Abstract
Increased bone marrow angiogenesis and vascular endothelial growth factor (VEGF) levels are adverse prognostic features in patients with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDSs). VEGF is a soluble circulating angiogenic molecule that stimulates signaling via receptor tyrosine kinases (RTKs), including VEGF receptor 2 (VEGFR-2). AML blasts may express VEGFR-2, c-kit, and FLT3. SU5416 is a small molecule RTK inhibitor (RTKI) of VEGFR-2, c-kit, and both wild-type and mutant FLT3. A multicenter phase 2 study of SU5416 was conducted in patients with refractory AML or MDS. For a median of 9 weeks (range, 1-55 weeks), 55 patients (33 AML: 10 [30%] primary refractory, 23 [70%] relapsed; 22 MDS: 15 [68%] relapsed) received 145 mg/m2 SU5416 twice weekly intravenously. Grade 3 or 4 drug-related toxicities included headaches (14%), infusion-related reactions (11%), dyspnea (14%), fatigue (7%), thrombotic episodes (7%), bone pain (5%), and gastrointestinal disturbance (4%). There were 11 patients (20%) who did not complete 4 weeks of therapy (10 progressive disease, 1 adverse event); 3 patients (5%) who achieved partial responses; and 1 (2%) who achieved hematologic improvement. Single agent SU5416 had biologic and modest clinical activity in refractory AML/MDS. Overall median survival was 12 weeks in AML patients (range, 4-41 weeks) and not reached in MDS patients. Most observed toxicities were attributable to drug formulation (polyoxyl 35 castor oil or hyperosmolarity of the SU5416 preparation). Studies of other RTKI and/or other antiangiogenic approaches, with correlative studies to examine biologic effects, may be warranted in patients with AML/MDS.
Introduction
The role of angiogenesis in the pathophysiology of hematologic malignancies, including acute myeloid leukemia (AML) and myelodysplastic syndromes (MDSs), is being intensively investigated.1-7 Vascular endothelial growth factor (VEGF) is a pivotal angiogenic molecule.8 VEGF regulates several endothelial cell functions, including mitogenesis, permeability, and the production of vasoactive molecules. VEGF also is a survival factor required for the maintenance of new blood vessels.9 The activity of VEGF is mediated through 3 receptor tyrosine kinases (RTKs): VEGF receptor 1 (VEGFR-1; Flt-1), VEGFR-2 (KDR/Flk-1), and VEGFR-3 (Flt-4).10 The endothelial proliferative and mitogenic activities of VEGF, as well as vascular permeability, are mediated primarily through VEGFR-2.11 Bone marrow VEGF and VEGFR-2 levels are significantly elevated in patients with untreated AML, while VEGFR-1 levels are normal.12 Bone marrow levels of VEGF and VEGFR-2 correlate with increased microvessel density (MVD) in these patients.10,12 VEGFR-2 levels are reduced to normal levels in patients with AML who achieve a complete remission (CR) following induction chemotherapy.12 Bone marrow VEGFR-2 levels are equivalently elevated in patients with AML and MDS—in neither disease is there a significant correlation between VEGFR levels and duration of survival.10 Plasma and marrow VEGF protein levels are significantly higher in MDS than in AML.10
Functional VEGF receptors are present on more than 50% of AML blasts; it has therefore been hypothesized that VEGF may participate in autocrine or paracrine loops in this disease.5,13-16 For example, VEGF directly stimulates the production of several hematopoietic growth factors from human umbilical vein endothelial cells (HUVECs), including granulocyte-macrophage colonystimulating factor (GM-CSF) and stem cell factor (SCF).5,17 Increased VEGF levels in the bone marrow at time of diagnosis are associated with reduced CR rates, disease-free (after CR) survival, and overall survival in patients with previously untreated AML.18 Its negative impact is independent of established prognostic factors, including age, cytogenetics, performance status, or presence of an antecedent hematologic disorder.18 Elevated VEGF levels also correlate with reduced survival in patients with MDS.10 These data suggest that the VEGF/VEGFR-2 pathway is a potential therapeutic target in these diseases.16
SU5416 (Z-3-{(2,4-dimethylpyrrol-5-yl) methylidenyl}-2-indolinone) is a small, lipophilic, highly protein-bound, synthetic RTK inhibitor (RTKI) of VEGFR-2.19,20 It acts as a competitive inhibitor with regard to adenosine triphosphate within the kinase domain of the receptor. SU5416 inhibits VEGF-dependent endothelial cell proliferation in vitro and in animal models.21,22 Although it has no direct cytotoxic properties, SU5416 produces a dose-dependent inhibition of tumor growth in a variety of xenograft models, including malignant melanoma, glioma, fibrosarcoma, and carcinomas of the lung, breast, prostate, and skin.21 In a human colon cancer xenograft model, SU5416 inhibited tumor metastases, microvessel formation, and proliferation.23
SU5416 also targets 2 other split kinase family RTKs, FLT3 (fms-related tyrosine kinase Flk2) and c-kit, both of which are expressed on AML blasts. C-kit is essential for normal hematopoietic cell development.24,25 Binding of the c-kit ligand, SCF, initiates a signal transduction cascade that includes receptor autophosphorylation and subsequent phosphorylation of numerous intracellular substrates. C-kit is expressed in leukemia blasts in approximately 60% to 80% of AML patients.24-27 SCF inhibits apoptosis in several myeloid leukemia cell lines established from patients with AML.28 In both MO7E cells, a human AML cell line that expresses c-kit, and blasts from patients that express c-kit, SU5416 inhibits SCF-induced tyrosine phosphorylation, reduces cell proliferation, and induces apoptosis.29 FLT3 is also expressed on hematopoietic progenitors, and dysregulation of FLT3 signaling is associated with myeloproliferative diseases.30-32 Internal tandem duplication (ITD) mutations in FLT3 result in constitutive FLT3 tyrosine kinase activity and are associated with a poor prognosis in patients with AML.33-36 FLT3-ITD is the most frequently observed molecular defect in AML (25%-30% of patients). Recent in vitro studies have shown that SU5416 is a potent inhibitor of both wild-type and mutant forms of FLT3.37 SU5416 may therefore directly target bone marrow angiogenesis via VEGFR-2 and blast cell proliferation via FLT3 and c-kit in AML. Recent data show that there are differences between patients with AML and MDS in terms of angiogenic and apoptotic parameters that would support a separate analysis of the efficacy of targeted therapies in these diseases.38 Mesters et al39 have recently reported the achievement of a CR in a patient in second, chemorefractory AML relapse following a 12-week course of twice-weekly SU5416 therapy. At time of achieving CR, normalization of the patient's elevated bone marrow MVD was noted. Based on these data, a multicenter phase 2 study to evaluate SU5416 in patients with refractory AML or MDS was conducted.
Patients, materials, and methods
This was an open-label multicenter study to assess the efficacy and toxicity of a twice-weekly infusion of SU5416 in patients with refractory AML or advanced MDS (refractory anemia with excess blasts [RAEB] or RAEB in transformation [RAEBT]). All study patients gave witnessed written consent for study participation and the institutional review boards at all participating institutions approved this protocol.
Patients
Patients eligible for enrollment in the study were 18 years or older with AML, excluding acute promyelocytic leukemia, RAEB, or RAEBT of the following types: (1) relapsed with an initial CR duration of less than 12 months, including those who failed to achieve CR after the first cycle of induction (primary resistant); (2) second or subsequent AML relapse; and (3) newly diagnosed patients older than 70 years, with karyotype other than inv16, 8:21, diploid,-X,-Y, or insufficient metaphases, who did not accept chemotherapy. Patients were required to have a baseline serum bilirubin level lower than 1.5 times the upper limit of normal (ULN) and a serum creatinine level lower than 176.8 μM.
Treatment regimen
SU5416 was supplied by Sugen (South San Francisco, CA) as a yellow-orange liquid formulation in vials containing 180 mg SU5416 in 40 mL vehicle for a final concentration of 4.5 mg/mL—thus the final volume of infused solution was dependent on patient body surface area. Additional components of the formulation included polyethylene glycol 400; polyoxyl 35 castor oil (Cremophor); and benzyl alcohol and dehydrated alcohol. SU5416 was diluted with either water for injection, 0.45% sodium chloride, or 0.9% sodium chloride prior to administration. Doses of SU5416 were administered via infusion pump at a rate of 200 cc/h except for the initial infusion, which was given at 100 cc/h for the first 15 minutes in an attempt to decrease the incidence of immediate-onset hypersensitivity reactions to polyoxyl 35 castor oil.40,41
SU5416 was administered at the dose, established on solid-tumor phase 1 and 2 studies,42 of 145 mg/m2 twice weekly through a central venous catheter (CVC) or peripherally inserted central catheter (PIC) for a total of 8 infusions in each 4-week cycle. Patients in the study were to receive a minimum of one 4-week cycle of therapy. Patients received premedication followed by SU5416 infusion (after at least a 30-minute delay for intravenous premedication). Premedication included oral or intravenous administration of 25 mg diphenhydramine or equivalent H1-blocker, and 20 mg famotidine or alternate H2-blocker. Patients also received dexamethasone at an initial dose of 10 mg intravenously (at least 30 minutes prior to infusion) for the first 3 infusions. This dose was subsequently reduced to 4 mg and again to 2 mg on subsequent occasions, as tolerated. For grade IV hypersensitivity reactions, the patient was withdrawn from the study. In the event of a grade I-III hypersensitivity reaction, the infusion was stopped and appropriate care given.
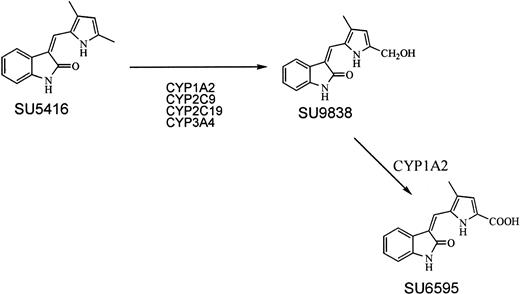
The primary pathway for metabolism of SU5416 is through sequential oxidative reactions of the 5-methyl group on the pyrrole ring (Figure 1).43 SU5416 is metabolized via the P-450 liver enzyme, CYP1A2 (an inducible enzyme), and to a lesser extent, by CYP3A4, CYP2C9, and CYP2C19. Patients who are receiving drugs and agents that inhibit or induce these enzymes, including some antifungal agents44 and macrolide antibiotics,45 may have altered plasma levels of SU5416. Recent data suggest that beverages such as coffee and grapefruit juice also inhibit CYP3A.46 These drugs or beverages were avoided while patients were in the study. Patients received full supportive care including transfusion of blood and blood products, antibiotics, antiemetics, antidiarrheals, and analgesics as appropriate. Colony-stimulating factors were not given to patients in the study.
Evaluation and statistics
Complete history and physical examination were performed within 3 days of study entry. The following baseline laboratory parameters were obtained: complete blood count with differential; biochemistry panel; coagulation profile; urinalysis; bone marrow aspirate and biopsy with histochemical, cytogenetic, and immunophenotypic analysis; chest x-ray; and, for women of childbearing potential, a pregnancy test. Bone marrow and peripheral blood slides were centrally reviewed. Bone marrow specimens were obtained at baseline, day 15, and day 29 of the first cycle of therapy, every 4 weeks thereafter, as clinically indicated, and at the time of leaving the study. Physical examination, adverse event evaluation, and hematologic, biochemical, and coagulation panels were repeated at weekly intervals and as clinically indicated.
All patients who received SU5416 were considered evaluable for toxicity analyses. Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria Version 2.0.
CR was defined as normalization of the peripheral blood and bone marrow with 5% or less blasts; normo- or hypercellular marrow; absolute neutrophil count (ANC) 1.0 × 109/L or higher; and platelet count 100 × 109/L or higher. Partial remission (PR) was defined as per CR except for the presence of 6% to 25% marrow blasts. Hematologic improvement (HI) was defined as per CR but with platelet count lower than 100 × 109/L. All other responses were considered failures.
Because this agent has a novel mechanism of action, a response rate of 10% in a very poor prognosis group of patients was considered of sufficient interest to warrant further investigation. A maximum total of 25 evaluable patients each with AML or MDS were to be entered in the study. This sample size yielded an 82% posterior credibility interval for probability of response of width approximately 0.16. For each group, there was one interim analysis after 14 patients had been evaluated. The study would terminate in that patient population if no responses had been observed. As a further measure to guard against exposure of patients to an agent with minimal activity, the aggregate response rate (CR/PR/HI) was reviewed periodically. If total response rates of 1 or less in 26 patients or 2 or less in 42 patients were observed, this would be evidence that the probability of less than 10% activity in this group of diseases was more than 90%.
Pharmacokinetics
Pharmacokinetic sampling of SU5416 and its metabolites (Figure 1) was performed on patients who, after one cycle of therapy (4 weeks), were considered to be responding. Blood samples were drawn according to a sparse sampling model and obtained on any of the 8 infusions in the second cycle of therapy. Plasma sample analysis was performed by high performance liquid chromatography as previously described.42 Modeling of the plasma concentration time data was performed using a nonlinear mixed effects program that uses an extended least squares algorithm (NONMEM, University of California San Francisco). The model assumed a 2-compartment disposition for SU5416. Parameters measured included peak concentration, area-under-the-curve (AUC), clearance, volume of distribution, and elimination half-life.
Results
Patients
Between October 2000 and November 2001, 55 patients were enrolled and commenced therapy. Of these, 33 patients (60%) had AML (10 [30%] with an antecedent MDS) and 22 had MDS. Baseline demographic data for AML and MDS patients are presented in Table 1. Of the patients, 10 (30%) had primary refractory AML and 12 (36%) had relapsed with an initial CR duration of less than 12 months.47
Clinical and laboratory characteristics of study AML (n = 33) and MDS (n = 22) patients
Characteristic . | Median . |
|---|---|
| AML patients | |
| Age, y (range) | 64 (23-76) |
| WBC count, × 109/L (range) | 2.3 (1.0-49.1) |
| Hemoglobin level, g/L (range) | 98 (73-171) |
| Platelets, × 109/L (range) | 30 (6-283) |
| % Peripheral blood blasts (range) | 14 (1-75) |
| % Bone marrow blasts (range) | 42 (0-90) |
| Bone marrow cellularity | |
| Hypocellular, less than 20% | 7 |
| Normocellular, 20% to 50% | 9 |
| Hypercellular, more than 50% | 17 |
| LDH, IU/L (range) | 461 (94-6763) |
| Creatinine, μM (range) | 88.4 (17.68-167.96) |
| Bilirubin, μM (range) | 8.55 (1.71-20.52) |
| SGPT, IU/L (range) | 20 (2-101) |
| Albumin, g/L (range) | 35 (18-44) |
| Male/female (%) | 20/13 (61/39) |
| Performance status, % | |
| 0/1 | 5/18 (15/55) |
| 2 | 10 (30) |
| Prior MDS (%) | 10 (30) |
| Primary refractory (%) | 10 (30) |
| Relapsed with CR duration, less than 1 y (%) | 12 (36) |
| Relapsed with CR duration, 1 y or longer (%) | 11 (33) |
| CR1 duration, mo, median (range) | 6 (1-36) |
| No. of prior salvage regimens, % | |
| None | 7 |
| 1 | 12 |
| 2 to 3 | 14 |
| MDS patients | |
| Age, y (range) | 66 (22-80) |
| WBC count, × 109/L (range) | 2.5 (0.4-5.7) |
| Hemoglobin level, g/L (range) | 89 (69-114) |
| Platelets, × 109/L (range) | 29 (3-540) |
| % Peripheral blood blasts (range) | 1 (0-27) |
| % Bone marrow blasts (range) | 6 (0-29) |
| Bone marrow cellularity | |
| Hypocellular, less than 20% | 4 |
| Normocellular, 20% to 50% | 17 |
| Hypercellular, more than 50% | 1 |
| LDH, IU/L (range) | 342 (117-2526) |
| Creatinine, μM (range) | 70.72 (44.2-114.92) |
| Bilirubin, μM (range) | 11.97 (5.13-34.2) |
| SGPT, IU/L (range) | 29 (6-108) |
| Albumin, g/L (range) | 36 (21-48) |
| Male/female (%) | 13/9 (59/41) |
| Performance status (%) | |
| 0/1 | 4/12 (18/55) |
| 2 | 6 (27) |
| Diagnoses | |
| RAEBT | 8 |
| RAEB | 14 |
| No. of prior regimens, % | |
| None | 7 |
| 1 | 7 |
| 2-3 | 8 |
| Median (range) | 1 (0-3) |
Characteristic . | Median . |
|---|---|
| AML patients | |
| Age, y (range) | 64 (23-76) |
| WBC count, × 109/L (range) | 2.3 (1.0-49.1) |
| Hemoglobin level, g/L (range) | 98 (73-171) |
| Platelets, × 109/L (range) | 30 (6-283) |
| % Peripheral blood blasts (range) | 14 (1-75) |
| % Bone marrow blasts (range) | 42 (0-90) |
| Bone marrow cellularity | |
| Hypocellular, less than 20% | 7 |
| Normocellular, 20% to 50% | 9 |
| Hypercellular, more than 50% | 17 |
| LDH, IU/L (range) | 461 (94-6763) |
| Creatinine, μM (range) | 88.4 (17.68-167.96) |
| Bilirubin, μM (range) | 8.55 (1.71-20.52) |
| SGPT, IU/L (range) | 20 (2-101) |
| Albumin, g/L (range) | 35 (18-44) |
| Male/female (%) | 20/13 (61/39) |
| Performance status, % | |
| 0/1 | 5/18 (15/55) |
| 2 | 10 (30) |
| Prior MDS (%) | 10 (30) |
| Primary refractory (%) | 10 (30) |
| Relapsed with CR duration, less than 1 y (%) | 12 (36) |
| Relapsed with CR duration, 1 y or longer (%) | 11 (33) |
| CR1 duration, mo, median (range) | 6 (1-36) |
| No. of prior salvage regimens, % | |
| None | 7 |
| 1 | 12 |
| 2 to 3 | 14 |
| MDS patients | |
| Age, y (range) | 66 (22-80) |
| WBC count, × 109/L (range) | 2.5 (0.4-5.7) |
| Hemoglobin level, g/L (range) | 89 (69-114) |
| Platelets, × 109/L (range) | 29 (3-540) |
| % Peripheral blood blasts (range) | 1 (0-27) |
| % Bone marrow blasts (range) | 6 (0-29) |
| Bone marrow cellularity | |
| Hypocellular, less than 20% | 4 |
| Normocellular, 20% to 50% | 17 |
| Hypercellular, more than 50% | 1 |
| LDH, IU/L (range) | 342 (117-2526) |
| Creatinine, μM (range) | 70.72 (44.2-114.92) |
| Bilirubin, μM (range) | 11.97 (5.13-34.2) |
| SGPT, IU/L (range) | 29 (6-108) |
| Albumin, g/L (range) | 36 (21-48) |
| Male/female (%) | 13/9 (59/41) |
| Performance status (%) | |
| 0/1 | 4/12 (18/55) |
| 2 | 6 (27) |
| Diagnoses | |
| RAEBT | 8 |
| RAEB | 14 |
| No. of prior regimens, % | |
| None | 7 |
| 1 | 7 |
| 2-3 | 8 |
| Median (range) | 1 (0-3) |
WBC indicates white blood cell; LDH, lactate dehydrogenase; and SGPT, serum glutamic pyruvic-transaminase
Responses
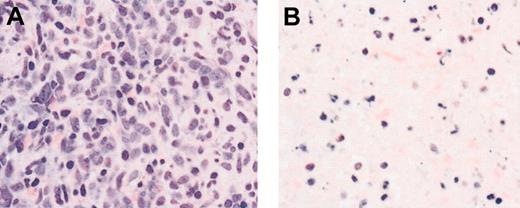
Bone marrow morphologic evaluation showed areas of marked apoptosis and necrosis in some patients with AML between days 7 and 14 after beginning therapy (Figure 2). There were 4 patients (7%) who had an objective response; 3 patients achieved a PR, 2 with AML, 1 with MDS; and 1 further patient with AML achieved an HI. Clinical details of the responding patients are contained in Table 2. Of 4 responding patients, 3 required 2 or more cycles of therapy before response was documented.
Induction of apoptosis and necrosis in a patient with AML following SU5416 therapy. Bone marrow biopsy from a patient with AML shows sheets of leukemic cells before therapy (A) and generalized necrosis and apoptosis on day 29 of SU5416 therapy. Hematoxylin and eosin stain. Magnification × 400.
Induction of apoptosis and necrosis in a patient with AML following SU5416 therapy. Bone marrow biopsy from a patient with AML shows sheets of leukemic cells before therapy (A) and generalized necrosis and apoptosis on day 29 of SU5416 therapy. Hematoxylin and eosin stain. Magnification × 400.
Characteristics of patients achieving objective response to SU5416
Diagnosis . | Age, y/sex . | FLT3-ITD/Asp835 . | Karyotype . | PS . | CR 1 duration, mo . | Induction regimen . | No. prior salvage regimens . | Response . | No. therapy cycles to response . | Survival, mo . | Eventfree survival, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AML | 64/M | N/N | + 8 | 0 | N/A | DA | None | PR | 3 | >8 | 4 |
| Prior MDS | BM blasts reduced from 43% to 13% PB blasts normalized from 9% of 11.7 WBC | ||||||||||
| AML | 62/F | N/N | Diploid | 1 | 4 | CAT + LT | 1 | PR | 4 | 7 | 6.5 |
| Prior MDS | BM blasts reduced from 33% to 7% PB blasts normalized from 2% of 1.2 WBC | ||||||||||
| AML | 74/M | N/Mut | + 14 | 1 | 14 | DA | 4 | HI | 2 | 3.5 | 3 |
| Prior MDS | - 7 | BM blasts reduced from 38% to 3% with normal CBC PB blasts normalized from 70% of 19.1 WBC Baseline platelets < 50 × 109 persisted | |||||||||
| RAEBT | 77/F | N/N | - 5 | 1 | 36 (from initial diagnosis) | (Prior erythropoietin, GCSF) | None | PR | 1 | >16 | 11 |
| - 7 | BM blasts reduced from 28% to 8% Normal baseline CBC |
Diagnosis . | Age, y/sex . | FLT3-ITD/Asp835 . | Karyotype . | PS . | CR 1 duration, mo . | Induction regimen . | No. prior salvage regimens . | Response . | No. therapy cycles to response . | Survival, mo . | Eventfree survival, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AML | 64/M | N/N | + 8 | 0 | N/A | DA | None | PR | 3 | >8 | 4 |
| Prior MDS | BM blasts reduced from 43% to 13% PB blasts normalized from 9% of 11.7 WBC | ||||||||||
| AML | 62/F | N/N | Diploid | 1 | 4 | CAT + LT | 1 | PR | 4 | 7 | 6.5 |
| Prior MDS | BM blasts reduced from 33% to 7% PB blasts normalized from 2% of 1.2 WBC | ||||||||||
| AML | 74/M | N/Mut | + 14 | 1 | 14 | DA | 4 | HI | 2 | 3.5 | 3 |
| Prior MDS | - 7 | BM blasts reduced from 38% to 3% with normal CBC PB blasts normalized from 70% of 19.1 WBC Baseline platelets < 50 × 109 persisted | |||||||||
| RAEBT | 77/F | N/N | - 5 | 1 | 36 (from initial diagnosis) | (Prior erythropoietin, GCSF) | None | PR | 1 | >16 | 11 |
| - 7 | BM blasts reduced from 28% to 8% Normal baseline CBC |
PS indicates Eastern Cooperative Oncology Group (ECOG) performance score; N, normal Flt3; N/A, not applicable; D, daunorubicin; A, ara-C; BM, bone marrow; PB, peripheral blood; WBC, white cell count; T, topotecan; C, cyclophosphamide; LT, liposomal tretinoin; CBC, complete blood count; and Mut, Flt3 mutation at Asp835
At the end of the first cycle of therapy in 33 patients with AML, 11 (33%) were removed from the study (10 had progressive disease and 1 refused further therapy after the first infusion), and 22 (66%) had stable disease and remained in the study. At the end of the first cycle of therapy, of 22 patients with MDS, 4 were removed from the study (3 had progressive disease and 1 was removed for noncompliance with protocol requirements), and 18 had stable disease and remained in the study.
In both the AML and MDS groups, the median time in the study was 9 weeks (range, 1-55 weeks), equating to 18 infusions of SU5416. In patients with AML, the maximum time in the study was 30 weeks. No dose reductions were required, even for those patients on long-term therapy (7 patients, > 100 days). In patients with MDS, the maximum time in the study was 55 weeks; again no dose reductions were required, with 8 patients in the study for more than 100 days. Median survival for AML patients was 12 weeks (range, 4-41 weeks); median survival was not yet reached in the MDS population.
Pharmacokinetics
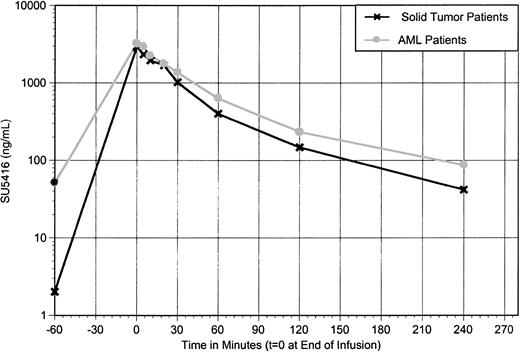
On day 1 of the first cycle of SU5416 therapy, 25 patients with AML had end-of-infusion blood draws with analysis of parent drug and 2 primary (inactive) metabolite levels. For SU5416, the end-of-infusion sampling point (consistently the maximal plasma concentration observed) was 3533 ± 1410 ng/mL (range, 440-6589 ng/mL). In patients with solid tumors treated with the study SU5416 regimen (145 mg/m2), the end-of-infusion plasma concentration on the first day of dosing was equivalent (n = 14) at 3636 ng/mL (±658).48
The initial metabolite (an alcohol group added to one of the methyl groups on the pyrrole ring) was observed at low concentrations (57 ± 28 ng/mL; range, 23-133 ng/mL) (Figure 1). The carboxylic acid derivative, arising from sequential oxidation and the primary metabolite observed in plasma, was observed at a concentration of 1105 ± 697 ng/mL (range, 370-3300 ng/mL). This later metabolite is observed at a wider range of concentrations (coefficient of variation of 63% compared with 40% for the parent drug), probably reflecting individual variability in activity of the metabolizing enzymes (primarily CYP1A2, with contributions from CYP3A4, CYP2C9, and CYP2C19). These metabolite data are also equivalent to those seen in patients with solid tumors.
During cycle 2, 7 patients underwent full pharmacokinetic sampling (from before infusion to 4 hours following the infusion). In solid tumor patients, clearance of parent drug increases between 4% and 308% in all patients with parallel declines in maximum plasma concentration (Cmax) and AUC upon chronic dosing at 3- to 4-day intervals (these values are 3636 ng/mL and 7325 μg/h/L, decreasing to 2997 ng/mL and 4416 μg/h/L, respectively, an 18% and a 40% decline).48 In patients with AML, the decrease in Cmax was less pronounced: comparing data obtained in these 7 patients, Cmax decreased from 3395 to 3211 ng/mL (5%). The AUC upon chronic dosing was also higher in AML patients, compared with data obtained in solid tumor patients (5860 to 4416 μg/h/L; Figure 3). As PK sampling was performed only in patients who were stable or responding to SU5416, this may have selected for patients who maintained higher systemic exposure to the drug.
SU5416 levels at 145 mg/m2 repeat schedule: comparison of patients with AML or solid tumors. SU5416 at a dose of 145 mg/m2 upon chronic dosing; mean plasma concentration versus time profile comparing patients with acute myeloid leukemia versus patients with miscellaneous solid tumors.
SU5416 levels at 145 mg/m2 repeat schedule: comparison of patients with AML or solid tumors. SU5416 at a dose of 145 mg/m2 upon chronic dosing; mean plasma concentration versus time profile comparing patients with acute myeloid leukemia versus patients with miscellaneous solid tumors.
Adverse events
All patients were premedicated prior to each SU5416 infusion with antihistamines (oral or intravenous) and intravenous steroids. There were 3 patients (5%) who experienced hypersensitivity reactions associated with the SU5416 infusion. These consisted of grade 3 flushing (n = 2), grade 3 pruritus (n = 1), and hypotension (respective grades 2 and 3 in 2 patients). All hypersensitivity reactions were successfully treated; all patients were subsequently retreated with SU5416 (using a higher dose of steroid premedication) without sequelae.
Injection site reactions were frequently observed (grade 1 or 2 in 43% of patients), consisting of pain at the site (frequently described as burning), erythema, or induration. There were 2 patients who experienced necrosis of the overlying skin, necessitating removal of the catheter. These toxicities occurred mainly in patients who received SU5416 via PICs rather than CVCs.
Nonhematologic grade 3 or 4 toxicities (summarized in Table 3) were more common in patients with AML than in those with MDS. Toxicities included headache (14%), dyspnea (14%), fatigue (7%), thromboembolic events (7%; 2 deep venous thrombosis [DVT] and 2 ischemic strokes), bone pain (5%), gastrointestinal events (nausea, diarrhea, and vomiting; 4%), and abdominal pain (5%). All drug-attributed toxicities were reversed by appropriate supportive care.
Nonhematologic grade 3 or 4 toxicities: percent by diagnostic group and overall
. | Headache . | Dyspnea . | Fatigue . | Chest pain . | Bone pain . | Diarrhea . | Nausea . | Vomiting . | Abdominal pain . |
|---|---|---|---|---|---|---|---|---|---|
| AML, n = 33 | 21 | 18 | 12 | 3 | 9 | 3 | 6 | 4 | 0 |
| MDS, n = 22 | 5 | 9 | 0 | 9 | 0 | 9 | 0 | 5 | 9 |
| Overall, n = 55 | 14 | 14 | 7 | 5 | 5 | 5 | 4 | 4 | 4 |
. | Headache . | Dyspnea . | Fatigue . | Chest pain . | Bone pain . | Diarrhea . | Nausea . | Vomiting . | Abdominal pain . |
|---|---|---|---|---|---|---|---|---|---|
| AML, n = 33 | 21 | 18 | 12 | 3 | 9 | 3 | 6 | 4 | 0 |
| MDS, n = 22 | 5 | 9 | 0 | 9 | 0 | 9 | 0 | 5 | 9 |
| Overall, n = 55 | 14 | 14 | 7 | 5 | 5 | 5 | 4 | 4 | 4 |
Discussion
This is the first report on a phase 2 study of a selective inhibitor of VEGFR-2, c-kit, and FLT3 in patients with refractory myeloid leukemia. This phase 2 study was conducted to assess the toxicity and efficacy of SU5416 in patients with refractory AML or MDS. Single agent SU5416 had biologic and modest clinical activity in these patients—it was associated with a PR or HI in 4 (7%) of 55 patients. This response, unremarkable for a traditional cytotoxic agent, is of interest when it occurs in response to an agent with no general direct cytotoxic properties.
SU5416 targets the split kinase domain RTKs VEGFR-2, FLT3, and c-kit RTKs. In a recent in vitro study, Spiekermann et al49 analyzed the expression of VEGFR and VEGF in AML cell lines and examined the inhibitory activity of SU5614, an indoline with similar target profile to SU5416, on human endothelial and leukemia cells. Although SU5614 was a potent inhibitor of VEGF-induced endothelial cell sprouting in vitro, the sensitivity of leukemia cells toward its growth inhibitory activity was determined by the level of c-kit, not by the level of VEGFR-2 expression. The ability of SU5416 to inhibit both wild-type and mutant FLT3 has been recently reported.37 ITD mutations in FLT3, which result in constitutive kinase activity, occur in 20% to 30% of patients with AML and are associated with a poor prognosis.50-52 Thus, in addition to VEGFR-2 and c-kit inhibition, SU5416 may also have an anti-AML activity by FLT3 inhibition.
SU5416 inhibits VEGF-driven mitogenesis of HUVECs in a dose-dependent manner, with a 50% inhibitory concentration (IC50) value of 0.04 μM at the end of a 48-hour assay. SU5416 also inhibits VEGF-mediated HUVEC survival, inducing apoptosis in a dose-dependent manner, with an IC50 of 0.5 μM in a 24-hour assay. The growth of MO7E human myeloid leukemia cells stimulated by SCF is inhibited by SU5416 in a dose-dependent manner with an IC50 of 0.1 μM. Kinetic studies indicate that the inhibitory effect of SU5416 on VEGF-dependent HUVEC mitogenic response is seen after incubations as short as 5 minutes, with maximal activity seen after 1 to 2 hours of incubation. SU5416 plasma levels of higher than 0.5 μM are achieved for a 1- to 2-hour period following each infusion on the study regimen.
In this study, 4 (7%) patients developed thromboembolic events (2 of ischemic stroke, 2 DVT). Such complications, including DVT, pulmonary embolism, transient ischemic attack, and cerebrovascular accidents, have been reported primarily in patients receiving SU5416 coadministered with other cytotoxic agents.53-55 Reported rates include 42% for patients treated with SU5416 plus gemcitabine and cisplatin,53 22% when combined with bolus 5-fluorouracil and leucovorin,54 and 29% when combined with paclitaxel and carboplatin.55 The relatively low rate of thrombosis (7%) observed in this study may be the result of SU5416 being delivered as monotherapy, baseline thrombocytopenia in most patients, and/or the use of low-dose prophylactic anticoagulation in those patients (n = 13, 24%) with a normal platelet count. Patient coagulation studies were monitored closely in the study—no pattern of abnormality was observed.
In a separate phase 2 study of SU5416 in patients with refractory AML who express c-kit, Fiedler et al56 have reported severe bone pain in 3 (14%) of 22 patients; the toxicity was considered unexpected and possibly related to SU5416. This toxicity has not been reported in patients with solid tumors receiving SU5416. In this study, grade 1 or 2 bone pain occurred in 30% of patients, grade 3 or 4 in 3 patients (9%) with AML, and none with MDS. Thus, bone pain appears to be a leukemia-specific adverse effect of SU5416.
Grade 1 or 2 pain, burning, or erythema at the catheter site was reported in 43% of patients in this study. There were 2 patients (4%) who experienced necrosis of the skin, necessitating removal, and 18 patients (33%) required insertion of a second catheter during their time in the study, primarily because of infection. For a median time in the study of 9 weeks, this rate of catheter failure appears high. The potential impact of angiogenesis modulators on wound healing and tissue repair is a clinical concern.57-60 In animal models, SU5416 is associated with significant reductions in the amount of granulation tissue and angiogenesis accompanying wound repair.57 Granulation tissue may have decreased collagen deposition, reported to be associated with a major reduction in active transforming growth factor–β1 (TGF-β1) tissue levels.57 Haroon et al57 have recently reported that SU5416 inhibits tissue transglutaminase, a crosslinking enzyme involved in TGF-β1 activation and matrix stabilization. The study data suggest that SU5416 may delay wound healing in patients, that CVC should be used for SU5416 administration, and that further studies are needed to evaluate the effects on wound healing of angiogenesis modulators.
A major challenge in developing SU5416 is the drug product itself. Its high concentration of polyoxyl 35 castor oil, and the hyperosmolarity of the diluted drug product, materially contributed to the incidence of hypersensitivity and injection site reactions observed in this study. Adverse events attributable to polyoxyl 35 castor oil are a major problem with other agents (eg, taxanes).40,41 A weekly infusion of SU5416 at a dose of 145 mg/m2 has been recently established to be safe and to maintain a comparatively higher systemic exposure for a given dose of SU5416.42
Aside from VEGFR RTKIs, multiple approaches to inhibition of VEGF activity, including antisense or ribozymes that target VEGF or VEGFR mRNA, soluble recombinant VEGFR, and antibodies that directly neutralize VEGF or block its receptors, are being investigated in patients with malignant disease.16 The various anti–VEGF RTKIs have differing patterns of inhibition of other angiogenesis-related receptors, including those for SCF (c-kit), platelet-derived growth factor (PDGFR), and hepatocyte growth factor (c-met).16 The investigation of these novel agents represents a formidable challenge.
Prepublished online as Blood First Edition Paper, March 20, 2003; DOI 10.1182/blood-2002-10-3023.
Supported in part by research funding from Sugen.
Several authors (A.L.H., J.M.C., A.-M.O., H.A.Y., S.G.L, and W.H.) are currently employed by Sugen, whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal