Abstract
The Chuvash form of polycythemia is an autosomal recessive disorder common to a large number of families in central Russia. Affected individuals have been reported to be homozygous for an Arg200Trp mutation in the von Hippel–Lindau (VHL) gene. We have screened 78 patients with erythrocytosis and found 8 of Bangladeshi and Pakistani origin to be homozygous for the Arg200Trp mutation and another of English descent to be heterozygous. Of these patients, 5 have elevated serum erythropoietin (Epo) levels, while the other 4 have Epo values in the normal range. The heterozygous patient does not fulfill the Chuvash criterion for homozygosity of the Arg200Trp mutation and consequently may harbor a further, as yet uncharacterized, mutation. This mutation has a wider geographic distribution than originally presumed and haplotype analysis suggests a common origin of the Arg200Trp mutation in the 4 families, but it still remains to be established if it has arisen independently of the Chuvash population.
Introduction
The autosomal recessive disorder known as the Chuvash form of familial polycythemia affects more than 100 individuals from more than 80 families in the mid-Volga region of European Russia.1 It is characterized by high hemoglobin (Hb) levels, usually more than 200 g/L, and normal to raised plasma erythropoietin (Epo) levels. A genome-wide screen indicated the location of a candidate gene on chromosome 3 and sequencing the von Hippel–Lindau gene (VHL) detected a homozygous mutation (Arg200Trp) in Chuvash polycythemic individuals.2 Subsequently this and 3 other VHL mutations (Asp126Tyr, Val130Ile, and Pro192Ala) were detected in 5 polycythemic children of Dutch-Italian, English, and Russian origins.3,4
Epo production is tightly regulated by a negative feedback loop5 involving the transcription complex hypoxia-inducible factor-1 (HIF-1).6 This complex is composed of 2 subunits, HIF-1α and the constitutively stable HIF-1β (also known as aryl hydrocarbon receptor nuclear translocator [ARNT]).7 Under normoxia the level of HIF-1α is maintained at a low level as it binds to the VHL protein, becomes ubiquitinated, and is targeted by the elongin C complex for degradation by the proteasome.8 A point mutation in the VHL gene is known to disrupt its association with HIF-1α and increase Epo production.9 Thus we decided to screen for VHL mutations in a group of erythrocytosis patients with normal to raised Epo levels, who were negative for Epo receptor (EpoR) mutations.
Study design
Patients and case history
Idiopathic erythrocytosis patients from the United Kingdom and Ireland with elevated Hb levels (> 180 g/L in males and 165 g/L in females), raised packed cell volume (PCV; above 0.51 in males and 0.48 in females), and with no splenomegaly and the absence of known secondary causes were recruited to our registry. Epo levels were measured locally and the normal range (NR) for each assay is given. Approval was obtained from the Queen's University research ethics committee for these studies. Informed consent was provided according to the Declaration of Helsinki.
Family A. A Bengali-speaking family from Bangladesh (Figure 1A) was investigated following the identification of the propositus (A1) who presented at the age of 21 years with peripheral neuropathy. A computed tomographic (CT) scan of his head was normal. He had a Hb level of 226 g/L, PCV of 0.67, normal P50 value, and an Epo level of 48 mU/mL (NR, 3.7-15.2 mU/mL). There were 3 other siblings (A2, A3, and A4) who had normal Epo levels (7.2-10.1 mU/mL, NR, 3.7-15.2 mU/mL) and elevated Hb levels. There is no consanguinity in the family. None of the patients have a history of cerebrovascular complications.
Pedigrees of the 4 families with the 598C> T mutation. Circles denote female family members, squares denote male family members, and symbols with a diagonal line indicate deceased family members. Black symbols indicate individuals with erythrocytosis and shaded symbols, nonerythrocytosis carriers for the 598C>T mutation. The genotype of individuals is indicated by wt (normal) and 598C>T for mutant. Hemoglobin level (g/L), PCV, red blood cell count (RBC), and Epo (mU/mL) values are given when known.
Pedigrees of the 4 families with the 598C> T mutation. Circles denote female family members, squares denote male family members, and symbols with a diagonal line indicate deceased family members. Black symbols indicate individuals with erythrocytosis and shaded symbols, nonerythrocytosis carriers for the 598C>T mutation. The genotype of individuals is indicated by wt (normal) and 598C>T for mutant. Hemoglobin level (g/L), PCV, red blood cell count (RBC), and Epo (mU/mL) values are given when known.
Family B. A32-year-old Punjabi-speaking female (B1, Figure 1B) from the Toba Tek Singh district of Pakistan attending an infertility clinic was found to have an Hb of 207 g/L and PCV of 0.66 with a normal P50 value. A CT scan following a stroke showed a cerebral infarct but no evidence of cerebrovascular malformation. Her 20-year-old sister (B2) was admitted to hospital with a large left parietal intracerebral hematoma. Cerebral angiograms and magnetic resonance image scans did not indicate any cerebrovascular abnormalities. B2's red cell count was elevated at 6 × 1012/L with an Hb level of 133 g/L and PCV of 0.43. A second sibling (B3) presented with an Hb level of 167 g/L and PCV of 0.67 with no history of stroke and so had no cerebrovascular investigation. Both B2 and B3 were iron deficient. Epo levels for B1, B2, and B3 were 369 mU/mL, 128 mU/mL, and 105 mU/mL, respectively (NR, 2.5-10.5 mU/mL). The older members of the next generation are healthy and there is no history of erythrocytosis in either maternal or paternal relatives, although both parents are first cousins.
Patient C1. An 8-year-old Urdu-speaking boy from the Punjab region of Pakistan (Figure 1C) presented with an Hb level of 244 g/L and PCV of 0.61. His Epo level was 96 mU/mL (NR, 9.1-30.8 mU/mL). Both parents are cousins and there is a family history of infant mortality, with 1 baby born dead, 3 dying within a few days of birth of unclear causes, and another sibling who died at the age of 6 with a cerebrovascular accident. There is one healthy surviving female now who is 8 years old.
Patient D1. A white male of English ancestry was diagnosed with erythrocytosis at the age of 34 years without a family history (Figure 1D). At presentation his Hb level was 214 g/L and PCV was 0.67. His Epo level was 26.1 mU/mL (NR, 8-28 mU/mL). All other parameters were normal. One year after diagnosis, D1 experienced a thrombosis in the left leg.
Sequencing the VHL gene
Genomic DNAwas isolated from peripheral blood using a Nucleon BACC 1 DNA extraction kit (Nucleon Biosciences, Manchester, United Kingdom). Polymerase chain reaction (PCR) was performed in 100 μL reaction containing 10% dimethyl sulfoxide, 1.5 mM MgCl2, 200 μM deoxynucleotide triphosphate, 0.5 μM each of forward and reverse primer, 2.5 units of Thermo-Start DNA Polymerase (ABgene, Epsom, Surrey, United Kingdom), and 100 ng genomic DNA, in 1 × Thermo-Start Standard Buffer provided by the manufacturer. The following primer sets were used: VHL F1, 5′-AGCGCGTTCCATCCTCTAC-3′ and VHL R1, 5′-GCTTCAGACCGTGCTATCGT-3′ for exon 1; VHL F2, 5′-GAGGTTTCACCACGTTAGCC-3′ and VHLR2, 5′-AGCCCAAAGTGCTTTTGAGA-3′ for exon 2; and VHL F3, 5′-CAGAGGCATGAACACCATGA-3′ and VHL R3, 5′-AAGGAAGGAACCAGTCCTGT-3′ for exon 3. Amplification was performed with an initial heat activation step of 15 minutes at 95°C followed by 35 cycles for 1 minute at 95°C, for 1 minute at 52°C, and for 1 minute at 72°C in the GeneAmp PCR system 2700 (Applied Biosystems, Warrington, United Kingdom). PCR products were purified using Concert Rapid PCR Purification System (Life Technologies, Paisley, United Kingdom) and were sequenced using ABI Prism Big Dye Terminator Cycle Sequencing Ready Reaction Kit Version 3 on ABI 3100 DNA Genetic Analyzer (Applied Biosystems).
Mutation screen for 598C> T base change
The 598C>T mutation destroys the restriction site for BsrBI enzyme, and 16 μL of PCR-amplified exon 3 was digested with 10 U BsrBI (New England Biolabs, Hitchin, United Kingdom) for 2 hours at 37°C and visualized by agarose electrophoresis.
Haplotype analysis
Single nucleotide polymorphisms (SNPs) located in introns 1 and 2 of the VHL gene, 3-kb 5′, and 3-kb and 8-kb 3′ to the VHL gene were selected from chromosome 3 reference contig NT_005927 (National Center for Biotechnology Information [NCBI]). The following primer sets were used: rs776517 F, 5′-GCCACCCCTTCCTCTTAAAT-3′ and rs776517 R, 5′-CCCCAAAGTAGGACTTCTGTTA-3′; for rs265318 primers rs775617F/R were used; rs374645 F, 5′-TTTACATTTCTTAAAATTTCCCATCA-3′ and rs374645 R, 5′-TCAGTCCTCACAACAGATCCA-3′; rs2600005 F, 5′-CCCCAAATACATGGGTTTCA-3′ and rs2600005 R, 5′-CAACGACTACCACCAGCAGA-3′; rs166538 F, 5′-CAAGGTGGTGAAACCCTGTC-3′ and rs166538 R, 5′-TTGGGCAATCTCCCATACAT-3′; and rs458952 F, 5′-TTGATCTTGAGCGTTTTGGA-3′ and rs458952 R, 5′-GTATTCTGACCCCAGGCTCTC-3′. Amplification was as described in “Sequencing the VHL gene” with an annealing temperature of 55°C, and PCR products were sequenced.
Results and discussion
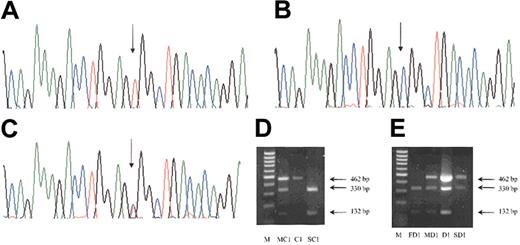
A group of 46 patients with raised and normal Epo levels were chosen from our registry of erythrocytosis patients. Sequencing all 3 exons of the VHL gene detected a homozygous C to T transition at nucleotide 598 in 4 siblings from family A (Figure 2A), 3 siblings from Family B, and patient C1. A ninth patient (D1) was found to be heterozygous for this mutation (Figure 2C). The 598C>T mutation destroys the restriction site for BsrBI enzyme, and a further group of 32 erythrocytosis patients with low to normal Epo levels were screened for the 598C>T mutation and found to be negative.
Detection of 598C>T mutation. Sequencing of exon 3 of the VHL gene detected a homozygous base change of C to T at nucleotide 598 in patients A1 to A3, B1 to B3, and C1 (A) when compared with the normal sequence (B). One patient, D1, was heterozygous for this base change (C). The position of the mutation is indicated by an arrow. The 598C>T mutation destroys the restriction site for BsrBI enzyme and the normal exon 3 PCR product is restricted into 2 fragments of 330 and 132 bp, but in the presence of the 598C>T mutation only a 462-bp fragment is obtained. Screening of family members of C1 indicated that his mother was heterozygous and his sibling was normal (D). The father of D1 is normal but his mother and son are heterozygous for the 598C>T (E). M indicates 100-bp DNA ladder; MC1, mother of patient C1; C1, patient C1; SC1, sibling of C1; FD1, father of patient D1; MD1, mother of patient D1; D1, patient D1; and SD1, son of D1.
Detection of 598C>T mutation. Sequencing of exon 3 of the VHL gene detected a homozygous base change of C to T at nucleotide 598 in patients A1 to A3, B1 to B3, and C1 (A) when compared with the normal sequence (B). One patient, D1, was heterozygous for this base change (C). The position of the mutation is indicated by an arrow. The 598C>T mutation destroys the restriction site for BsrBI enzyme and the normal exon 3 PCR product is restricted into 2 fragments of 330 and 132 bp, but in the presence of the 598C>T mutation only a 462-bp fragment is obtained. Screening of family members of C1 indicated that his mother was heterozygous and his sibling was normal (D). The father of D1 is normal but his mother and son are heterozygous for the 598C>T (E). M indicates 100-bp DNA ladder; MC1, mother of patient C1; C1, patient C1; SC1, sibling of C1; FD1, father of patient D1; MD1, mother of patient D1; D1, patient D1; and SD1, son of D1.
The 598C>T mutation predicts an amino acid change of arginine to tryptophan at codon 200 (Arg200Trp), which is located outside the 7-stranded β domain of pVHL that binds HIF-1α (residues 63-154) but lies within the α helix (residues 193-204).10 Although this helix does not bind directly to HIF-1α, it may contribute to the association of pVHL and HIF-1α.10 The Arg200Trp mutation affects the affinity of pVHL for HIF-1α, thereby prolonging the stability of HIF-1α and up-regulating Epo production.11
All patients reported here, except D1, fulfill the Chuvash polycythemia criteria clinically, with a recessive mode of inheritance. A further unidentified mutation may contribute to D1's erythrocytosis. Although the mother and the 20-year-old son of D1 are heterozygous for the 598C>T mutation, they have not been diagnosed with erythrocytosis, and D1's son has an Hb level of 173 g/L, which is at the upper end of the normal range but he is a smoker. In common with previous reports we have found the Arg200Trp mutation is associated with both raised or inappropriately normal serum Epo values.1-4,11
The Arg200Trp mutation has been implicated as the cause of autosomal recessive Chuvash polycythemia,2 and we have detected the same mutation in families of Bangladeshi, Pakistani, and English origin. It appears that the 598C>T mutation has not arisen de novo in these patients as several members in families A and B possess the mutation and mothers from families A, C, and D are heterozygous (Figure 2D-E). To complete the study, analysis of the surviving parent of family B and patient C1's father is required. To date the Arg200Trp mutation has been reported in Chuvashian,2 Dutch-Italian, English, Russian, and African-American individuals,3,4 and patients with VHL syndrome12,13 and clear cell carcinoma14,15 (http://www.umd.necker.fr:2005/), thus suggesting that this mutation has arisen independently in different ethnic groups or that it has a wider geographic distribution than initially presumed. To identify the origin of the Arg200Trp mutation, haplotype analysis has been performed with families A, B, C, and D using SNPs located in introns 1 and 2 of the VHL gene, and 3-kb 5′, and 3-kb and 8-kb 3′ to the VHL gene (Table 1).
Allele frequencies of 6 dimorphic SNPs located within the VHL gene and flanking regions in healthy individuals and subjects bearing the 598C>T base change
Polymorphism . | Allele . | Normal N = 4 . | Heterozygotes N = 10 . | Homozygotes N = 16 . |
|---|---|---|---|---|
| rs776517 | A | 1.0 | 0.3 | 0.0 |
| 3 kb 5′ to VHL | G | 0.0 | 0.7 | 1.0 |
| (10, 109, 403 bp) | ||||
| rs265318 | A | 1.0 | 0.4 | 0.0 |
| 3 kb 5′ to VHL | C | 0.0 | 0.6 | 1.0 |
| (10, 109, 505 bp) | ||||
| rs374645 | C | 1.0 | 0.5 | 0.0 |
| Intron 1 VHL | T | 0.0 | 0.5 | 1.0 |
| (10, 116, 017) | ||||
| rs2600005 | A | 0.0 | 0.6 | 1.0 |
| Intron 2 VHL | G | 1.0 | 0.4 | 0.0 |
| (10, 119, 507) | ||||
| rs166538 | C | 0.5 | 0.9 | 1.0 |
| 3 kb 3′ to VHL | T | 0.5 | 0.1 | 0.0 |
| (10, 127, 692 bp) | ||||
| rs458952 | A | 0.0 | 0.7 | 1.0 |
| 8 kb 3′ to VHL | G | 1.0 | 0.3 | 0.0 |
| (10, 132, 640 bp) |
Polymorphism . | Allele . | Normal N = 4 . | Heterozygotes N = 10 . | Homozygotes N = 16 . |
|---|---|---|---|---|
| rs776517 | A | 1.0 | 0.3 | 0.0 |
| 3 kb 5′ to VHL | G | 0.0 | 0.7 | 1.0 |
| (10, 109, 403 bp) | ||||
| rs265318 | A | 1.0 | 0.4 | 0.0 |
| 3 kb 5′ to VHL | C | 0.0 | 0.6 | 1.0 |
| (10, 109, 505 bp) | ||||
| rs374645 | C | 1.0 | 0.5 | 0.0 |
| Intron 1 VHL | T | 0.0 | 0.5 | 1.0 |
| (10, 116, 017) | ||||
| rs2600005 | A | 0.0 | 0.6 | 1.0 |
| Intron 2 VHL | G | 1.0 | 0.4 | 0.0 |
| (10, 119, 507) | ||||
| rs166538 | C | 0.5 | 0.9 | 1.0 |
| 3 kb 3′ to VHL | T | 0.5 | 0.1 | 0.0 |
| (10, 127, 692 bp) | ||||
| rs458952 | A | 0.0 | 0.7 | 1.0 |
| 8 kb 3′ to VHL | G | 1.0 | 0.3 | 0.0 |
| (10, 132, 640 bp) |
rs number indicates reference number in SNPs database from NCBI; bp, location on chromosome 3 reference contig NT-005927 (NCBI); and N, number of alleles
A common haplotype of GCTACA was found to be associated with the Arg200Trp mutation (Table 2), thus suggesting a founder mutation in our group of patients. A comprehensive haplotype analysis of individuals from Chuvashia and the other ethnic groups will establish if there was a common founder mutation that has been distributed by migration.
Frequency of the founder haplotype (GCTACA) in normal 598C and 598T alleles
Haplotype . | . | . | . | . | . | Homozygote normal-598C . | Heterozygote normal-598C . | Homozygote mutant-598T . | Heterozygote mutant-598T . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs776517 . | rs265318 . | rs374645 . | rs2600005 . | rs166538 . | rs458952 . | N . | N . | N . | N . | |||||
| A | A | C | G | C | G | 2 | 1 | 0 | 0 | |||||
| A | A | C | G | T | G | 2 | 1 | 0 | 0 | |||||
| G | A | C | G | C | A | 0 | 1 | 0 | 0 | |||||
| G | C | C | A | C | A | 0 | 1 | 0 | 0 | |||||
| G | C | C | A | C | A | 0 | 1 | 0 | 0 | |||||
| G | C | T | A | C | A | 0 | 0 | 16 | 5 | |||||
Haplotype . | . | . | . | . | . | Homozygote normal-598C . | Heterozygote normal-598C . | Homozygote mutant-598T . | Heterozygote mutant-598T . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs776517 . | rs265318 . | rs374645 . | rs2600005 . | rs166538 . | rs458952 . | N . | N . | N . | N . | |||||
| A | A | C | G | C | G | 2 | 1 | 0 | 0 | |||||
| A | A | C | G | T | G | 2 | 1 | 0 | 0 | |||||
| G | A | C | G | C | A | 0 | 1 | 0 | 0 | |||||
| G | C | C | A | C | A | 0 | 1 | 0 | 0 | |||||
| G | C | C | A | C | A | 0 | 1 | 0 | 0 | |||||
| G | C | T | A | C | A | 0 | 0 | 16 | 5 | |||||
Prepublished online as Blood First Edition Paper, April 17, 2003; DOI 10.1182/blood-2002-10-3246.
Supported by the Northern Ireland Leukaemia Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank all the clinicians who have referred erythrocytosis patients from the United Kingdom and Ireland to our registry and provided patient samples.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal