Abstract
The mitogen-activated protein kinase (MAPK) (also called extracellular signal–regulated kinase [ERK]) pathway has been implicated in malignant transformation and in the regulation of cellular growth and proliferation of several tumor types, but its expression and function in Hodgkin disease (HD) are unknown. We report here that the active phosphorylated form of MAPK/ERK is aberrantly expressed in cultured and primary HD cells. Inhibition of the upstream MAPK kinase (also called MEK) by the small molecule UO126 inhibited the phosphorylation of ERK and demonstrated a dose- and time-dependent antiproliferative activity in HD cell lines. UO126 modulated the levels of several intracellular proteins including B-cell lymphoma protein 2 (Bcl-2), myeloid cell leukemia–1 (Mcl-1) and caspase 8 homolog FLICE-inhibitory protein (cFLIP), and induced G2M cell-cycle arrest or apoptosis. Furthermore, UO126 potentiated the activity of apoliprotein 2/tumor necrosis factor–related apoptosis-inducing ligand (APO2L/TRAIL) and chemotherapy-induced cell death. Activation of CD30, CD40, and receptor activator of nuclear kappaβ (RANK) receptors in HD cells by their respective ligands increased ERK phosphorylation above the basal level and promoted HD cell survival. UO126 inhibited basal and ligand-induced ERK phosphorylation, and inhibited ligand-induced cell survival of HD cell lines. These findings provide a proof-of-principle that inhibition of the MEK/ERK pathway may have therapeutic value in HD.
Introduction
The mitogen-activated protein kinases (MAPKs) are a group of protein serine/threonine kinases that are activated in response to a variety of extracellular stimuli and mediate signal transduction from the cell surface to the nucleus.1,2 Under physiologic conditions, activation of the MAPK cascades by surface receptor/ligand interaction is tightly controlled and is involved in regulating cell proliferation, differentiation, and apoptosis. In mammalian cells, 3 major types of MAPK cascades have been identified: extracellular signal–regulated kinase 1/2 (ERK) 1/2 MAPK, Jun N-terminal kinase (JNK)/stress-activated protein kinase (SAPK) kinase, and p38 kinase.3 In the ERK1/2 (p44/42) MAPK pathway, activation of receptor tyrosine kinases by growth factors induces son of sevenless (SOS) translocation to the plasma membrane and phosphorylation and activation of a cascade of kinases, including Ras, Raf, MAP/ERK kinase (MEK), and ERK. The active phosphorylated form of ERK1/2 (p-ERK) translocates to the nucleus and phosphorylates transcription factors that control cell proliferation and differentiation.
Constitutive activation of MEK/ERK kinases has previously been reported in primary and cultured human cancer cells, including leukemia and breast carcinoma.4-10 Inhibition of Ras/MEK/ERK signaling has been shown to induce cell-cycle arrest and cell death by interfering with several intracellular survival and cell-cycle proteins.6,7,11 Furthermore, this activated pathway has been implicated in cancer cell resistance to chemotherapy and tumor necrosis factor–apoptosis inducing ligand (TRAIL/APO2L).7,12 These observations, in addition to recent data demonstrating that MEK/ERK inhibitors can potentiate the effect of other anticancer therapeutic agents including chemotherapy and STI571,13-16 have generated enthusiasm about the potential role of MEK/ERK inhibitors in cancer drug development.
TRAIL (APO2L) is a potent death protein belonging to the tumor necrosis factor (TNF) family.17,18 The ability of APO2L/TRAIL to preferentially kill a wide variety of tumor cells while sparing normal cells, and to act synergistically with chemotherapeutic drugs, has generated excitement for its potential use in cancer therapy.19-23 APO2L/TRAIL has 4 exclusive receptors: APO2L/TRAIL receptor 1 (R1) (death receptor 4 [DR4]); R2 (DR5, Killer, TRICK2); R3 (decoy receptor 1 [DcR1], TRID, LIT); and R4 (DcR2, TRUNDD).24-29 TRAIL-R1 and TRAIL-R2 are death receptors that contain a death domain (DD) in their intracellular tail. R3 and R4 receptors are decoy receptors that do not transduce apoptotic signals. Both receptors recruit a Fas-associated DD adapter protein and activate several upstream caspases including caspase 8 and caspase 10, in addition to cleaving Bid with subsequent activation of caspase 9.24,30,31 APO2L/TRAIL has been reported to induce cell death in a wide variety of primary and cultured hematologic tumors,29,32-35 but its activity in Hodgkin disease (HD) remains unknown.
The malignant Reed-Sternberg cells of Hodgkin disease (HD) are known to express several TNF family receptors, including CD30, CD40, and receptor activator of nuclear factor–κB (RANK).36-39 Many of these survival and apoptotic pathways are currently under investigation for targeted therapy of cancer. CD30, CD40, and RANK share overlapping biologic functions such as activation of nuclear factor–κB (NF-kB), regulation of cytokine and chemokine secretion, and regulation of HD cell growth and survival.37,40,41 However, because these receptors have overlapping biologic functions, identifying a shared signaling pathway among these receptors could simplify treatment strategies.
In this study, we report that ERK is aberrantly active in HD, is involved in regulating HD cell proliferation and survival, and is shared among CD30, CD40, and RANK signaling pathways, making it attractive for targeted therapy. We also report that inhibition of the MEK/ERK pathway potentiates the activity of chemotherapy and APO2L/TRAIL in HD cells.
Materials and methods
Cell lines and cell culture
The human Hodgkin- and Reed-Sternberg (H/RS)–derived cell lines HD-MYZ, HD-LM-2, L-428, and KM-H2 were obtained from the German Collection of Microorganisms and Cell Cultures, Department of Human and Animal Cell Cultures, Braunschweig, Germany. The phenotypes and genotypes of these cell lines have been previously published.42 All cell lines were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, l-glutamine, and penicillin/streptomycin (Gibco BRL, Gaithersburg, MD) in a humid environment of 5% CO2 at 37°C. Peripheral blood lymphocytes were obtained from healthy donors, and immunohistochemistry on archived HD tissue was performed after obtaining an institutional review board (IRB)–approved consent form and according to institutional guidelines.
Antibodies and recombinant proteins
Recombinant human RANK ligand (RANKL, TNF-related activation-induced cytokine [TRANCE]), CD40 ligand (CD40L, CD154), CD30 ligand (CD30L, CD153), and the pancaspase inhibitor Z-VAD-FMK were obtained from Alexis (San Diego, CA). Recombinant human tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL; Apo2 ligand) trimer was kindly provided by Dr Avi Ashkenazi (Genentech, South San Francisco, CA). Antibodies to ERK1/2, phosphorylated ERK1/2 (Thr202, Tyr204); caspases 7, 8, 9, and 10; cleaved poly(adenosine diphosphate [ADP]–ribose) polymerase (PARP); MEK inhibitor UO126; and phospho p53 antibody sampler kit containing antibodies to total p53 and phosphospecific antibodies to Ser6, Ser9, Ser15, Ser20, Ser37, Ser46, and Ser392 were obtained from Cell Signaling Technology (Beverly, MA). Antibodies to myeloid cell leukemia–1 (Mcl-1), B-cell lymphoma–x (Bcl-x), Bcl-2, Bcl-2–associated X protein (Bax), Bak, cellular inhibitor of apoptosis–2 (cIAP2), p14ARF, p21WAF, p27Kip1, cell division cycle 25A (cdc25A), and cdc25C were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); antibodies to caspase 8 homolog FLICE-inhibitory protein (cFLIP) and cIAP1, survivin, neuronal apoptosis inhibitor protein (NAIP), and BID were from R&D Systems (Minneapolis, MN); to X-linked IAP (XIAP) from Transduction Laboratories (San Diego, CA); and antibodies to β-actin were from Sigma Chemical (St Louis, MO).
In vitro proliferation assay
Cells were cultured in 24-well plates in volumes of 500 μLat 5 × 105/mL. Cell viability was assessed with a nonradioactive cell-proliferation MTS assay by using CellTiter96 Aqueous One Reagent (Promega, Madison, WI), as described previously.43 In this assay, formazan absorbance was measured at 490 nm on a μQuant plate reader equipped with KC4 software (BioTek Instruments, Winooski, VT). Each experiment was set up in triplicate, and the mean value of the 3 measurements was determined.
Flow cytometry
Cell-cycle fractions and apoptosis were determined by the propidium iodide (PI) staining method as previously described,44,45 or by combining PI staining with the terminal uridine deoxynucleotidyl nick end labeling (TUNEL) assay with the use of the DeadEnd Fluorometric TUNEL System (Promega) according to the manufacturer's instructions. Data were collected on a Becton Dickinson FACScan flow cytometer with the use of CellQuest software (BD Biosciences, San Jose, CA), and analyzed by WinMDI 2.8 software (Joseph Trotter, Scripps Research Institute, La Jolla, CA).
Western blot analysis
Cellular protein was extracted by incubation in lysis buffer (Cell Signaling Technology, catalog no. 9803) for 15 minutes at 4°C and then centrifuged to remove cellular debris. The protein in the resulting supernatant was quantified by the bicinchonic acid (BCA) method according to the manufacturer's instructions (Pierce Chemical, Rockford, IL), diluted 1:1 in protein-loading buffer (0.25 M Tris-HCl [tris(hydroxymethyl)aminomethane–HCl], 2% sodium dodecyl sulfate [SDS], 4% β-mercaptoethanol, 1% glycerol, and 0.2 mg/mL bromophenol blue), and boiled for 30 minutes. A total of 30 μg protein was loaded onto 10% or 15% Tris-HCl SDS–polyacrylamide electrophoresis Ready Gels (Bio-Rad, Hercules, CA), transferred to a nitrocellulose transfer membrane (Pierce Chemical), and detected by means of ECL-Plus (Amersham, Buckinghamshire, England).
Immunohistochemical analysis
Paraffin-embedded lymph node biopsy sections were immunostained by using a Techmate 1000 automatic immunostainer (Ventana, Tucson, AZ) as described previously.37 Briefly, sections were deparaffinized in xylene, rehydrated with decreasing concentrations of alcohol and finally phosphate-buffered saline (PBS), and subjected to steam-heat epitope retrieval in 10 mM citrate buffer (pH 6.0) for 30 minutes in a commercially available vegetable steamer. The sections were then rinsed in distilled water, washed in PBS for 5 minutes, and incubated for 15 minutes with DAKO protein block (DAKO, Carpinteria, CA). Next, they were incubated for 2 hours with antibodies to total ERK1/2 or to phosphorylated ERK1/2 (p-ERK) (Cell Signaling Technology) diluted 1:250 or 1:500 in 0.1% bovine serum albumin/PBS. Sections were then washed, and bound antibodies were detected by means of an LSAB2 peroxidase kit (primary rabbit/mouse; DAKO) with diaminobenzidine as chromogen. Finally, the sections were counterstained with hematoxylin, dehydrated, and mounted. Negative controls were biopsy sections that were immunostained either with nonreactive mouse immunoglobulin G diluted to the same concentration as the anti-ERK antibody or with no antibody.
Results
Expression of ERK1/2 in cultured and primary Hodgkin disease cells
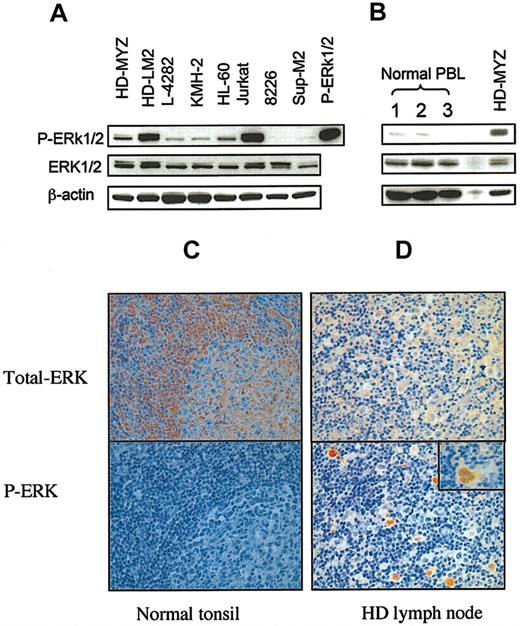
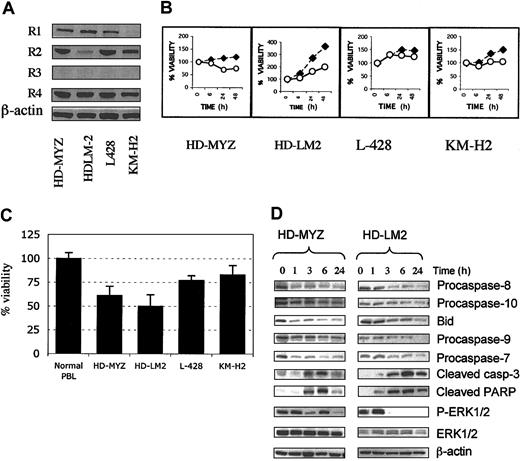
To determine whether HD cell lines aberrantly express activated ERK1/2, we examined whole cell lysates from 4 HD-derived cell lines for the expression of total ERK1/2 and phosphorylated ERK1/2 (p-ERK) by Western blot. Purified p-ERK1/2 protein was used as positive control. We found that all 4 HD cell lines expressed p-ERK1/2, with HD-LM2 expressing the highest level (Figure 1A). HL-60 cells and Jurkat cells also expressed p-ERK1/2. In contrast, 2 CD30+ cell lines (8226 myeloma and Sup-M2 anaplastic large-cell lymphoma), and peripheral blood lymphocytes from 3 healthy donors either did not express or weakly expressed p-ERK1/2 (Figure 1A-B).
Expression of ERK in cultured and primary HD cells. Total cell lysates were examined for the expression of ERK and phosphorylated ERK (p-ERK) by Western blot. (A) Expression of ERK in 4 HD cell lines (HD-MYZ, HD-LM2, L-428, and KMH-2) and in 4 other CD30+ lymphoid cell lines. All HD-derived cell lines expressed p-ERK, but HD-LM2 expressed the highest level. The Jurkat cell line also expressed high levels of p-ERK. The multiple myeloma cell line 8226 and the anaplastic large-cell lymphoma cell line SUP-M2 did not express p-ERK. The p-ERK protein was used as a positive control. (B) Expression of p-ERK in peripheral blood lymphocyte (PBL) samples from 3 healthy individuals was either weak or undetectable. (C) Histochemical staining of a normal tonsil for total ERK (upper panel) and p-ERK (lower panel) (× 40). Normal tonsillar cells expressed total ERK, but they did not express p-ERK. (D) A section of lymph node involved with nodular sclerosis HD was stained for total ERK (upper panel) or p-ERK (lower panel). Both benign and malignant cells expressed total ERK. However, only the malignant Reed-Sternberg cells expressed p-ERK (lower panel, × 10). Inset in the lower panel shows a Reed-Sternberg cell with intense nuclear staining of p-ERK (× 40).
Expression of ERK in cultured and primary HD cells. Total cell lysates were examined for the expression of ERK and phosphorylated ERK (p-ERK) by Western blot. (A) Expression of ERK in 4 HD cell lines (HD-MYZ, HD-LM2, L-428, and KMH-2) and in 4 other CD30+ lymphoid cell lines. All HD-derived cell lines expressed p-ERK, but HD-LM2 expressed the highest level. The Jurkat cell line also expressed high levels of p-ERK. The multiple myeloma cell line 8226 and the anaplastic large-cell lymphoma cell line SUP-M2 did not express p-ERK. The p-ERK protein was used as a positive control. (B) Expression of p-ERK in peripheral blood lymphocyte (PBL) samples from 3 healthy individuals was either weak or undetectable. (C) Histochemical staining of a normal tonsil for total ERK (upper panel) and p-ERK (lower panel) (× 40). Normal tonsillar cells expressed total ERK, but they did not express p-ERK. (D) A section of lymph node involved with nodular sclerosis HD was stained for total ERK (upper panel) or p-ERK (lower panel). Both benign and malignant cells expressed total ERK. However, only the malignant Reed-Sternberg cells expressed p-ERK (lower panel, × 10). Inset in the lower panel shows a Reed-Sternberg cell with intense nuclear staining of p-ERK (× 40).
To determine whether primary Reed-Sternberg cells also aberrantly express p-ERK1/2, we studied sections of HD lymph nodes by immunohistochemical techniques using antibodies to total ERK and phospho-specific antibody to p-ERK1/2. Sections from normal tonsils (n = 4) were stained for total ERK and p-ERK and were used as controls. As shown in Figure 1C, cells of a normal tonsil expressed total ERK protein, which was predominantly localized in the cytoplasm (upper panel), but did not express p-ERK (lower panel). Similarly, total ERK was expressed in the cellular element (both the benign cells and Reed-Sternberg cells) in sections from lymph nodes involved with nodular sclerosis HD (n = 13); however, only the malignant Reed-Sternberg cells expressed p-ERK, which was predominantly localized in the nucleus (Figure 1D). Thirteen lymph node sections from patients with nodular sclerosis HD were examined. The percentage of Reed-Sternberg cells that expressed p-ERK varied widely among the different sections, ranging from below 10% to more than 75%, and did not correlate with disease stage. In some cases, p-ERK was also expressed in dendritic cells and some endothelial cells (data not shown). The malignant cells of follicular lymphoma and small lymphocytic lymphoma did not express p-ERK (data not shown).
Effect of MEK inhibition on cell proliferation and survival
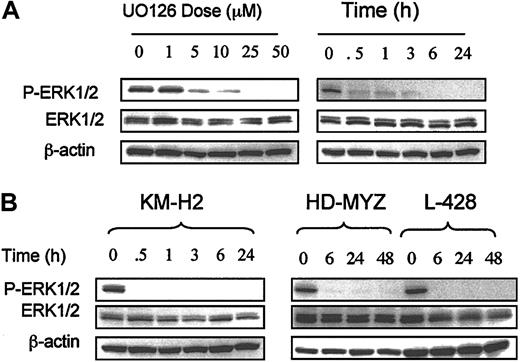
To determine whether aberrantly activated MEK1/2 can be inhibited by the small molecule U0126,46 we incubated HD cell lines with increasing concentrations of UO126 for 24 hours and determined the effects on ERK phosphorylation by Western blot. Control cells were incubated with dimethyl sulfoxide (DMSO) alone. As shown in Figure 2A, UO126 decreased ERK1/2 phosphorylation in the HD-LM2 cell line in a dose- and time-dependent manner, with complete dephosphorylation of ERK at a concentration of 25 μM. In contrast, UO126 had no effect on total ERK protein. Similar results were observed in the remaining 3 HD cell lines (Figure 2B). When HD cell lines were incubated with 25 μM UO126, ERK phosphorylation was significantly reduced within 30 minutes, was complete in all cell lines by 6 hours, and lasted for as long as 48 hours (Figure 2).
Effect of MEK inhibition on p-ERK in HD cell lines. (A) Effect of the MEK inhibitor UO126 on the HD-LM2 cell line. UO126 inhibited ERK phosphorylation in a dose- and time-dependent manner. When the cells were incubated with UO126 for 24 hours, the maximal effect was observed at a dose of 25 μM (p-ERK1/2 panel). When the cells were incubated with 25 μM UO126, the effect was nearly complete after 30 minutes of incubation and was complete after 6 hours of incubation. (B) A similar effect was observed in the 3 remaining HD cell lines, with complete inhibition of p-ERK that lasted for as long as 48 hours.
Effect of MEK inhibition on p-ERK in HD cell lines. (A) Effect of the MEK inhibitor UO126 on the HD-LM2 cell line. UO126 inhibited ERK phosphorylation in a dose- and time-dependent manner. When the cells were incubated with UO126 for 24 hours, the maximal effect was observed at a dose of 25 μM (p-ERK1/2 panel). When the cells were incubated with 25 μM UO126, the effect was nearly complete after 30 minutes of incubation and was complete after 6 hours of incubation. (B) A similar effect was observed in the 3 remaining HD cell lines, with complete inhibition of p-ERK that lasted for as long as 48 hours.
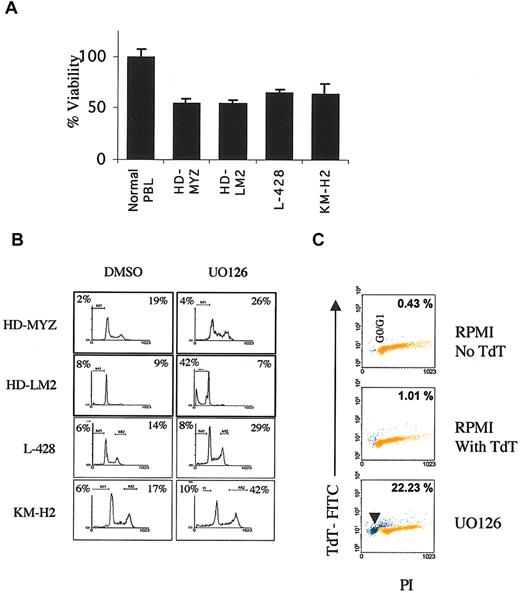
To determine the biologic consequences of MEK1/2 inhibition in HD cell lines, we determined the effect of UO126 on cell proliferation using the MTS assay. Within 48 hours, UO126 (25 μM) reduced viable cell numbers by 30% to 50% in all HD cell lines while sparing normal peripheral blood lymphocytes (Figure 3A). UO126 was most effective in the HD-MYZ and HD-LM2 cell lines, which expressed the highest levels of phosphorylated ERK1/2. Next, we determined whether the antiproliferative effect of UO126 was due to cell-cycle arrest or induction of apoptosis. Using the PI staining method, we found that UO126 induced G2M cell-cycle arrest in the HD-MYZ and L-428 cells, while it induced apoptosis in HD-LM2 cells (Figure 3B). In the KM-H2 cell line, UO126 induced modest apoptosis and cell-cycle arrest effects. The ability of UO126 to induce apopotosis in the HD-LM2 cells was confirmed in subsequent experiments by means of the TUNEL assay combined with PI staining (Figure 3C).
Effect of MEK inhibition on HD cell line viability. (A) Normal peripheral blood lymphocytes (PBLs) and HD cell lines were incubated with UO126 (25 μM) for 48 hours; UO126 had no significant effect on PBLs compared with DMSO alone, while it reduced the number of viable cells of all 4 HD cell lines by 30% to 50%. (B) With the use of the PI staining method, the antiproliferative effect of UO126 (25 μM for 48 hours) was found to be due to either cell-cycle arrest at the G2M phase (percentages shown in the right upper area) or induction of apoptosis (percentages shown in the left upper area). The apoptotic activity of UO126 was most prominent in the HD-LM2, and to a lesser extent, the KM-H2 cell lines. Cell-cycle arrest at the G2M phase was most prominent in the HD-MYZ, L-428, and KM-H2 cell lines. Data shown are representative of 3 independent experiments. Histogram analysis and statistics were generated by using Cellquest software. (C) Induction of apoptosis by UO126 in HD-LM2 cells was confirmed by dual staining with PI and TUNEL. Apoptotic cells are shown in the sub-G0 area (arrowhead), and the percentage of apoptotic cells is shown. The upper 2 boxes show data with RPMI with and without terminal deoxynucleotidyl transferase (TdT). The lower panel shows results obtained after incubation with UO126 for 48 hours.
Effect of MEK inhibition on HD cell line viability. (A) Normal peripheral blood lymphocytes (PBLs) and HD cell lines were incubated with UO126 (25 μM) for 48 hours; UO126 had no significant effect on PBLs compared with DMSO alone, while it reduced the number of viable cells of all 4 HD cell lines by 30% to 50%. (B) With the use of the PI staining method, the antiproliferative effect of UO126 (25 μM for 48 hours) was found to be due to either cell-cycle arrest at the G2M phase (percentages shown in the right upper area) or induction of apoptosis (percentages shown in the left upper area). The apoptotic activity of UO126 was most prominent in the HD-LM2, and to a lesser extent, the KM-H2 cell lines. Cell-cycle arrest at the G2M phase was most prominent in the HD-MYZ, L-428, and KM-H2 cell lines. Data shown are representative of 3 independent experiments. Histogram analysis and statistics were generated by using Cellquest software. (C) Induction of apoptosis by UO126 in HD-LM2 cells was confirmed by dual staining with PI and TUNEL. Apoptotic cells are shown in the sub-G0 area (arrowhead), and the percentage of apoptotic cells is shown. The upper 2 boxes show data with RPMI with and without terminal deoxynucleotidyl transferase (TdT). The lower panel shows results obtained after incubation with UO126 for 48 hours.
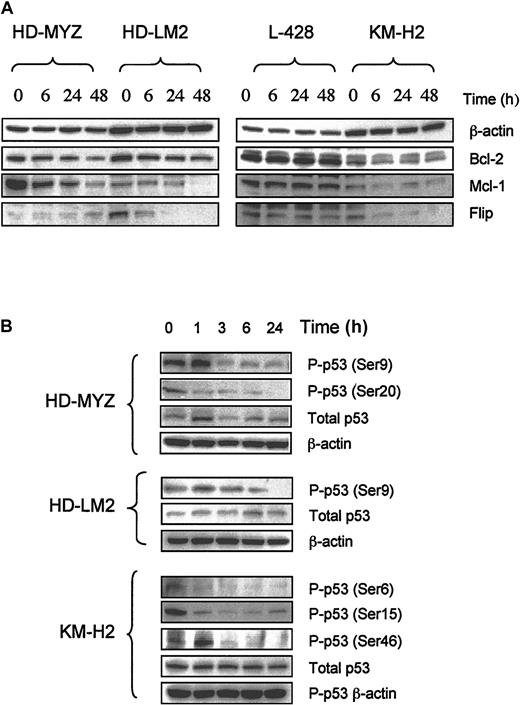
To understand the molecular mechanisms involved in the antiproliferative activity of UO126 (25 μg) in HD cells, we studied its effect on several intracellular proteins that are known to regulate cell cycle and/or apoptosis. In 3 of the 4 cell lines, UO126 down-regulated Bcl-2 and Mcl-1 proteins, whereas the antiapoptotic protein cFLIP was down-regulated in 2 cell lines (Figure 4A). However, UO126 had no effect on levels of Bcl-x, Bax, Bak, BID, IAP family members, p14, p21, p27, cdc25a, cdc25c, or cyclin D1 in any of the HD cell lines (data not shown). Furthermore, UO126 had no effect on caspase activation (caspase 8, 10, 9, 7, or 3) or on PARP cleavage. Preincubating HD cell lines with the pancaspase inhibitor Z-VAD FMK (2 μM) did not inhibit UO126 effects in any of the cell lines (data not shown). These data suggest that the ability of UO126 to induce apoptosis in HD cells was caspase independent. In all cell lines, p53 was aberrantly phosphorylated on several sites (Figure 4B). UO126 had mixed effects on p53 phosphorylation: it increased p53 phosphorylation above basal levels within 1 hour of incubation in the HD-LM2 (Ser9) and KM-H2 (Ser46), followed by a decrease in p53 phosphorylation with extended incubation time, while it decreased p53 phosphorylation in the HD-MYZ (Ser9, Ser20) and KM-H2 (Ser6, Ser15) cell lines (Figure 4B).
Effect of UO126 (25 μM) on intracellular proteins that regulate cell cycle and survival. (A) UO126 reduced intracellular levels of Bcl-2, Mcl-1, and FLIP proteins in 3 of 4 HD cell lines. (B) UO126 resulted in loss of p53 phosphorylation at several sites in 3 HD cell lines.
Effect of UO126 (25 μM) on intracellular proteins that regulate cell cycle and survival. (A) UO126 reduced intracellular levels of Bcl-2, Mcl-1, and FLIP proteins in 3 of 4 HD cell lines. (B) UO126 resulted in loss of p53 phosphorylation at several sites in 3 HD cell lines.
MEK inhibition potentiates the effect of APO2L/TRAIL and chemotherapy on Hodgkin disease cell lines
Because UO126 down-regulated cell-survival proteins, we next studied the effect of UO126 on APO2L/TRAIL and chemotherapy-induced cell death in HD cell lines. We first determined APO2L/TRAIL receptor expression and the activity of APO2L/TRAIL in HD cell lines. As shown in Figure 5A, all HD cell lines expressed the APO2L/TRAIL receptors R1 (weakly expressed in the KM-H2), R2, and R4 and lacked R3 expression. APO2L/TRAIL (1 μg/mL) was most effective in the HD-MYZ and HD-LM2 cell lines, in which it reduced the viable cell number by 39% (± 10%), and 50% (± 12%), respectively (Figure 5B-C). In contrast, TRAIL had minimal effect on resting peripheral blood lymphocytes of 3 healthy individuals (Figure 5C). The sensitivity to APO2L/TRAIL could not be predicted by the pattern of APO2L/TRAIL-receptor expression or by c-FLIP levels (Figure 4A). Furthermore, sensitivity to APO2L/TRAIL could not be predicted by the levels of caspase 8, 9, 10, Bcl-2, Bcl-xL, Bax, survivin, XIAP, NAIP, or cIAP (data not shown). APO2L/TRAIL activated the extrinsic and mitochondrial apoptotic pathways, as evidenced by its activation of caspases 8, 10, 9, 7, and 3 and cleavage of BID and PARP (Figure 5D). Interestingly, APO2L/TRAIL also inhibited ERK phosphorylation without significant effect on total cellular ERK levels, therefore mimicking the effect of UO126 (Figure 5D).
Activity of APO2L/TRAIL on 4 Hodgkin disease cell lines. (A) Western blot analysis of the TRAIL receptor expression in HD cell lines. The TRAIL death receptors R1 and R2 were expressed in all cell lines (R1 was weakly expressed in KM-H2). The decoy receptor R4 but not R3 was also expressed in all 4 HD cell lines. (B) A representative experiment demonstrating APO2L/TRAIL–induced apoptosis in HD cell lines. Cells were incubated with 1 μg/mL TRAIL for 48 hours, and cell viability was determined by MTS assay. ⋄ indicates RPMI; ○, TRAIL. (C) Summary data showing TRAIL activity in HD cell lines. Data for each cell line and normal peripheral blood lymphocytes represent a mean (± SEM) of at least 3 independent experiments performed in triplicate. The viable cell number was determined by MTS assay after 48 hours of incubation. TRAIL was most effective in the HD-MYZ and HD-LM2 cell lines. (D) In the 2 sensitive cell lines, APO2L/TRAIL activated caspases 8, 10, 9, 7, and 3 and cleaved BID and PARP. APO2L/TRAIL also inhibited the phosphorylation of ERK without significant effect on the total amount of ERK in these 2 cell lines.
Activity of APO2L/TRAIL on 4 Hodgkin disease cell lines. (A) Western blot analysis of the TRAIL receptor expression in HD cell lines. The TRAIL death receptors R1 and R2 were expressed in all cell lines (R1 was weakly expressed in KM-H2). The decoy receptor R4 but not R3 was also expressed in all 4 HD cell lines. (B) A representative experiment demonstrating APO2L/TRAIL–induced apoptosis in HD cell lines. Cells were incubated with 1 μg/mL TRAIL for 48 hours, and cell viability was determined by MTS assay. ⋄ indicates RPMI; ○, TRAIL. (C) Summary data showing TRAIL activity in HD cell lines. Data for each cell line and normal peripheral blood lymphocytes represent a mean (± SEM) of at least 3 independent experiments performed in triplicate. The viable cell number was determined by MTS assay after 48 hours of incubation. TRAIL was most effective in the HD-MYZ and HD-LM2 cell lines. (D) In the 2 sensitive cell lines, APO2L/TRAIL activated caspases 8, 10, 9, 7, and 3 and cleaved BID and PARP. APO2L/TRAIL also inhibited the phosphorylation of ERK without significant effect on the total amount of ERK in these 2 cell lines.
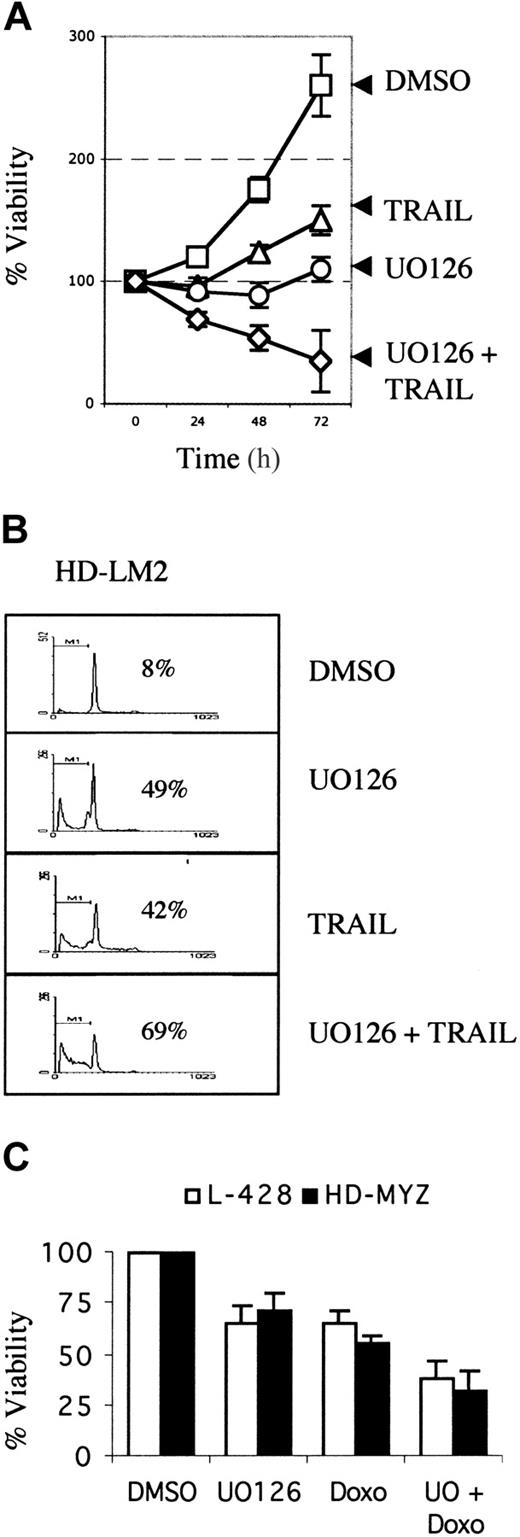
We then evaluated whether UO126 potentiates the activity of APO2L/TRAIL in HD cell lines. HD cell lines were incubated with UO126 (25 μM), TRAIL (1 μg/mL), or both for 72 hours. Cell viability was determined by the MTS assay, and cell death was determined by PI staining and fluorescence-activated cell sorter (FACS) analysis. As shown in Figure 6A-B, the combination of APO2L/TRAIL and UO126 was more effective than either drug alone in inducing cell death. In contrast, UO126 did not potentiate the toxicity of APO2L/TRAIL to normal peripheral blood lymphocytes (data not shown). The interaction between UO126 and doxorubicin chemotherapy was also evaluated. For these experiments, HD cell lines were incubated with increasing concentrations of doxorubicin (0.005 to 10 μg/mL). The maximum effective dose was between 1 and 5 μg/mL, and the 50% inhibitory concentration (IC50) was between 0.05 and 0.25 μg/mL (data not shown). Thus, for subsequent experiments, a submaximal doxorubicin dose of 0.1 μg/mL was selected. As shown in Figure 6C, UO126 enhanced the activity of doxorubicin chemotherapy in 2 HD cell lines. This effect was less prominent in the remaining 2 HD cell lines (data not shown). The ability of UO126 to enhance APO2L/TRAIL– and chemotherapy-induced cell death in HD cell lines may be due to its ability to down-regulate c-FLIP, Bcl2, and Mcl-1 in these cell lines (Figure 4).
Effect of MEK inhibition on activity of of APO2L/TRAIL and chemotherapy in HD cell lines. MEK inhibition potentiated the effect of APO2L/TRAIL and chemotherapy in HD cell lines. (A) The HD-LM2 cell line was incubated with DMSO, UO126 (25 μM), APO2L/TRAIL (1 μg/mL), or the combination of UO126 plus TRAIL for 72 hours. Viable cell numbers were determined by the MTS assay. Results are mean of 3 independent experiments performed in triplicate (± SEM). (B) A representative experiment showing the effect of UO126 and TRAIL on the HD-LM2 cells. Tumor cells were incubated as described in the legend to panel A for 48 hours. Cell-cycle analysis and cell death were determined by the PI staining method. Percentages of dead cells are shown for each condition. The added activity of the combination of APO2L/TRAIL and UO126 was due to increased cell death. (C) Effect of UO126 (25 μM) on doxorubicin chemotherapy (0.1 μg/mL)–induced cell death in the L-428 and HD-MYZ cell lines. Results are presented as the mean of 3 independent experiments performed in triplicate (± SEM). Cell viability was determined by MTS assay after 48 hours of incubation.
Effect of MEK inhibition on activity of of APO2L/TRAIL and chemotherapy in HD cell lines. MEK inhibition potentiated the effect of APO2L/TRAIL and chemotherapy in HD cell lines. (A) The HD-LM2 cell line was incubated with DMSO, UO126 (25 μM), APO2L/TRAIL (1 μg/mL), or the combination of UO126 plus TRAIL for 72 hours. Viable cell numbers were determined by the MTS assay. Results are mean of 3 independent experiments performed in triplicate (± SEM). (B) A representative experiment showing the effect of UO126 and TRAIL on the HD-LM2 cells. Tumor cells were incubated as described in the legend to panel A for 48 hours. Cell-cycle analysis and cell death were determined by the PI staining method. Percentages of dead cells are shown for each condition. The added activity of the combination of APO2L/TRAIL and UO126 was due to increased cell death. (C) Effect of UO126 (25 μM) on doxorubicin chemotherapy (0.1 μg/mL)–induced cell death in the L-428 and HD-MYZ cell lines. Results are presented as the mean of 3 independent experiments performed in triplicate (± SEM). Cell viability was determined by MTS assay after 48 hours of incubation.
MEK/ERK signaling pathway is shared by CD30, CD40, and RANK in Hodgkin disease cell lines
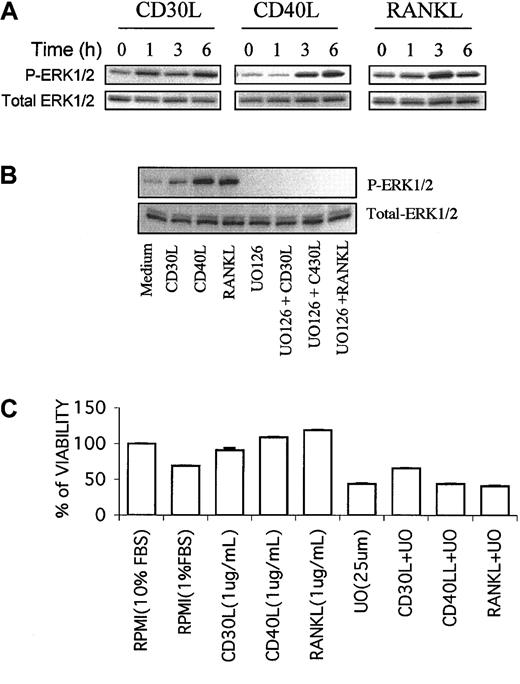
The malignant Reed-Sternberg cells of HD and HD cell lines express several TNF-family receptors, including CD30, CD40, and RANK.36,37,47,48 Signaling through these receptors regulates HD cell survival and cytokine and chemokine secretion.37,41,47,49,50 We have recently reported that RANK ligand (RANKL) activates NF-kB and MEK/ERK pathways in HD cell lines.37,51 Similarly, CD30 ligand has been reported to activate the ERK pathway in the HD-LM2 cell line.52 Whether CD30 activation in other HD cell lines and whether CD40 activation can also activate MEK/ERK pathway are currently unknown. Thus, we studied the effects of CD30 ligand (CD30L) and CD40 ligand (CD40L) on the MEK/ERK pathway in HD cell lines. As expected, RANKL (1 μg/mL) phosphorylated ERK (Figure 7A). Similarly, both CD30L (1 μg/mL) and CD40L (1 μg/mL) activated MEK and induced phosphorylation of ERK in a time-dependent manner (Figure 7A).
Sharing of MEK/ERK signaling pathway by CD30, CD40, and RANK in HD cell lines. MEK/ERK signaling pathway is shared by CD30, CD40, and RANK in HD cell lines. (A) The L-428 cell line, which expresses CD30, CD40, and RANK receptors, was incubated with 1 μg/mL of CD30 ligand (CD30L), CD40 ligand (CD40L), and RANK ligand (RANKL) for 1 to 6 hours. Total ERK and p-ERK were determined by Western blot. All 3 ligands increased the phosphorylation of ERK in a time-dependent manner. (B) When the L-428 cell line was incubated with CD30L, CD40L, or RANKL for 6 hours, ERK phosphorylation was induced. UO126 (25 μM) inhibited the basal level of p-ERK, and the combination of UO126 with any of these 3 ligands inhibited the ligand-induced ERK phosphorylation. (C) Under serum deprivation conditions (1% FBS), any of the studied ligands (CD30L, CD40L, RANKL) enhanced the survival of the L-428 HD cell line, an effect that was inhibited by UO126.
Sharing of MEK/ERK signaling pathway by CD30, CD40, and RANK in HD cell lines. MEK/ERK signaling pathway is shared by CD30, CD40, and RANK in HD cell lines. (A) The L-428 cell line, which expresses CD30, CD40, and RANK receptors, was incubated with 1 μg/mL of CD30 ligand (CD30L), CD40 ligand (CD40L), and RANK ligand (RANKL) for 1 to 6 hours. Total ERK and p-ERK were determined by Western blot. All 3 ligands increased the phosphorylation of ERK in a time-dependent manner. (B) When the L-428 cell line was incubated with CD30L, CD40L, or RANKL for 6 hours, ERK phosphorylation was induced. UO126 (25 μM) inhibited the basal level of p-ERK, and the combination of UO126 with any of these 3 ligands inhibited the ligand-induced ERK phosphorylation. (C) Under serum deprivation conditions (1% FBS), any of the studied ligands (CD30L, CD40L, RANKL) enhanced the survival of the L-428 HD cell line, an effect that was inhibited by UO126.
Because the malignant Reed-Sternberg cells of HD may receive signaling through these receptors in vivo, we determined whether UO126 could inhibit MEK activity in the presence of active signaling through these receptors. As shown in Figure 7B, UO126 not only inhibited the basal level of p-ERK, but also inhibited ligand-induced ERK phosphorylation. When HD cells were cultured in 10% heat-inactivated fetal bovine serum (FBS), none of the studied ligands (CD30L, CD40L, RANKL) induced cell proliferation (data not shown). However, under serum deprivation conditions (1% FBS), all ligands enhanced HD cell survival, and UO126 inhibited ligand-induced cell survival (Figure 7C).
Discussion
This study provides the first evidence of the potential role of the MEK/ERK pathway in regulating the growth and survival of HD cells. All 4 HD cell lines that we have studied aberrantly expressed p-ERK. Furthermore, primary Reed-Sternberg cells of HD also aberrantly expressed p-ERK. In any given lymph node section that was involved with HD, however, not all the Reed-Sternberg cells expressed p-ERK, suggesting that the malignant cells may be in different phases of proliferation or are exposed to different levels of inducing cytokines.11 Inhibition of MEK activity, which is upstream of ERK, by the small molecule UO126 decreased ERK phosphorylation in a dose- and time-dependent manner, and was associated with inhibition of cell proliferation in all 4 HD cell lines that we tested. In one cell line (HD-LM-2) the antiproliferative effect was due to induction of apoptosis. The induction of apoptosis was caspase independent as the pancaspase inhibitor Z-VAD did not counteract the effect of UO126. UO126 decreased the levels of Bcl-2 and Mcl-1, suggesting that this may have been the underlying mechanism for induction of cell death. A similar effect of MEK inhibitors on Mcl-1 protein has previously been reported in leukemic cells.53 In a different study on leukemia cells, MEK inhibition did not decrease Bcl-2 levels,7 but pharmacologic disruption of Bcl-2 protein acted synergistically with MEK inhibitors to induce apoptosis.15 This suggests that MEK inhibitors may induce cell death by different mechanisms in different cell types.
Treatment of HD cell lines with UO126 also resulted in cell-cycle arrest at the G2M phase. The underlying mechanism for the observed cell-cycle arrest is not clear, as MEK inhibition did not alter the total cellular content of several cell-cycle regulatory proteins, including p53, p14, p21, p27, cdc25A, cdc25C, and cyclin D1. This does not, however, exclude a potential role of any of these proteins in the mechanism of UO126 action. In fact, the phosphorylated state of these proteins may be involved in the regulation of the cell cycle without changes of the total cellular content of these proteins. In this study, we showed that UO126 indeed altered the phosphorylated state of p53 without affecting the total cellular content of p53 protein. The effect of UO126 on other cell-cycle proteins warrants further investigation.54 The ability of UO126 to inhibit HD cell line proliferation without affecting the survival of normal peripheral blood lymphocytes suggests that MEK inhibition may preferentially affect tumor but not benign cells, which makes it appealing for therapeutic purposes.
The primary function of APO2L/TRAIL is to induce cell death by activating its death receptors TRAIL-R1 and TRAIL-R2.24 TRAIL induces cell death by direct activation of caspases (extrinsic receptor-mediated pathway), but it can also activate the mitochondria-intrinsic pathway by cleaving Bid.24,30,31 Activated caspase 8 can cleave Bid, which promotes Bax and Bak activation and oligomerization, which lead to mitochondrial membrane damage and cytochrome-C release with subsequent activation of caspase 9.55-57 Active caspase 9 can then activate the execution caspases 3, 6, and 7. Although the activity of APO2L/TRAIL in other lymphoid malignancies has been previously examined,29,32 the activity of APO2L/TRAIL in HD has not been previously reported. In this study, we found that, like other lymphoid malignancies, HD cells expressed R1, R2, and R4, but not R3, receptors. APO2L/TRAIL was variably effective in inducing cell death in all 4 cell lines, and its activity was mediated by activating both the extrinsic (caspase 8 and 10) and the intrinsic (caspase 9) death pathways.30 The link between the extrinsic and intrinsic pathways was through cleavage of BID, as previously reported in other cell systems.31
MEK inhibition by the small molecule UO126 potentiated the effects of APO2L/TRAIL and chemotherapy, perhaps by decreasing levels of Bcl-2, Mcl-1, and cFLIP, which are known to mediate resistance to chemotherapy and APO2L/TRAIL.58-60 This finding is consistent with the previous observation that ERK may mediate resistance to APO2L/TRAIL.12 Although ERK has been reported to mediate resistance to APO2L/TRAIL in other tumor cell types,12 it is interesting to note that APO2L/TRAIL inhibited ERK phosphorylation in HD cell lines in a time-dependent manner, mimicking the effect of UO126. The decrease in p-ERK induced by APO2L/TRAIL was not related to cleavage of the ERK protein, because the total amount of ERK did not significantly change over the same time frame. This suggests that other kinases that are upstream of ERK may be cleaved by the activated caspases, or that APO2L/TRAIL may activate a caspase-independent signaling pathway that somehow regulates ERK activity, such as MAPK phosphatase.
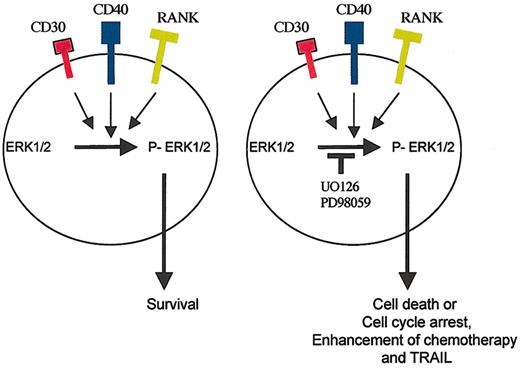
The malignant Reed-Sternberg cells of HD are known to express several TNF-family receptors, including CD30, CD40, and RANK.36-39 In this study, we showed that activating these receptors by their respective ligands activates MEK/ERK pathways, as evinced by phosphorylation of ERK in a time-dependent manner. Because Reed-Sternberg cells are exposed to these ligands in vivo, we examined whether the small molecule UO126 is capable of inhibiting MEK in the presence of active signaling through CD30, CD40, and RANK. We have shown that UO126 is indeed capable of inhibiting MEK and cell survival in the presence of these ligands, suggesting that it may maintain its biologic and therapeutic function in vivo (Figure 8). However, the UO126 concentrations used for these in vitro studies were at micromolar levels, which obviously precludes its clinical use. Newer MEK inhibitors are currently being designed that have higher affinity to MEK and can be used at lower concentrations suitable for use in vivo. This study, however, provides a first proof-of-principle that MEK inhibition may be of therapeutic value in patients with HD.
A model for ERK activation in HD cells. The malignant Reed-Sternberg cells express CD30, CD40, and RANK receptors, which can activate the MEK/ERK survival pathway. Inhibition of this pathway by the small molecule UO126 induces cell death or cell-cycle arrest and potentiates the killing effect of APO2L/TRAIL and chemotherapy.
A model for ERK activation in HD cells. The malignant Reed-Sternberg cells express CD30, CD40, and RANK receptors, which can activate the MEK/ERK survival pathway. Inhibition of this pathway by the small molecule UO126 induces cell death or cell-cycle arrest and potentiates the killing effect of APO2L/TRAIL and chemotherapy.
Prepublished online as Blood First Edition Paper, April 10, 2003; DOI 10.1182/blood-2002-11-3507.
Supported in part by M.D. Anderson's core grant from the National Cancer Institute (NCI) (CA-16672).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Dr Avi Ashkenazi for providing APO2L/TRAIL, and Kathryn Hale for editorial assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal