytosis. After entering the cell, the antigens are enclosed in the cell's endosomal compartment and shipped from neutral to subsequently more acidic endosomal vesicles. In a late acid endosomal compartment, the antigens undergo proteolysis and this involves the aspartic protease cathepsin D, which appears to be essential for cross-presentation of MP. Cathepsin D may be required for degradation of the antigens or antigen complexes into sizes/forms that can be transported out to the cytosol. When the antigen has entered the cytosol it is processed by the proteasome. The proteasome products are transported via TAP into the lumen of the ER, where the peptides are loaded on newly synthesized MHC class I molecules and subsequently transported out to the DC surface for presentation. To our knowledge, this is the first study that has fully characterized the cross-presentation pathway in human DCs.
Materials and methods
Culture medium, cytokines, and reagents
Culture medium RPMI 1640 (Gibco BRL, Gaithersburg, MD) was supplemented with 20 μg/mL gentamicin (Gibco BRL), 1 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; Mediatech, Herndon, VA) and 1% human plasma, 5% pooled human serum (PHS, c-Six Diagnostics, Mequon, WI) or 5% single-donor serum. Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF; 5.6 × 103 IU/μg; Immunex, Seattle, WA) and recombinant human interleukin 4 (IL-4; 0.5 × 105 U/μg; R&D Systems, Minneapolis, MN) were purchased for in vitro generation of DCs. Cytochalasin D (CCD), dimethyl amiloride (DMA), NH4Cl, cathepsin D, pepstatin A, leupeptin, lactacystin, and brefeldin A were purchased from Sigma (St Louis, MO).
Cell isolation
Leukocyte-enriched buffy coats were obtained from the New York Blood Center. Peripheral blood human mononuclear cells (PBMCs) were separated by density gradient centrifugation on Ficoll-Hypaque (Amersham Pharmacia Biotech, Piscataway, NJ). Monocyte-enriched and T cell-enriched fractions were prepared by rosetting PBMCs with neuraminidase-treated (Calbiochem-Novabiochem, La Jolla, CA) sheep red blood cells as previously described.24
DCs
DCs were generated by culturing the DC progenitor with 100 IU/mL GM-CSF (5.6 × 103 IU/μg; Immunex) and 300 U/mL IL-4 (0.5 × 105 U/μg; Schering-Plough, Kenilworth, NJ) for 5 days. The cytokines were added to the cultures at day 0, 2, and 4. On day 5, nonadherent immature DCs (IDCs) were collected, transferred to new plates, and cultured with 20 ng/mL lipopolysaccharide (LPS; Sigma) as a maturation stimulus.
MP-specific CD8+ T-cell clone
An HLA A*0201-restricted, influenza A matrix peptide-specific CD8+ T-cell clone was prepared as previously described.25 In brief, cultures containing MP-specific T cells were cloned by limiting dilution in the presence of irradiated feeder cells, phytohemagglutinin (PHA) and IL-2 (Chiron Therapeutics, Emeryville, CA) to yield clone Flu16. The clone was used at least 15 days after restimulation in vitro, when it was at a resting state.
HLA-A*0201 typing
Expression of HLA A*0201 molecules on PBMCs was determined by staining with a monoclonal antibody (BB7.2, HB-82; American Type Culture Collection, Manassas, VA) against HLA-A*0201, followed by phycoerythrin (PE)-conjugated goat antimouse (GAM F(ab′)2; Biosource, Camarillo, CA). The stained cells were analyzed on a FACScan (Becton Dickinson, San Jose, CA) using CellQuest software.
Recombinant vaccinia virus vectors
Recombinant vaccinia vectors consisted of vaccinia-MP (Vac-MP) encoding the influenza A virus matrix 1 protein and Vac-ICP47 encoding ICP47 protein from herpes simplex virus (HSV) and were kind gifts from Professors B. Moss and J. Yewdell (NIH) and a control vector, Vac WR-eco gpt (Vac-ctr, Virogenetics, Troy, NY).
Virus infection of cells
PBMCs were washed in RPMI and the recombinant vaccinia viruses were added to cells (107/mL) at a multiplicity of infection (MOI) of 2. The infection was carried out at 37°C for 1 hour in RPMI with 1% human plasma, after which infected cells were washed 3 times in RPMI containing 5% PHS. Vaccinia-infected monocytes were recultured for 12 to 16 hours and IDCs for 4 to 5 hours before they were used in assays.
Induction of apoptosis and necrosis in vaccinia-infected monocytes
To generate apoptotic or necrotic cells, we used human monocytes previously infected with recombinant vaccinia virus Vac-ctr or Vac-MP. UV-triggered apoptosis was induced using a 60-mJ UVB lamp (Derma Control, Dalton, IL), calibrated to provide 2 mJ/cm2/s and necrosis was induced by 4 to 5 repetitive freeze and thaw cycles. More than 80% of the cells were apoptotic within 4 to 8 hours and used in assays about 4 hours after induction of cell death (M. Larsson, unpublished data, January 19, 2000).
Cross-presentation by DCs
IDCs were pretreated for about 40 minutes at 37°C with no or increasing doses of the inhibitor used in the assay. After preincubation, DCs were added to wells containing apoptotic or necrotic monocytes or 100 nM MP peptide and the same doses of inhibitor and cultured for 10 to 12 hours. These inhibitors were used at the following doses: 0 to 10 μM CCD, 0 to 200 μM DMA, 0 to 60 mM NH4Cl, 0 to 2 μM cathepsin D, 0 to 300 μM pepstatin A, 0 to 200 μM leupeptin, 0 to 80 μM lactacystin, and 0 to 10 μg/mL brefeldin A. The maturation stimulus LPS (20 ng/mL) was added to all wells. The different DC groups were harvested after 10 to 12 hours, washed 2 times in cold phosphate-buffered saline (PBS), and fixed for 30 seconds in 0.01% glutaraldehyde (Sigma). Fixation was stopped by addition of 2 M l-lysine (Sigma) and the cells were washed 2 times in PBS.
ELISPOT assay for detection of IFN-γ release from antigen-specific T cells
The 96-well plates (Millititer, Millipore, Bedford, MA) were coated overnight at 4°C with 5 μg/mL anti-interferon γ (IFN-γ) monoclonal antibody (Mabtech, Stockholm, Sweden). The antibody-coated plates were washed 4 times with PBS and blocked with RPMI containing 5% PHS for 1 hour at 37°C. The different groups of glutaraldehyde-fixed DCs were added to the wells together with the HLA A*0201+ CD8+ T-cell clone Flu16 and incubated overnight (14-18 hours) at 37°C. Plates were washed, stained, and developed as described previously.26 Only spots with a fuzzy border and a brown color were counted.26
Results
Kinetics of uptake of dead cells: at low concentrations apoptotic cells are presented more efficiently by DCs compared with necrotic cells
We used a previously described system to investigate the cross-presentation pathway by human DCs.6 In brief, HLA mismatch allogeneic human monocytes were infected (approximately 12 hours) with Vac-MP after which they were subjected to induction of apoptosis or necrosis. We used Flu16, a CD8+ T-cell clone, which recognizes the GILGFVFTL epitope of MP in the context of HLA A*0201 to assess the cross-presentation.27
We first evaluated the cross-presentation kinetics of antigen from apoptotic and necrotic cells using this clone. IDCs were cocultured with Vac-MP-infected apoptotic or necrotic monocytes. IDCs were sampled between 0 to 48 hours after coculture with dead cells and fixed with glutaraldehyde to terminate further antigen uptake, processing, and presentation. After fixation, DCs were cocultured with the Flu16 in an IFN-γ ELISPOT assay to assess when the MP epitope was initially presented on MHC class I molecules. Processed antigen derived from dead cells was detected already after 2 to 4 hours. When we used low doses of dead cells per IDCs (0.6:1), the presentation of apoptotic cells was detected earlier (within 2 hours) and at a higher magnitude than antigen from necrotic cells (Figure 1A). In the presence of a maturation stimulus, the differences in presentation efficacy between apoptotic and necrotic cells decreased, especially at later time points (Figure 1B). At low doses, the apoptotic cells are a more efficient delivery system likely due to the fact that antigens are concentrated inside them, whereas for necrotic cells the antigens are dispersed in the culture media. At higher doses of dead cells per DCs (2:1), apoptotic and necrotic cells become equally efficient sources of antigens both for immature and matured DCs (Figure 1C-D). The cross-presentation maximum was reached between 12 and 24 hours, and followed by a plateau or decrease at 48 hours (Figure 1C-D and M. Larsson, unpublished data, June 28, 2000). For subsequent experiments, we chose to use 2 dead cells per DC and cocultured them for 12 hours in the presence of LPS, because this culture condition allowed efficient cross-presentation of both apoptotic and necrotic cell-derived antigens (Figure 1C-D).
Kinetics of human DC-mediated cross-presentation of antigens derived from apoptotic or necrotic cells infected with Vac-MP. Vaccinia-infected apoptotic or necrotic HLA-mismatched monocytes were cocultured with IDCs at a ratio of 0.6 (A-B) or 2 (C-D) per DC, with or without maturation stimuli. The monocytes had previously been infected with Vac-ctr or Vac-MP for 12 hours, prior to undergoing apoptosis or necrosis. DCs were harvested at different time points between 0 and 48 hours, washed, and fixed in glutaraldehyde. The ability of immature (A,C) or mature (B,D) DCs to cross-present MP was assessed with the clone Flu16 in an IFN-γ ELISPOT assay. Controls consisted of dead cells and Flu16 alone or together, which did not yield IFN-γ production (unpublished data). Panels A-D show 1 representative experiment of 4, respectively.
Kinetics of human DC-mediated cross-presentation of antigens derived from apoptotic or necrotic cells infected with Vac-MP. Vaccinia-infected apoptotic or necrotic HLA-mismatched monocytes were cocultured with IDCs at a ratio of 0.6 (A-B) or 2 (C-D) per DC, with or without maturation stimuli. The monocytes had previously been infected with Vac-ctr or Vac-MP for 12 hours, prior to undergoing apoptosis or necrosis. DCs were harvested at different time points between 0 and 48 hours, washed, and fixed in glutaraldehyde. The ability of immature (A,C) or mature (B,D) DCs to cross-present MP was assessed with the clone Flu16 in an IFN-γ ELISPOT assay. Controls consisted of dead cells and Flu16 alone or together, which did not yield IFN-γ production (unpublished data). Panels A-D show 1 representative experiment of 4, respectively.
Presentation of MP endogenously expressed by DCs ensues via the classical pathway for MHC class I presentation
Initially, we assessed the effectiveness of various chemical and biologic inhibitors on the presentation of endogenously expressed MP. These studies were undertaken to ensure that the inhibitors were not toxic in our culture systems. We tested the following reagents: lactacystin (40 μM), brefeldin A (1 μg/mL), vac-ICP47 (2 MOI), pepstatin A (200 μM), and cathepsin D (1 μM). Inhibition of the cytosolic events, that is, proteasome degradation, transport via TAP into ER, and transport on newly synthesized MHC class I molecules out of ER reduced presentation of endogenously expressed MP. This is in agreement with previous findings showing that presentation of the MP peptide requires access to the cytosol and that brefeldin A blocks its presentation28,29 (Figure 2). NH4Cl decreased the endogenous MP presentation, probably due to the reduced infectivity of the vaccinia virus when the endosomal acidification is inhibited.30-33 Pepstatin A and exogenous cathepsin D had no effect on the presentation of endogenously expressed MP by DCs (Figure 2).
Endogenous MP presentation. Immature HLA A*0201+ DCs were infected with Vac-ctr or Vac-MP and treated simultaneously with cathepsin D (1 μM), pepstatin A (200 μM) or NH4Cl (50 μM), brefeldin A (1 μg/mL), lactacystin (40 μM), or were previously infected with Vac-ctr or Vac-ICP47 (MOI 2). Noninfected DCs were incubated with MP peptide (100 nM) and the same dose of inhibitors. A maturation stimulus was given in the beginning of the incubation. All DC groups were fixed in glutaraldehyde and cultured with Flu16. Responses were assessed by IFN-γ ELISPOT. The left axis shows endogenous presentation and the right synthetic peptide presentation. One representative set of experiments of 3 is shown.
Endogenous MP presentation. Immature HLA A*0201+ DCs were infected with Vac-ctr or Vac-MP and treated simultaneously with cathepsin D (1 μM), pepstatin A (200 μM) or NH4Cl (50 μM), brefeldin A (1 μg/mL), lactacystin (40 μM), or were previously infected with Vac-ctr or Vac-ICP47 (MOI 2). Noninfected DCs were incubated with MP peptide (100 nM) and the same dose of inhibitors. A maturation stimulus was given in the beginning of the incubation. All DC groups were fixed in glutaraldehyde and cultured with Flu16. Responses were assessed by IFN-γ ELISPOT. The left axis shows endogenous presentation and the right synthetic peptide presentation. One representative set of experiments of 3 is shown.
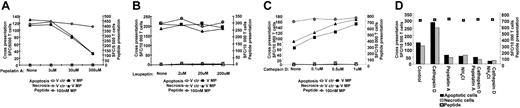
Uptake of dead cells requires functional macropinocytosis or phagocytosis
DCs can internalize exogenous antigens via different mechanisms such as clathrin-mediated endocytosis, fluid-phase endocytosis, macropinocytosis, or phagocytosis.34 To evaluate if uptake via macropinocytosis or phagocytosis is involved in the cross-presentation of cell-associated MP by DCs, we inhibited these processes using DMA and CCD, respectively. DCs were preincubated with DMA (0-300 μM) or CCD (0-10 μM) and subsequently cocultured for about 12 hours with dead cells. The control for MHC class I presentation was DCs pulsed with 100 nM MP peptide and treated in an identical fashion to the inhibitors. At 10 μM, CCD reduced the cross-presentation of antigens derived from dead cells more than 90%, but reduced synthetic peptide presentation only about 10% (Figure 3A). Furthermore, the cross-presentation of apoptotic and necrotic cells was completely blocked with DMA (Figure 3B), whereas MP peptide presentation was reduced 5% to 20% (Figure 3B). Altogether, these data show that cytoskeleton disruption via CCD, or inhibition of ion channel via DMA, prevents the cross-presentation of cell-associated antigens. Our data implicate both phagocytosis and macropinocytosis in this cross-presentation pathway.
Actin cytoskeletal reorganization and ion channels are necessary for uptake and presentation of antigen from apoptotic and necrotic cells. Immature HLA A*0201+ DCs were preincubated with increasing doses of CCD or DMA. Pretreated DCs were thereafter cocultured at a 1:2 ratio with HLA-mismatched apoptotic or necrotic monocytes expressing MP and the same dose of inhibitor for 10 to 12 hours. DCs pulsed with 100 nM MP peptide were used as controls. All groups of CCD-treated (A) or DMA-treated (B) DCs were fixed with glutaraldehyde cocultured with Flu16; responses were monitored by IFN-γ ELISPOT assay. The left axis shows cross-presentation and the right peptide presentation. Controls consisting of dead cells and Flu16 alone or together did not induce any IFN-γ production (unpublished data). One representative experiment of 4 is shown.
Actin cytoskeletal reorganization and ion channels are necessary for uptake and presentation of antigen from apoptotic and necrotic cells. Immature HLA A*0201+ DCs were preincubated with increasing doses of CCD or DMA. Pretreated DCs were thereafter cocultured at a 1:2 ratio with HLA-mismatched apoptotic or necrotic monocytes expressing MP and the same dose of inhibitor for 10 to 12 hours. DCs pulsed with 100 nM MP peptide were used as controls. All groups of CCD-treated (A) or DMA-treated (B) DCs were fixed with glutaraldehyde cocultured with Flu16; responses were monitored by IFN-γ ELISPOT assay. The left axis shows cross-presentation and the right peptide presentation. Controls consisting of dead cells and Flu16 alone or together did not induce any IFN-γ production (unpublished data). One representative experiment of 4 is shown.
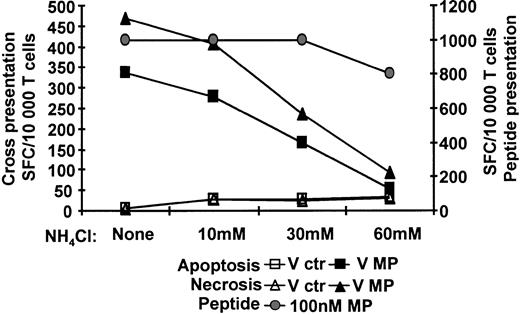
Inhibition of acidification of endosomal compartments blocks DC cross-presentation
After uptake, antigens enter the endocytic pathway and progressively pass through increasingly acidic compartments. Raising the endosomal pH reduces the proteolytic activity of most endosomal proteases and interferes with fusion between vesicles.35 We examined if acidification of the endosomal compartment of human DCs was required for cross-presentation. IDCs were preincubated with increasing concentrations of NH4Cl (0-60 mM), and then cocultured with apoptotic or necrotic monocytes previously infected with Vac-ctr or Vac-MP and maturation stimulus. Neutralization of endosomes with 60 mM NH4Cl blocked more than 75% of the cross-presentation but only 15% to 20% of synthetic peptide presentation (Figure 4). These data show that cross-presentation requires endosomal functions dependent on acidification such as proteolysis by proteases or endosomal fusion, for maximum efficiency. The decrease in synthetic peptide presentation may be due to mild toxicity or negative effects (or both) on the recycling of MHC class I molecules.
Acidification of endosomal compartment is needed for cross-presentation of MP by DCs. IDCs were preincubated with increasing doses of NH4Cl. The NH4Cl-pretreated DCs were thereafter cocultured with HLA-mismatched apoptotic or necrotic monocytes (1:2 ratio) expressing MP together with the same doses of inhibitor and a maturation stimulus for about 12 hours. All groups of DCs were fixed by glutaraldehyde and cultured with Flu16. Responses were determined in an IFN-γ ELISPOT assay. The left axis shows cross-presentation; the right, peptide presentation. One representative experiment of 4 is shown.
Acidification of endosomal compartment is needed for cross-presentation of MP by DCs. IDCs were preincubated with increasing doses of NH4Cl. The NH4Cl-pretreated DCs were thereafter cocultured with HLA-mismatched apoptotic or necrotic monocytes (1:2 ratio) expressing MP together with the same doses of inhibitor and a maturation stimulus for about 12 hours. All groups of DCs were fixed by glutaraldehyde and cultured with Flu16. Responses were determined in an IFN-γ ELISPOT assay. The left axis shows cross-presentation; the right, peptide presentation. One representative experiment of 4 is shown.
Cross-presentation of influenza A MP by DCs involves proteolysis by the aspartic protease cathepsin D but not cysteine and serine proteases
In view of our observation that acidification was necessary for the cross-presentation of MP by DCs, we examined the requirement for aspartic or serine/cysteine proteases in human DCs. We used pepstatin A, an inhibitor of aspartic proteases such as cathepsin D, and leupeptin, an inhibitor of cysteine and serine proteases such as cathepsin B and L,36,37 and tested their effect on cross-presentation. IDCs were preincubated with increasing doses of pepstatin A (0-300 μM) or leupeptin (0-200 μM) and then cocultured with Vac-ctr- or Vac-MP-infected dead cells. Pepstatin A reduced cross-presentation about 70% at 300 μM but had minimum effect on synthetic peptide presentation (Figure 5A). In contrast, leupeptin did not affect either cross-presentation or peptide presentation at the concentrations used (Figure 5B). These results indicate that aspartic but not serine and cysteine proteases are required for DC-mediated cross-presentation of MP. In addition, pepstatin A did not have any effect on endogenous MP presentation (Figure 2). Given the finding that particular cathepsins are required for maximal cross-presentation, we examined whether addition of exogenous cathepsin D could increase this presentation. Therefore, cathepsin D was exogenously added to cocultures of DCs and dead cells. Interestingly, the exogenous cathepsin D enhanced cross-presentation up to 100% at 1 to 2 μM in all experiments (Figure 5C; and M. Larsson, unpublished data, October 9, 2001). The synthetic MP peptide presentation was largely unaffected by cathepsin D (Figure 5C). Furthermore, endogenous MP presentation by the DCs was not affected by exogenous cathepsin D (Figure 2). This demonstrates that the effect on cross-presentation occurs in the endosomal compartments where exogenous MP proteins are located.
Endosomal preprocessing of antigen associated with apoptotic or necrotic cells involves cathepsin D. Immature HLA A*0201+ DCs were preincubated with increasing doses of pepstatin A (A), leupeptin (B), or cathepsin D (C). Pretreated DCs were cocultured about 12 hours with apoptotic or necrotic monocytes expressing MP or with MP peptide and the same doses of the inhibitors along with a maturation stimulus. All DC groups were fixed in glutaraldehyde and cultured with Flu16. Responses were assessed by IFN-γ ELISPOT. (D) IDCs were preincubated with cathepsin D (1 μM) alone, or combined with either pepstatin A (200 μM) or NH4Cl (50 μM). The pretreated DCs were cocultured with apoptotic or necrotic monocytes together with same doses of cathepsin D alone or combined with the inhibitors and exposed to maturation stimuli for about 12 hours (D). All groups of DCs were fixed by glutaraldehyde and assessed with Flu16 in IFN-γ ELISPOT assay. In panels A-D, the left axis shows cross-presentation; the right, peptide presentation. The ratio of dead cells to DCs was 2:1 and the concentration of MP peptide was 100 nM. One representative set of experiments of 4 is shown.
Endosomal preprocessing of antigen associated with apoptotic or necrotic cells involves cathepsin D. Immature HLA A*0201+ DCs were preincubated with increasing doses of pepstatin A (A), leupeptin (B), or cathepsin D (C). Pretreated DCs were cocultured about 12 hours with apoptotic or necrotic monocytes expressing MP or with MP peptide and the same doses of the inhibitors along with a maturation stimulus. All DC groups were fixed in glutaraldehyde and cultured with Flu16. Responses were assessed by IFN-γ ELISPOT. (D) IDCs were preincubated with cathepsin D (1 μM) alone, or combined with either pepstatin A (200 μM) or NH4Cl (50 μM). The pretreated DCs were cocultured with apoptotic or necrotic monocytes together with same doses of cathepsin D alone or combined with the inhibitors and exposed to maturation stimuli for about 12 hours (D). All groups of DCs were fixed by glutaraldehyde and assessed with Flu16 in IFN-γ ELISPOT assay. In panels A-D, the left axis shows cross-presentation; the right, peptide presentation. The ratio of dead cells to DCs was 2:1 and the concentration of MP peptide was 100 nM. One representative set of experiments of 4 is shown.
The heightened cross-presentation is likely due to enhanced proteolysis in the acid endosomal environment after internalization of cathepsin D by DCs, because cathepsin D has no or limited enzymatic activity at pH 7.5, the pH of the culture media.36 To verify this, we examined whether acidification of endosomes was required for the observed increase in cross-presentation by exogenous cathepsin D. DCs were preincubated with 1 μM cathepsin D alone or together with either 200 μM pepstatin A or 50 mM NH4Cl for about 40 minutes and subsequently cocultured with dead cells. Exogenous cathepsin D alone increased the cross-presentation as expected. Notably, cross-presentation was inhibited when cathepsin D was combined with either pepstatin A or NH4Cl (Figure 5D). These experiments demonstrate that exogenous cathepsin D exerts its effect in acidic intracellular compartments and points to an essential role of this enzyme in DC-mediated MHC class I cross-presentation. Based on all the experiments described in Figure 5, we propose that cross-presentation of exogenous antigens derived from dead cells requires preprocessing in late acid endosomal compartments by proteases and in the case of MP the aspartic protease cathepsin D.
Proteasome processing is required for MHC class I cross-presentation of MP-peptide epitope by DCs
In the cytosol, proteins are degraded by proteasomes into shorter peptides.35 To assess if MP derived from dead cells is delivered from endosomes to cytosol and require proteasome degradation for presentation, we used lactacystin. This drug blocks the catalytic activity of the β subunits of the proteasome.38,39 IDCs were preincubated with different doses of (0-80 μM) lactacystin and cocultured with dead cells. Lactacystin blocked more than 75% of the DC cross-presentation capacity at 80 μM but had no substantial effect on synthetic peptide presentation (Figure 6). Furthermore, in experiments with Vac-MP-infected DCs, lactacystin blocked the presentation of MP to the same degree as cross-presentation (Figure 2). Together, these data show that MP needs to be processed by the proteasome in the presenting DC to be presented on MHC class I molecules. More importantly, the results shown in Figures 4 and 5 demonstrate that antigen transferred from dead donor cells was intact to some degree and had not gone through extensive proteolytic degradation before cell death.
Cross-presentation of MP is dependent on protein processing by the proteasome and active transport into the ER via TAP. IDCs were preincubated with increasing doses of lactacystin. The lactacystin-pretreated DCs were cocultured approximately 12 hours with apoptotic or necrotic monocytes expressing MP together with the same doses of inhibitor and maturation stimulus (left axis). The ratio of dead cells to DCs was 2:1. Control DCs were pulsed with 100 nM MP peptide (right axis). All groups of DCs were fixed by glutaraldehyde, cultured with Flu16 and responses assessed by ELISPOT assay. The DC/T cell ratio was 2:1. One representative experiment of 4 is shown.
Cross-presentation of MP is dependent on protein processing by the proteasome and active transport into the ER via TAP. IDCs were preincubated with increasing doses of lactacystin. The lactacystin-pretreated DCs were cocultured approximately 12 hours with apoptotic or necrotic monocytes expressing MP together with the same doses of inhibitor and maturation stimulus (left axis). The ratio of dead cells to DCs was 2:1. Control DCs were pulsed with 100 nM MP peptide (right axis). All groups of DCs were fixed by glutaraldehyde, cultured with Flu16 and responses assessed by ELISPOT assay. The DC/T cell ratio was 2:1. One representative experiment of 4 is shown.
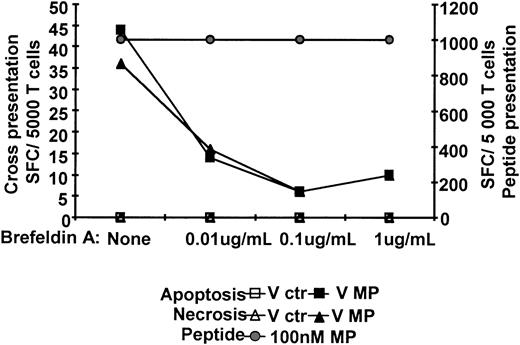
Transport of newly synthesized MHC I class molecules from the Golgi apparatus is required for DCs cross-presentation
Newly synthesized MHC class I molecules loaded with peptides are transported from the ER to the cell surface for presentation. In the presence of brefeldin A, MHC class I complexes were withheld in the ER.35 We examined if cross-presentation of MP requires access to newly synthesized MHC class I molecules in the ER. DCs were incubated with Vac-ctr- or Vac-MP-infected dead cells for 6 to 8 hours and then incubated with increasing doses of brefeldin A (0-1 μg/mL) for 6 hours. Incubation with 1 μg/mL brefeldin A completely blocked cross-presentation and synthetic peptide presentation decreased by 5% to 20% (Figure 7). Furthermore, endogenous MP presentation was blocked in a manner similar to cross-presentation (Figure 2). Therefore, cross-presentation of MP requires access to newly synthesized MHC class I molecules and transport to the cell surface from the ER.
Cross-presentation is dependent on peptide loading and transport of newly synthesized MHC class I from the ER. Immature HLA A*0201+ DCs were cocultured for about 6 hours with apoptotic or necrotic monocytes expressing MP, or MP peptide and a maturation stimulus. Brefeldin A was added at increasing concentrations to the DCs. The ratio of dead cells to DCs was 2:1 and MP peptide concentration 100 nM. All groups of DCs were fixed by glutaraldehyde and cocultured with Flu16 and responses assessed by IFN-γ ELISPOT assay. The DC/T cell ratio was 4:1. One representative experiment of 4 is shown.
Cross-presentation is dependent on peptide loading and transport of newly synthesized MHC class I from the ER. Immature HLA A*0201+ DCs were cocultured for about 6 hours with apoptotic or necrotic monocytes expressing MP, or MP peptide and a maturation stimulus. Brefeldin A was added at increasing concentrations to the DCs. The ratio of dead cells to DCs was 2:1 and MP peptide concentration 100 nM. All groups of DCs were fixed by glutaraldehyde and cocultured with Flu16 and responses assessed by IFN-γ ELISPOT assay. The DC/T cell ratio was 4:1. One representative experiment of 4 is shown.
Cross-presentation of MP-peptide by DCs is dependent on functioning TAP transport
Peptides from 8 up to 16 amino acid residues are transported from the cytosol into the ER lumen in an adenosine triphosphate (ATP)-dependent manner through the TAP.35 Cross-presentation of MP required peptide loading on newly synthesized MHC class I molecules in the lumen of ER. For that reason we examined if this was dependent on or independent of transport via TAP. To study the role of TAP in cross-presentation we used a vaccinia vector encoding the ICP47 protein from HSV (Vac-ICP47). This protein binds to the cytosolic side of TAP and blocks peptide transport into the ER.40 IDCs were infected with Vac-ctr or Vac-ICP47 for 4 to 5 hours and cocultured with apoptotic or necrotic monocytes previously infected with Vac-ctr or Vac-MP together with maturation stimulus for 12 to 16 hours. Sixty to 70% of IDCs infected with Vac-ICP47 expressed high levels of early vaccinia antigens and the recombinant antigen of interest (data not shown).41 ICP47 inhibition of TAP blocked DC cross-presentation up to 85% and MP synthetic peptide presentation about 7% to 20% (Figure 8). Furthermore, endogenous DC presentation of MP was affected in the same manner as cross-presentation (Figure 2). Therefore, functional transport of antigens from the cytosol into the ER via TAP is essential for cross-presentation of cell-associated influenza antigens.
Cross-presentation of MP is dependent on active transport into the ER by TAP. Immature HLA A*0201+ DCs were infected with Vac-ctr or Vac-ICP47 for 4 hours at an MOI of 2. The Vac-ctr- or Vac-ICP47-infected DCs were cocultured about 12 hours with apoptotic or necrotic monocytes expressing MP or MP peptide and a maturation stimulus. The left axis shows cross-presentation; the right, peptide presentation. The ratio of dead cells to DC was 2:1 and MP peptide 100 nM. All groups of DCs were fixed by glutaraldehyde, cocultured with Flu16 and responses assessed by IFN-γ ELISPOT assay. The DC/T cell ratio was 4:1. One representative experiment of 4 is shown.
Cross-presentation of MP is dependent on active transport into the ER by TAP. Immature HLA A*0201+ DCs were infected with Vac-ctr or Vac-ICP47 for 4 hours at an MOI of 2. The Vac-ctr- or Vac-ICP47-infected DCs were cocultured about 12 hours with apoptotic or necrotic monocytes expressing MP or MP peptide and a maturation stimulus. The left axis shows cross-presentation; the right, peptide presentation. The ratio of dead cells to DC was 2:1 and MP peptide 100 nM. All groups of DCs were fixed by glutaraldehyde, cocultured with Flu16 and responses assessed by IFN-γ ELISPOT assay. The DC/T cell ratio was 4:1. One representative experiment of 4 is shown.
In summary, this study is the first to characterize the MHC class I cross-presentation pathway in human DCs. We show for the first time the requirement for proteolysis by the aspartic protease cathepsin D in a late acid endosomal compartment before transport to the cytosol occurs. In the cytosol, the antigen requires degradation by the proteasome and is subsequently transported via TAP into ER for loading on newly synthesized MHC class I molecules (Figure 9).
Human DC cross-presentation of cell-associated antigens. Dead cells are internalized by DCs via mechanisms dependent on cytoskeletal actin rearrangements or functional ion channels, which are required for phagocytosis and macropinocytosis. After entering the cell, the antigens are enclosed in the cell's endosomal compartment and shipped from neutral to subsequently more acidic endosomal vesicles. In a late acid endosomal compartment the antigens undergo proteolysis; this involves the aspartic protease cathepsin D, which appears to be essential for cross-presentation of MP. Cathepsin D may be required for degradation of the antigens or antigen complexes into sizes/forms that can be transported out to the cytosol. When the antigen has entered the cytosol it is processed by the proteasome. The proteasome products are transported via TAP into the lumen of ER where the peptides are loaded on newly synthesized MHC class I molecules and subsequently transported out to the DC surface for presentation.
Human DC cross-presentation of cell-associated antigens. Dead cells are internalized by DCs via mechanisms dependent on cytoskeletal actin rearrangements or functional ion channels, which are required for phagocytosis and macropinocytosis. After entering the cell, the antigens are enclosed in the cell's endosomal compartment and shipped from neutral to subsequently more acidic endosomal vesicles. In a late acid endosomal compartment the antigens undergo proteolysis; this involves the aspartic protease cathepsin D, which appears to be essential for cross-presentation of MP. Cathepsin D may be required for degradation of the antigens or antigen complexes into sizes/forms that can be transported out to the cytosol. When the antigen has entered the cytosol it is processed by the proteasome. The proteasome products are transported via TAP into the lumen of ER where the peptides are loaded on newly synthesized MHC class I molecules and subsequently transported out to the DC surface for presentation.
Discussion
In vitro, several forms of antigen can access the exogenous pathway for cross-presentation, apparently through phagocytic or nonphagocytic mechanisms.2 Antigens such as particulate antigens, immune complexes, and HSPs have been shown to be loaded onto MHC class I molecules in fashions either independent of or dependent on proteasome degradation, transport into the ER via TAP, and MHC class I peptide loading in the ER.35 Up to now, however, there has been little information with regard to the cross-presentation pathway in human cells. Many of the antigens used to dissect cross-presentation pathways are not physiologic or have been used at nonphysiologic doses, for example, OVA protein coupled to beads, OVA-loaded spleen cells, or large amounts of soluble or particulate proteins. Furthermore, the dependence of TAP has been studied in TAP-/- knockout mice in vivo17 or in vitro,23 but not in a cell with functional TAP. The mechanisms by which naturally occurring sources of cell-associated antigens, for example, apoptotic cells, necrotic cells, and immune complex, are processed and presented remain therefore to be fully elucidated in human cells.
Our study of human DCs reveals many unique features of cross-presentation in vitro. Cross-presentation of MP from either apoptotic or necrotic cells was evident within 2 to 4 hours of coculture. Previous in vitro studies have shown that cross-presentation of soluble OVA by mouse monocytes was detectable after 0.5 hour, but was optimal by 4 hours, whereas presentation of OVA contained within immune complexes was optimal by 6 hours in the mouse DC line D1.22,42 Furthermore, cross-presentation by mouse DCs and macrophages of vaccinia-infected fibroblasts were seen after 1 hour of incubation.23 In vivo, cross-presentation is equally efficient. CD8α+ DCs recovered from the spleen 2 hours after injection could activate OVA-specific T cells.4 The minor variations seen in MHC class I cross-presentation in these different systems may rely on the source of antigen, type of APC, and the readout for MHC class I presentation. Our findings, the first in human cells to address the kinetics of cross-presentation in DCs, are consistent with the studies cited above, and indicate that the uptake processing and presentation of cell-associated antigens are rapid and efficient. The in vivo advantage of this rapid uptake by DCs may be prevention of widespread infection by processing and presentation of the pathogen before it can infect additional cells.43
Receptor-mediated endocytosis and macropinocytosis are efficient mechanisms that can guide exogenous antigens into the MHC class I and II presentation pathway in DCs.10,42 Cross-presentation was inhibited in the presence of CCD or DMA, consistent with the interpretation that internalization and possibly processing of dead cells require both cytoskeletal actin rearrangement and functional ion channels. Macropinocytosis efficiently concentrates and delivers antigens for MHC class I and II presentation in mouse DCs and macrophages,10,42 and our findings suggest that this may also be true for cell-associated antigens in human DCs. We show for the first time that interference with the cytoskeletal network and ion channels inhibits the cross-presentation of cell-associated antigens from both apoptotic and necrotic cells. Our findings indicate that multiple mechanisms are likely to interact or independently take part in the uptake of both apoptotic and necrotic cells.
After internalization by DCs, apoptotic and necrotic cells enter endosomal compartments. Acidification of this endosomal compartment is required for cross-presentation of exogenous MP, given that treatment with NH4Cl inhibited presentation. This may reflect the need for maturation from early to late endosomal compartments or proteolysis of the antigen in these compartments. Previous studies have shown that cross-presentation can be either dependent on or independent of acidification. For example, the presentation of OVA derived from immune complexes by a mouse DC line was dependent on endosomal acidification,22 whereas processing of soluble OVA by a mouse macrophage cell line was independent of acidification.21 These differences between the mouse macrophage cell line, human DCs, and mouse DCs may depend on the nature of the antigen or the mechanism for antigen delivery or both.
Studies examining the cross-presentation of HSPs in the mouse/human model have shown that that degradation of exogenous antigen into peptides can occur in the endosomal compartments followed by loading onto MHC class I molecules located in this compartment.44 Processing of proteins into antigenic fragments, both MHC class I- and II-restricted peptides, occurred in purified phagosome fractions obtained from a murine macrophage cell line pulsed with OVA-coated latex beads,45 suggesting the involvement of proteases such as cathepsins. Cathepsins possess broad substrate specificity and are abundant in the endosomal/lysosomal compartment, which should make them suitable for degradation of antigens.36 Our findings show that cathepsin D but not cathepsin B is involved in the cross-presentation of MP in human DCs and that exogenous added cathepsin D enhanced the cross-presentation but not endogenous MP presentation. Moreover, enhancement in cross-presentation required its internalization into acid endosomes. DC processing of proteins for presentation in the context of MHC class II has been shown to involve endo/lysosomal proteases such as cathepsin B, D, and S,46 which are active in immature human DCs.47 Vac A, a toxin from Helicobacter pylori, interferes with normal membrane trafficking and prevented vesicular transport from late endosomes to lysosomes in NIH3T3 cells and this inhibited the cleavage of procathepsin L in the lysosomes but had no effect on the cleavage of procathepsin B and D that occurs in late endosomes.48 We did not see any effects of Vac A on cross-presentation of MP in our studies and this may be due to the dependence on cathepsin D and not cathepsin L for MP cross-presentation.
However, cathepsins have not previously been shown to be involved in the proteolysis of antigens designated for the MHC class I cross-presentation. Furthermore, our data show that cross-presentation is due to degradation of antigen and not peptide contamination because it required degradation by proteases. The aspartic protease cathepsin D is present at high concentrations in endosomes such as the MHC class II-enriched compartments (MIICs) in human DCs49 and these compartments may be the sites where preprocessing of exogenous antigens along with transport out to the cytosol occur. Rodriguez et al found that transport of immune-complexed antigens from the endosomal compartments to cytosol was selective for molecules less than 40 kDa.22 Furthermore, delivery of antigen into the cytosol required disassociation from the immune complex, which was dependent on endosomal acidification.22 Similarly, enzymatic degradation of cell-associated antigens may be required, either to dissociate antigen aggregates or to directly cleave antigen into small enough fragments for delivery to the cytosol.
Most known MHC class I peptides are derived from proteins degraded by the proteasomes in the cytosol. Arrode et al have shown that cross-presentation by human DCs of exogenous human CMV antigens derived from apoptotic cells onto MHC class I could be blocked by inhibition of the proteasome.9 Our studies support this finding because we found that presentation of MP derived from apoptotic and necrotic cells also required processing by the proteasome. In addition, this shows that the processing of MP in endosomes in human DCs is not sufficient to produce the final MHC class I-restricted peptide (GILGFVFL). Endogenous MP in the cytosol of Vac-MP-infected DCs is also degraded by the proteasome so the handling of MP in cytosol is the same independent of endogenous or exogenous delivery.
Peptides are transported from the cytosol into the ER lumen in an ATP-dependent manner through the TAP. Evidence indicates that TAP-transported peptides are longer than the final peptide products (8-9 amino acids) displayed by MHC class I molecules on the cell surface50 and that further trimming can occur in the ER.51-53 Cross-presentation of cell-associated MP required access into the ER via TAP because it could be abolished when we inhibited active TAP transport by ICP47 expression in human DCs. ICP47 is an HSV protein that binds a domain of TAP on the outside of the ER with high affinity and therefore blocks the transport of all peptides into ER.40 Furthermore, MP-derived peptides also required transport out to the DC surface because inhibition of this transport via brefeldin A blocked cross-presentation. Endogenous presentation of MP by DCs showed the same dependence of TAP and ER as cross-presentation. Overall, these findings confirm studies in other models of cross-presentation demonstrating the dependence on TAP.5,10-12,17,23 However, ours are the first to show the genuine requirement for TAP (using ICP47) and the requirement for newly synthesized MHC class I in human DCs for MHC class I cross-presentation.
In summary, our studies show that antigens derived from apoptotic or necrotic cells are processed through a common pathway that involves active internalization of cell components, preprocessing in endosomes via cathepsin D, entry into the cytosol for processing by proteasome and TAP-dependent entry into the ER. We found little evidence to suggest that processing was TAP independent as found in other models.15,16 The sharing of a common pathway between apoptotic and necrotic cells is another piece of evidence that there is an overlap in their uptake and processing. This is supported by the finding of a common set of genes in Caenorhabditis elegans, which mediate the uptake of both apoptotic and necrotic cells.54 Further studies will be required to characterize the role of cathepsin D in the processing of cell-associated antigens.
Prepublished online as Blood First Edition Paper, August 21, 2003; DOI 10.1182/blood-2003-06-1801.
Supported by National Institutes of Health (NIH) grants AI 44628 and AI 39519 (N.B.), a Burroughs Welcome Clinical Investigator Grant, a Doris Duke Foundation Award, and a Cancer Research Institute grant (N.B.). N.B. is an Elisabeth Glaser Scientist. Funding was also provided by Foundation for AIDS & Immune Research (FAIR) (M.L.) and Association Pour le Recherche sur le Cancer (J.F.F.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal