Abstract
The present study was designed to investigate the expression of members of the toll-like receptor (TLR) family in human B cells. High-density, resting, and low-density activated tonsillar B cells expressed TLR9 and TLR10 mRNA transcripts at the highest levels. Expression was higher in activated B cells than in resting cells. Analysis of a range of resting and activated human leukocyte populations revealed that mRNA expression of TLR10 was restricted to B cells. Stimulation of resting B cells with anti-μ and anti-CD40 antibodies or with Staphylococcus aureus Cowan I bacteria (SAC) increased expression of TLR9 and TLR10. TLR1 and TLR4 expression were not significantly induced by B-cell activation. Interestingly, a CpG oligonucleotide, a TLR9 agonist, also stimulated TLR9 expression in B cells. Exposure to anti-μ antibodies augmented TLR9 expression, concomitantly and dramatically increasing the responsiveness of B cells to CpG oligonucleotides in terms of proliferation and chemokine (CC chemokine ligand 3 [CCL3] and CCL22) production. Epstein-Barr virus (EBV)–transformed cell lines and other cell lines representative of mature B-cell neoplasias (Burkitt lymphoma, follicular lymphoma, multiple myeloma) expressed TLR9 and/or TLR10, whereas pre-B cell lines were negative. These results show that normal and neoplastic human B lymphocytes express a distinct TLR repertoire including TLR9 and TLR10 and that expression is increased upon engagement of the antigen receptor complex or TLR9 itself. Regulated expression of selected TLRs in B cells is likely to play an important role in linking innate and adaptive immune responses in normal and pathologic conditions.
Introduction
Innate recognition of microbial products is the keystone to the vertebrate immunity and is orchestrated by germ line–encoded pattern-recognition receptors. Detection through these receptors of conserved pathogen-associated molecular patterns foreign to the host triggers a rapid and robust innate response, which directs the emanating adaptive reactions while also providing an immediate antipathogen response.1 The toll receptors are one such example of pattern-recognition receptors whose importance has been shown by their evolutionary conservation between invertebrates and vertebrates.2-5 The mammalian toll-like receptors (TLRs), originally described for their homology with the Drosophila toll,6 are a family of transmembrane receptors containing extracellular leucine-rich regions, which recognize various microbial components engaging a signaling cascade that results in the response versus such microbes. Of the 10 TLRs described to date (TLR1-10), the ligands for 9 of the receptors have been identified. TLR1, TLR2, TLR4, and TLR6 (both as homo- and heterodimers) recognize an array of microbial structures, whereas more specific recognition is mediated by TLR3, a signaling mediator for viral double-stranded RNA, and TLR5, which recognizes flagellin, a protein monomer found in the flagella of gram-negative bacteria.7,8 Synthetic antiviral compounds imiquimod and resiquimod (R-848) are recognized by TLR7 and TLR8.9,10 Unmethylated CpG motifs, characteristic of bacterial DNA, are detected by TLR9 and many recent studies have focused on CpG oligodeoxynucleotides and their role as stimulators of immune cells.11-24 These pathogen-associated molecular patterns (PAMPs) serve as effective signals of bacterial invasion and trigger the ensuing immune response. Engagement of the TLRs by their respective ligands triggers a multifaceted response involving antigen presentation, cell surface expression of costimulatory molecules, and synthesis and release of cytokines, which in turn stimulates specific adaptive responses involving T and B lymphocytes.7
Members of the TLR family are differentially expressed on hematopoietic and nonhematopoietic cells. In general, mononuclear phagocytes and dendritic cells express the widest TLR repertoire.25-28 Moreover, exposure to microbial products and cytokines regulates TLR expression with considerable species-related differences.29-31 Extensive characterization of basal and inducible expression of TLRs in various tissue and isolated cell types is the key to understanding TLR function and presents the opportunity for manipulation of host immune responses.
Several recent studies have suggested a role for TLRs in the stimulatory effects induced by microbial products on human B lymphocytes11,18,22 ; however, the expression of TLRs in B cells has not been systematically investigated. Here we report that normal and malignant B cells show a distinct TLR mRNA expression profile, which includes particularly high levels of TLR9 and TLR10, the latter being expressed specifically in this cell type. Engagement of the B-cell receptor or the costimulatory molecule CD40, as well as stimulation with the microbial products Staphylococcus aureus Cowan I bacteria (SAC) or CpG DNA, augmented the TLR transcript in resting B cells. Augmented TLR9 mRNA expression was associated with increased responsiveness to its agonist, CpG DNA, enhancing proliferation and chemokine production. The regulated expression of selected TLRs in B cells may play an important role in linking innate to adaptive immune responses.
Materials and methods
Cell culture
The cell culture medium routinely used was RPMI 1640 with 2 mM glutamine and 10% fetal calf serum (FCS). B lymphocytes were obtained from freshly excised human tonsils (courtesy of Ospedale Bollate, Milano, Italy) after patients provided informed consent according to the Declaration of Helsinki. The cultures were purified by rosetting with aminoethylisothio-uronium bromide–treated sheep red blood cells as described32 (≥ 95% purity as assessed by indirect immunofluorescence analysis using anti-CD20 monoclonal antibody [mAb]). The total B-cell fraction was further fractionated by discontinuous gradient centrifugation made up of 75%, 57%, 50%, 40%, and 30% Percoll (Pharmacia, Uppsala, Sweden). After centrifugation, the small, dense, resting B cells are found at the 57% to 75% interface while the buoyant fraction obtained at the 40% to 50% interface is composed of activated or germinal center (GC) B cells. The resting B-cell population was incubated at 2 × 109 cells/L with the indicated stimuli. Anti-CD40 antibody, kindly provided by Dr D. Vercelli (San Raffaele Hospital, Milan, Italy) was used at dilution of 1:500. F(ab′)2 fragment of goat antihuman μ-chain (anti-μ) was used at 100 μg/mL. SAC were used at 0.005%.
Circulating human monocytes, polymorphonuclear cells (PMNs), T lymphocytes, and natural killer (NK) cells were separated from blood of healthy donors (> 95% purity was assessed by morphology) as described previously.33 Dendritic cells (DCs) were derived in vitro from monocyte precursors by standard procedures.34 Lipopolysaccharide (LPS; Difco, Detroit, MI) was used at 10 ng/mL.
DNA and ODNs
Specific DNA probes for detection of TLR1-5 by Northern analysis were prepared as described previously.26 The full-length TLR6 cDNA coding region cloned into the expression vector pEF-BOS was kindly provided by S. Akira (Osaka University, Osaka, Japan) and a specific DNA probe was prepared by digestion with SalI. The full-length TLR7 cloned into pCR2.1 (Invitrogen, Carlsbad, CA) and TLR8 and TLR9 cloned into pT-Adv (Clontech, Palo Alto, CA) were generously provided by B. Beutler (Scripps Research Institute, La Jolla, CA). The probes used were 800 bp of TLR7 cut with AccI and NdeI, 800 bp of TLR8 cut with ClaI and XhoI, and 800 bp of TLR9 cut out with HindIII and NdeI. The full-length TLR10 cloned into pDisplay (Invitrogen) was a gift from E. Bates (Schering Plough, Levallois-Perret Cedex, France). The TLR10 probe was prepared by digesting this construct with AccI and BglII, yielding a 1200-bp segment.
Modified CpG oligodeoxynucleotides were purchased from Epoch Biosciences (San Diego, CA): phosphorothioate (PS) 2006-TCG TCG TTT TGT CGT TTT GTCGT and PS 2117-TC*G TC*G TTT TGT C*GT TTT GTC*GT (C* signifies methylated cytidine; underline represents CpG motif). The sequences used are designed with a phosphorothioate backbone, which confers nuclease resistance on the DNA protecting them from rapid degradation within the cells. The sequences were chosen on the basis of the study of Hartmann et al17 in which they showed PS 2006 to be an optimal sequence for stimulation of human B cells. PS 2117 is used as the control ODN (oligodeoxynucleotide) since stimulation by PS 2006 is CpG specific and thus methylation of the cytidines renders the DNA inactive.
Primers used for real-time polymerase chain reaction (PCR) were purchased from Invitrogen. The primers for human actin were used in a previous paper35 (hActin-forward: CCC GGCCAACCGCGAGAAGAT; hActin-reverse: GTCCCGGCCAGCCAGGTCCAG; product size 219 bp). The primers for the TLRs were designed using the Primer Express program from Applied Biosystems (Foster City, CA) (hTLR1-forward: ACACCAAGTTGTCAG CGATGTG; hTLR1-reverse: ACCATGCGTGTACCAGACACTG; hTLR4-forward: AGAGCCGCTGGTGTATCTTTGA; hTLR4-reverse: ACTTCTCCACCTTCTGCAGG AC; hTLR7-forward: CTAAAGACCCAGCTGTGACCAG; hTLR7-reverse: CCAGTCCC TTTCCTCG AGACAT; hTLR9-forward: CCTTCGTGGTCTTCGACAAAAC; hTLR9-reverse: TTGT ACACCCAGTCTGCCACTG; product size 52 bp [human TLR9 cDNA: GenBank accession code AF259262]; hTLR10-forward: GCCCAAGGATAG GCGTAAATG; hTLR10-reverse: ATAGCAGCTCGAAGGTTT GCC; product size 54 bp [human TLR10 cDNA: GenBank accession code AF296673]). The plasmids used as standards for absolute quantification of the real-time PCR data were TLR9 cloned into pTAdV (Clontech) and TLR10 cloned into pDisplay (Invitrogen).
Northern blot analysis
Total RNA was isolated by the guanidine isothiocyanate method with minor modifications.36 Total RNA (10 μg) was analyzed by electrophoresis through 1% agarose/formaldehyde gels followed by Northern blot transfer to Gene Screen Plus membranes (New England Nuclear, Boston, MA). The DNA probes against human β-actin, TLR9, and TLR10 were labeled with α-[32P] deoxycytosine triphosphate (dCTP) (3000 Ci/mmol [111 TBq/mmol]; Amersham, Little Chalfont, Buckinghamshire, United Kingdom). Membranes were pretreated and hybridized in 50% formamide (Merck, Rahway, NJ) with 10% dextran sulfate (Sigma, St Louis, MO), 1% sodium dodecyl sulfate (SDS; Merck), 1 M NaCl, and 100 μg/mL salmon sperm DNA at 42°C; washed twice with 2 × SSC (SSC; 0.15 M NaCl, 0.015 M sodium citrate), and 1% SDS at 60°C for 30 minutes; and finally rinsed in 0.1 × SCC at room temperature. Membranes were exposed to autoradiograph film for 24 to 72 hours at -80°C. Even loading of the membranes was checked by visualizing the ethidium bromide–stained bands under UV light, as shown in each figure.
Real-time PCR
RNA (1 μg) isolated by guanidine isothiocyanate was reverse transcribed using avian myeloblastosis virus (AMV) reverse transcriptase in a final reaction volume of 50 μL (Taqman cDNA synthesis kit; Applied Biosystems). Two microliters obtained cDNA was used per 25-μL reverse transcriptase (RT) reaction. The reaction was carried out using the SYBR Green kit from Applied Biosystems according to the manufacturer's instructions. Each sample was measured in triplicate. A range of primer concentrations was tested to find the combination giving the optimum amplification efficiency. The amplification efficiency of the PCR was determined by running log dilutions of the samples and plotting them against threshold concentration (Ct) values. The slope of the curve is converted to amplification efficiency (E) by the following algorithm: E = 10-1/slope. All used primer sets were used at a concentration where efficiency was 1.8 to 2.3. The copy number of the TLRs was calculated from a standard curve obtained by plotting known concentrations of different plasmids containing the sequence to be amplified at log dilutions against the Ct values. The final calculated copy number values have been standardized with the values for β-actin for the same samples and then expressed as copy number per μg of RNA added to the reaction. Specificity of the amplification products was controlled by melting curve analysis. No amplification of nonspecific products was observed for any of the primer sets used.
[3H]-thymidine uptake assay
Resting B lymphocytes were seeded (1 × 109 cells/L, 250 μL/well) in a flat-bottomed 96-well plate (Falcon, Oxnard, CA) in complete RPMI. Cells were incubated in medium or prestimulated (in triplicate) with anti-μ (10.5 μg/mL) for 12 hours. Cells were then stimulated for a further 48 hours with a range of concentrations of CpG ODN 2006 or with the control ODN 2117. Proliferation was determined by measuring the uptake into the cells of [3H]-thymidine (Amersham) added at 0.5 μCi/well (0.0185 MBq/well). Control cells were incubated in medium alone for the duration of the experiment. Cells were harvested 8 hours later with a Titertek cell harvester (Skatron, Lyerbyen, Norway).
ELISA
Supernatants collected from resting and stimulated B cells were analyzed for the chemokines macrophage inflammatory protein-1α (MIP-1α, CCL3), macrophage-derived chemokine (MDC, CCL22) using enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems (Minneapolis, MN) following the manufacturer's instructions.
Results
The TLR repertoire expressed in B cells
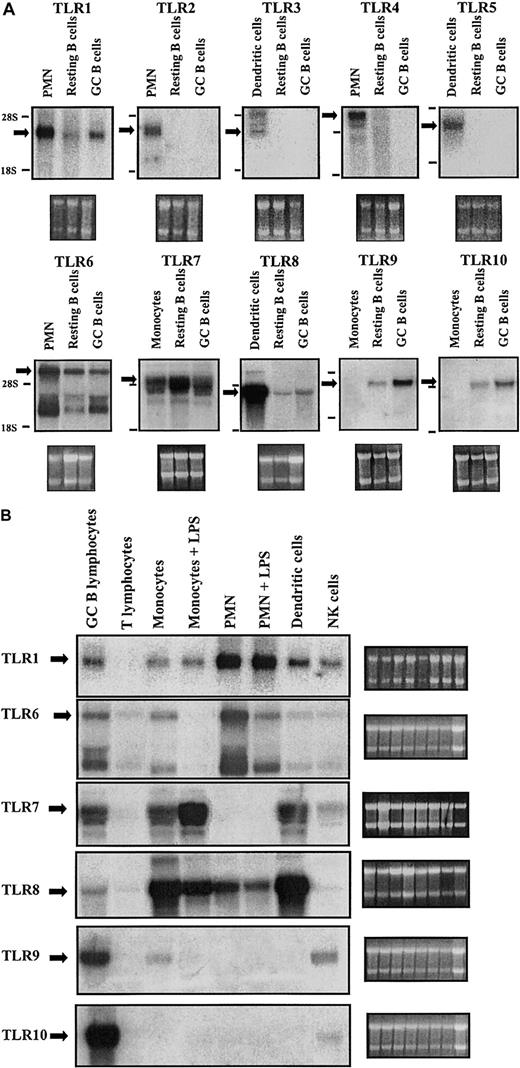
In a first series of experiments, human B cells were screened for expression of the 10 known TLRs. Tonsillar B lymphocytes were purified and then further separated on a discontinuous Percoll gradient to obtain a buoyant population enriched for germinal center cells (thereafter called GC B cells) and a dense population enriched for resting B cells.32,37,38 We then investigated TLR expression in these ex vivo–isolated resting B cells and the GC B cells. Figure 1A shows Northern blot analysis of resting and GC B cells for TLRs 1 through 10. Positive expression controls (extracts from PMNs, monocyte-derived dendritic cells) were included for each of the TLRs from 1 to 8. Monocytes were included as a negative control for TLR9 and TLR10, because of the leukocyte subtypes analyzed (Figure 1B), expression of these TLRs was highest in B cells. These results reveal that human B cells express TLR1 and TLR6 through TLR10.
TLR expression in immunocompetent cells. (A) Resting B cells and GC B cells were purified from total tonsillar B lymphocytes. Total RNA was prepared and subjected to Northern analysis employing radiolabeled probes complementary to TLR1-10. Specific TLR transcripts are indicated by an arrow. The bottom panels show the ribosomal 28S and 18S subunits as seen under UV after staining with ethidium bromide. PMNs, dendritic cells, and monocytes are included as positive controls for TLR1-8 expression. Monocytes are included as a negative control for TLR9 and TLR10, which among the cell types tested, show highest expression in B cells. (B) Fresh human leukocyte subpopulations were separated and cultured in vitro in the absence or presence of the indicated stimuli for 3 hours. Total RNA was prepared and subjected to Northern analysis. Specific TLR transcripts are indicated by an arrow. The side panels show the ribosomal 28s and 18s subunits as seen under UV after staining with ethidium bromide. Results are representative of 3 independent experiments.
TLR expression in immunocompetent cells. (A) Resting B cells and GC B cells were purified from total tonsillar B lymphocytes. Total RNA was prepared and subjected to Northern analysis employing radiolabeled probes complementary to TLR1-10. Specific TLR transcripts are indicated by an arrow. The bottom panels show the ribosomal 28S and 18S subunits as seen under UV after staining with ethidium bromide. PMNs, dendritic cells, and monocytes are included as positive controls for TLR1-8 expression. Monocytes are included as a negative control for TLR9 and TLR10, which among the cell types tested, show highest expression in B cells. (B) Fresh human leukocyte subpopulations were separated and cultured in vitro in the absence or presence of the indicated stimuli for 3 hours. Total RNA was prepared and subjected to Northern analysis. Specific TLR transcripts are indicated by an arrow. The side panels show the ribosomal 28s and 18s subunits as seen under UV after staining with ethidium bromide. Results are representative of 3 independent experiments.
Notable are the multiple mRNA bands always detected while probing for TLR6. This is a phenomenon noted previously in the analysis of TLR639 and may be due to cross-reaction of the probe with TLR1 mRNA (high homology between the nucleotide sequences of TLR1 and TLR6) or, as in murine TLR6, production of multiple mRNAs by alternative splicing.
The subsequent systematic screening of different human leukocyte populations for the expression of these TLR transcripts (Figure 1B) revealed that TLR9 and TLR10 are expressed predominantly in B cells. TLR10 expression is specifically restricted to B cells (with a faint band in NK cells), whereas TLR9 is also expressed in NK cells and to a lesser extent in monocytes. It must be noted that the dendritic cells included in this study are monocyte-derived dendritic cells, which were shown to lack TLR9. Another DC subtype, plasmacytoid DCs, has been shown to express TLR9, thus these cells must also be added to the expression profile of TLR9.40 The predominance of TLR9 and TLR10 in B cells in comparison to other cell types led us to initially concentrate our study on the basal and inducible expressions of these TLR subtypes in B cells.
In Figure 1A, significant basal levels of mRNA expression were observed in resting B cells in the case of both TLR9 and TLR10. In GC B cells, an up-regulation of expression of both genes is apparent. This suggests that activation of B cells in vivo leads to augmented TLR9 and TLR10 gene expression.
TLR gene expression in response to in vitro activation of B cells
Having found that ex vivo–isolated GC B cells express higher TLR9 and TLR10 than resting cells, it was important to ascertain whether signals representative of different polyclonal B-cell activators regulate the TLR expression. Costimulation of the antigen receptor and the CD40 molecule is known to induce a strong mitogenic response in B cells.32,41,42 Using a combination of anti-μ and anti-CD40 antibody, we determined whether this signal was sufficient to up-regulate expression of TLR9 and TLR10. Northern analysis of RNA extracted from B cells following 24-hour and 48-hour stimulation with these mitogens revealed a strong induction of expression of the genes encoding TLR9 and TLR10 (Figure 2A). These results were confirmed by real-time PCR analysis. As shown in Figure 2B, anti-μ or anti-CD40 alone were found to induce an up-regulation of expression of both TLR9 and TLR10, with anti-μ being more potent than anti-CD40 particularly in induction of TLR9. The combination of the 2 mitogens induced TLR9 to levels of expression intermediate to those induced by either mitogen alone. In addition, although costimulation produced significant induction of TLR10 expression, levels were lower than with anti-μ or anti-CD40 alone. In a parallel experiment, costimulation with anti-μ and anti-CD40 was found to induce differentiation in B lymphocytes (results not shown) indicating that progression of B cells through the maturation process results in up-regulation of the genes encoding TLR9 and TLR10.
Regulation of TLR9/10 expression by the mitogens anti-μ and anti-CD40. (A) Purified resting tonsillar B lymphocytes were stimulated in vitro for 24 hours and 48 hours (2 ×109 cells/L) with or without anti-μ (10.5 μg/mL) + CD40 Ab (0.5 μg/mL). Total RNA was prepared from control resting B cells (R), buoyant in vivo–activated B cells (GC), and αμ/CD40-stimulated B cells.24,48 (B) Purified resting tonsillar B lymphocytes were stimulated in vitro for the indicated time periods (2 × 109 cells/L) with anti-μ (10.5 μg/mL), anti-CD40 Ab (0.5 μg/mL), or anti-μ in combination with CD40 Ab (0.5 μg/mL). Total RNA was prepared and cDNA synthesized and analyzed for expression of TLR9 and TLR10 by real-time PCR as outlined in “Materials and methods.” β-Actin expression was used to standardize all results. Results are expressed as copy number × 106/μg RNA added to the cDNA synthesis reaction (mean ± SEM [n = 3] for a representative experiment). These results are representative of 3 independent experiments.
Regulation of TLR9/10 expression by the mitogens anti-μ and anti-CD40. (A) Purified resting tonsillar B lymphocytes were stimulated in vitro for 24 hours and 48 hours (2 ×109 cells/L) with or without anti-μ (10.5 μg/mL) + CD40 Ab (0.5 μg/mL). Total RNA was prepared from control resting B cells (R), buoyant in vivo–activated B cells (GC), and αμ/CD40-stimulated B cells.24,48 (B) Purified resting tonsillar B lymphocytes were stimulated in vitro for the indicated time periods (2 × 109 cells/L) with anti-μ (10.5 μg/mL), anti-CD40 Ab (0.5 μg/mL), or anti-μ in combination with CD40 Ab (0.5 μg/mL). Total RNA was prepared and cDNA synthesized and analyzed for expression of TLR9 and TLR10 by real-time PCR as outlined in “Materials and methods.” β-Actin expression was used to standardize all results. Results are expressed as copy number × 106/μg RNA added to the cDNA synthesis reaction (mean ± SEM [n = 3] for a representative experiment). These results are representative of 3 independent experiments.
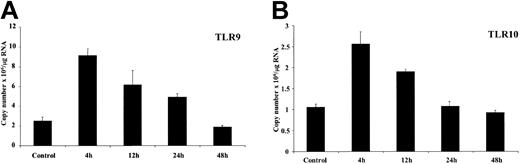
We went on to test the response to SAC, which potently induces proliferation of dense, resting B lymphocytes at least in part by interacting with B-cell surface immunoglobulin (Ig) via protein A.32 Upon activation of resting B cells by SAC we observed a powerful, rapid but transient induction of TLR expression with levels peaking at 4 hours followed by a return to control levels after 48 hours of stimulation (Figure 3). Patient variability was observed with peak expression ranging from 4 to 12 hours; however, the overall pattern of expression was always identical with levels returning to control after 48 hours.
Regulation of TLR9/10 expression by the mitogen SAC. Purified resting tonsillar B lymphocytes were stimulated in vitro for the indicated time periods (2 × 109 cells/L) with SAC (1/20 000). Total RNA was prepared, converted to cDNA, and analyzed for expression of (A) TLR9 and (B) TLR10 by real-time PCR as outlined in “Materials and methods.” β-Actin expression was used to standardize all results. Results are expressed as copy number × 106/μg RNA added to the cDNA synthesis reaction and represent the mean ± SEM (n = 3) for a representative experiment. These results are representative of 3 independent experiments.
Regulation of TLR9/10 expression by the mitogen SAC. Purified resting tonsillar B lymphocytes were stimulated in vitro for the indicated time periods (2 × 109 cells/L) with SAC (1/20 000). Total RNA was prepared, converted to cDNA, and analyzed for expression of (A) TLR9 and (B) TLR10 by real-time PCR as outlined in “Materials and methods.” β-Actin expression was used to standardize all results. Results are expressed as copy number × 106/μg RNA added to the cDNA synthesis reaction and represent the mean ± SEM (n = 3) for a representative experiment. These results are representative of 3 independent experiments.
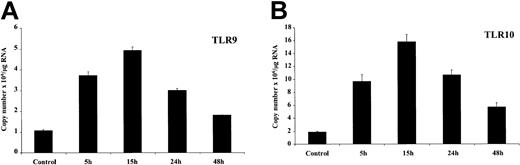
Bacterial DNA acts as a potent activator of B lymphocytes. Oligonucleotides containing unmethylated CpG motifs characteristic of bacterial DNA have been previously shown to induce proliferation and differentiation of B cells.11,18,22 Of particular relevance to this study is the fact that the recognition receptor for bacterial DNA is TLR9.16 We therefore investigated the effect of CpG ODN, a synthetic oligonucleotide containing unmethylated CpG motifs, on expression of its own recognition receptor in B cells (Figure 4). Up-regulation of TLR9 expression was detected after 5 hours of stimulation and peaked after 15 hours of stimulation and remained elevated relative to control even after 48 hours of stimulation. Hence, CpG DNA causes long-term up-regulation of its recognition receptor, thus potentially positively regulating its own effects. TLR10 expression is also augmented by the addition of CpG DNA with a similar pattern but an even more marked induction than TLR9.
Regulation of TLR9/10 expression by CpG DNA. Purified resting tonsillar B lymphocytes were stimulated in vitro for the indicated time periods (2 × 109 cells/L) with CpG (6 μg/mL). Total RNA was prepared, converted to cDNA, and analyzed for expression of (A) TLR9 and (B) TLR10 by real-time PCR as outlined in “Materials and methods.” β-Actin expression was used to standardize all results. Results are expressed as copy number × 106/μg RNA added to the cDNA synthesis reaction and represent the mean ± SEM (n = 3) for a representative experiment. These results are representative of 3 independent experiments.
Regulation of TLR9/10 expression by CpG DNA. Purified resting tonsillar B lymphocytes were stimulated in vitro for the indicated time periods (2 × 109 cells/L) with CpG (6 μg/mL). Total RNA was prepared, converted to cDNA, and analyzed for expression of (A) TLR9 and (B) TLR10 by real-time PCR as outlined in “Materials and methods.” β-Actin expression was used to standardize all results. Results are expressed as copy number × 106/μg RNA added to the cDNA synthesis reaction and represent the mean ± SEM (n = 3) for a representative experiment. These results are representative of 3 independent experiments.
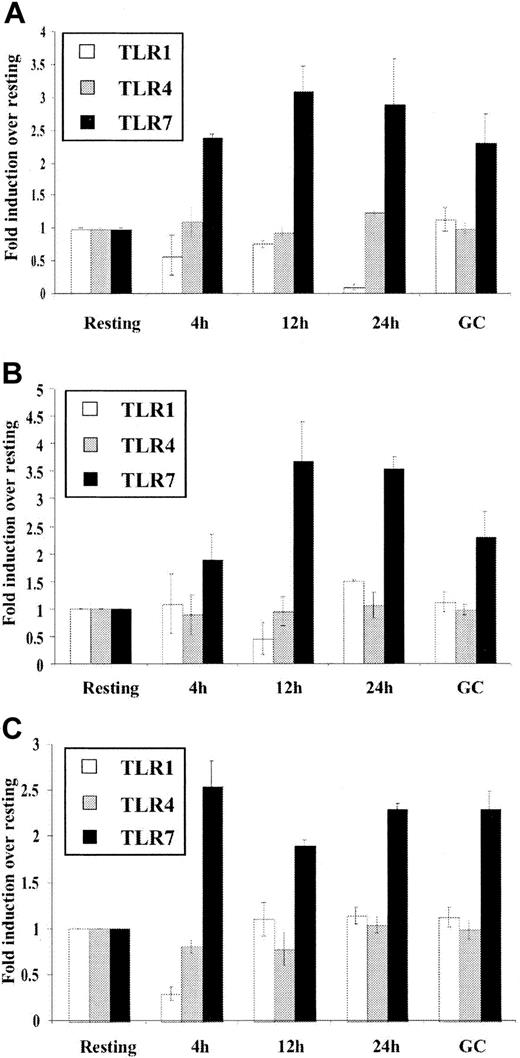
In order to consider the possibility that B-cell activation may simply nonspecifically up-regulate TLR expression, we also examined expression of other important TLRs. Due to their significant expression levels in B cells (Figure 1A), TLR1 and TLR7 were examined along with TLR4, the receptor for bacterial LPS and one of the most extensively characterized toll receptors.8 It should be noted that LPS, a TLR4 agonist, is a potent mitogen for mouse but not for human B cells.43 As shown in Figure 5, TLR1 and TLR4 mRNA expression was not significantly up-regulated upon in vitro stimulation by mitogens or in ex vivo GC or activated B cells. In contrast, TLR7 expression was higher in GC than in resting B cells and was up-regulated by in vitro mitogen stimulation. Taken together, the data thus far indicate that in human B cells the expression of TLR is differentially regulated giving a clue as to which TLR subtypes are central to B-cell function.
Differential regulation of different TLR expression in B cells. Purified resting tonsillar B lymphocytes were stimulated in vitro for the indicated time periods (2 × 109 cells/L) with (A) anti-μ (10.5 μg/mL), (B) CpG (6 μg/mL), and (C) SAC (1/20 000). Total RNA was prepared from control resting B cells (Resting), buoyant in vivo–activated B cells (GC), and in vitro–stimulated B cells (4h, 12h, 24h) converted to cDNA and analyzed for expression of TLR1, TLR4, and TLR7 by real-time PCR as outlined in “Materials and methods.” β-Actin expression was used to standardize all results. Results are expressed as fold induction over levels of RNA in resting cells and represent the mean ± SEM (n = 3) for a representative experiment. These results are representative of 3 independent experiments.
Differential regulation of different TLR expression in B cells. Purified resting tonsillar B lymphocytes were stimulated in vitro for the indicated time periods (2 × 109 cells/L) with (A) anti-μ (10.5 μg/mL), (B) CpG (6 μg/mL), and (C) SAC (1/20 000). Total RNA was prepared from control resting B cells (Resting), buoyant in vivo–activated B cells (GC), and in vitro–stimulated B cells (4h, 12h, 24h) converted to cDNA and analyzed for expression of TLR1, TLR4, and TLR7 by real-time PCR as outlined in “Materials and methods.” β-Actin expression was used to standardize all results. Results are expressed as fold induction over levels of RNA in resting cells and represent the mean ± SEM (n = 3) for a representative experiment. These results are representative of 3 independent experiments.
Augmentation of TLR9 expression by anti-μ is associated with increased responsiveness to CpG oligonucleotides
We chose to focus on TLR9 function since expression profile analysis indicates its importance in B-cell function and due to the availability of the potent agonist, CpG ODN 2006. In order to demonstrate the functional consequence of up-regulation of the TLR9 receptor, we investigated the effects of CpG ODN 2006 before and after induction of TLR9 expression. CpG DNA is well established as a proliferative agent for B cells,11 therefore as one functional output we determined the proliferative response to CpG by measuring [3H]-thymidine uptake into the cells. As shown in Figure 6A, we prestimulated resting lymphocytes with anti-μ to allow induction of the gene for TLR9. The cells were then stimulated with a range of concentrations of CpG 2006 for 48 hours. Thymidine uptake was low in lymphocytes incubated in medium alone and only moderately elevated in the presence of anti-μ alone (results not shown). In cells preincubated with medium alone and then exposed to CpG 2006, there was a concentration-dependent increase in uptake until 0.6 μg/mL after which levels decreased slightly with increasing concentration. The effects of ODN 2006 were CpG specific since the control ODN, an oligo identical in sequence to 2006 but with methylated cytidines (2117) did not induce proliferative effects in resting B cells (thymidine uptake × 103 counts per minute [cpm] 0.245 ± 0.036). Notable though is the 4-fold increase in response to CpG after prestimulation with anti-μ. These results were substantiated by a similar increase in responsiveness to CpG apparent in cytokine production (Figure 6B). Prestimulation of cells with either anti-μ (or anti-CD40) antibody causes a 3- to 4-fold increase in the levels of CCL3 and a 2- to 3-fold increase in the levels of CCL22 secreted by resting B cells upon stimulation with CpG. Overall, the above results suggest that the increased expression of TLR9 in these cells results in increased responsiveness to CpG DNA, which has implications for the role of TLR9 in differentiating and mature B lymphocytes. Furthermore, the observed TLR9-mediated production of chemokines by B cells provides a key link between B cells and mechanisms of innate immunity.
Augmented TLR9 expression correlates with increased responsiveness to CpG DNA. (A) Resting B lymphocytes were seeded in a 96-well plate (1 × 109 cells/L) in complete RPMI. Cells were incubated in medium or prestimulated with anti-μ (10.5 μg/mL) for 12 hours. Cells were then stimulated for a further 48 hours with the indicated concentrations of CpG DNA (2006). Proliferation was determined by measuring the uptake of [3H]-thymidine (0.5 μCi/well [0.0185 MBq/well]) into the cells. Control cells were incubated in medium alone for the duration of the experiment. Results are expressed as cpm × 103 ± SEM and are representative of 3 independent experiments. (B-C) Resting B lymphocytes were seeded in a 24-well plate (1 × 109 cells/L) in complete RPMI. Cells were incubated in medium or prestimulated with anti-μ (10.5 μg/mL) or anti-CD40 (0.5 μg/mL) for 12 hours. Cells were then stimulated for a further 24 hours with CpG DNA (2006; 0.6 μg/mL). Supernatants were removed from cells and analyzed for CCL3 (B) and CCL22 (C) production by ELISA. Results are expressed in pg/mL (mean ± SEM) and are representative of 2 independent experiments.
Augmented TLR9 expression correlates with increased responsiveness to CpG DNA. (A) Resting B lymphocytes were seeded in a 96-well plate (1 × 109 cells/L) in complete RPMI. Cells were incubated in medium or prestimulated with anti-μ (10.5 μg/mL) for 12 hours. Cells were then stimulated for a further 48 hours with the indicated concentrations of CpG DNA (2006). Proliferation was determined by measuring the uptake of [3H]-thymidine (0.5 μCi/well [0.0185 MBq/well]) into the cells. Control cells were incubated in medium alone for the duration of the experiment. Results are expressed as cpm × 103 ± SEM and are representative of 3 independent experiments. (B-C) Resting B lymphocytes were seeded in a 24-well plate (1 × 109 cells/L) in complete RPMI. Cells were incubated in medium or prestimulated with anti-μ (10.5 μg/mL) or anti-CD40 (0.5 μg/mL) for 12 hours. Cells were then stimulated for a further 24 hours with CpG DNA (2006; 0.6 μg/mL). Supernatants were removed from cells and analyzed for CCL3 (B) and CCL22 (C) production by ELISA. Results are expressed in pg/mL (mean ± SEM) and are representative of 2 independent experiments.
TLR9 and TLR10 mRNA expression in transformed B lymphocytes
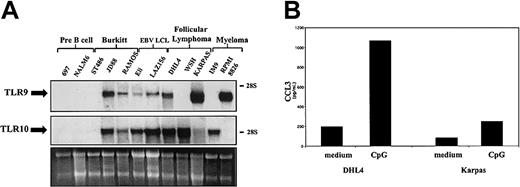
Neoplastic B cells are to a large extent thought to represent normal B cells blocked at various stages of differentiation. As such, they can be used as a model of their normal counterparts. In order to further characterize the pattern of expression of TLR9 and TLR10 during B-cell differentiation, we have tested a panel of cell lines derived from different leukemia/lymphoma cell types. TLR expression was examined in a range of pre-B cell, Burkitt lymphoma (BL), EBV-lymphoblastoid cell line (EBV-LCL), follicular lymphoma (FL), and myeloma cell lines (Figure 6). In all EBV-transformed B-cell lines studied, high levels of TLR9 and TLR10 transcripts were detected. Cell lines established from B-cell neoplasias (Burkitt lymphoma, follicular lymphoma, myeloma), which represent the neoplastic equivalents of activated B cells located mainly in the germinal center, express TLR9 and/or TLR10, the sole exception being the Burkitt cell line ST486, which expressed neither receptor. Conversely, in both pre-B cell lines studied TLR9 and TLR10 were undetectable. These results suggest that TLR9 and 10 expression is induced as B lymphocytes progress through the maturation process from pre-B cells to mature B cells.
To demonstrate the functionality of TLR9 mRNA expression in neoplastic B cells, we again measured chemokine production upon stimulation with CpG. As seen in Figure 7B, the production of the chemokine CCL3 is induced in both DHL-4 and Karpas lymphoma cell lines by CpG 2006. Thus, in lymphoma-derived cell lines TLR9 is not only highly expressed but displays functional characteristics also observed in normal cells. In an initial study on a short-term cultured Burkitt lymphoma sample, high expression of TLR9 was observed as in established lines, whereas transcript levels were low in resting B-cell chronic lymphocytic leukemia (9 cases).
Expression and function of TLRs in transformed B cells. (A) Total RNA was isolated from transformed cell lines representing B cells at various stages of differentiation. RNA (7 μg) was subjected to Northern analysis employing a radiolabeled probe complementary to TLR9 (top) and TLR10 (middle). Ribosomal RNA was used as the loading control (bottom). The electrophoretic mobility of the 18S and 28S subunits of ribosomal RNA is indicated. These results are representative of 2 independent experiments. (B) DHL-4 and Karpas cell lines were seeded in a 24-well plate (0.5 × 109 cells/L) in complete RPMI. Cells were stimulated for 24 hours with CpG DNA (2006) (0.6 μg/mL). Supernatants were removed from cells and analyzed for chemokine production by ELISA. Results are expressed in pg/mL (mean ± SEM) and are representative of 2 independent experiments.
Expression and function of TLRs in transformed B cells. (A) Total RNA was isolated from transformed cell lines representing B cells at various stages of differentiation. RNA (7 μg) was subjected to Northern analysis employing a radiolabeled probe complementary to TLR9 (top) and TLR10 (middle). Ribosomal RNA was used as the loading control (bottom). The electrophoretic mobility of the 18S and 28S subunits of ribosomal RNA is indicated. These results are representative of 2 independent experiments. (B) DHL-4 and Karpas cell lines were seeded in a 24-well plate (0.5 × 109 cells/L) in complete RPMI. Cells were stimulated for 24 hours with CpG DNA (2006) (0.6 μg/mL). Supernatants were removed from cells and analyzed for chemokine production by ELISA. Results are expressed in pg/mL (mean ± SEM) and are representative of 2 independent experiments.
Discussion
The results presented demonstrate that human B lymphocytes express a distinct TLR expression profile in which TLR9 and TLR10 predominate. We found that resting human B lymphocytes express significant basal levels of TLR1 and TLR6-10. Expression of TLR7, TLR9, and TLR10 was dramatically up-regulated by activation of the B cells in vivo as indicated by high expression of these receptors in the buoyant or germinal center population of B lymphocytes freshly isolated from human tonsillar tissue. Expression of the other TLR subtypes was unaffected by B-cell activation. Interestingly, among the leukocyte populations examined in the present study, expression of TLR10 was restricted to normal and malignant mature B cells, thus identification of the ligand for this receptor presents the potential to selectively target B cells during immunotherapy. During processing of this article, a study was published showing polyclonal activation of memory B cells by CpG DNA and suggesting this as a mechanism of maintenance of serologic memory.44 Moreover, more recently Bernasconi et al45 found that TLR9 and TLR10 are induced by triggering of the B-cell receptor and that memory B cells express high levels of these receptors. Proliferation and differentiation to Ig production paralleled TLR9 expression consistently with data reported here.
The present study focused mainly on TLR9 due to its high expression in B cells and the well-characterized mitogenic effects of its ligand on B cells. TLR9 recognizes unmethylated CpG motifs characteristic of bacterial DNA,16,19,21 and this receptor is therefore involved in the immediate innate responses to a diverse range of invading microbes. By expressing and producing inflammatory chemokines in response to TLR9 agonists, in addition to their well-established role in adaptive immunity, B cells may also participate in the mechanisms of innate resistance.
Expression of components of TLR receptor complexes can be regulated by immunologic or microbial agents.25,26,29,30,46 For instance, it has recently been shown that interferon γ (IFNγ) augments expression of TLR4, myeloid differentiation factor 88 (MyD88), and interleukin-1 receptor–associated kinase (IRAK) in human monocytes, whereas interleukin-10 (IL-10) has an inhibitory effect.26,29,30 Here we report that engagement of the B-cell antigen–receptor complex, as well as activation by the polyclonal B-cell activators SAC and CpG DNA, increase the expression of TLR7, TLR9, and TLR10. Thus, both in vivo and in vitro activation of B cells up-regulate expression of these receptors. Interestingly, anti–μ-induced augmented expression of TLR9-primed resting B cells results in an increased response to CpG. Therefore, up-regulation of TLR9 mRNA expression is functional resulting in increased responsiveness to the TLR9 ligand in vitro. This has implications for the potential role of such a system in vivo. Induction of TLR9 in mouse dendritic cells by LPS results in a similar augmentation of CpG ODN responsiveness.46 There is, however, evidence that regulation of TLR expression can differ considerably among animal species.31 Therefore, it will be important to investigate whether regulation of TLR9 in murine B cells is similar to that reported here for human cells.
Our observation that the synthetic oligodeoxynucleotide ODN 2006 induces up-regulation of expression of its own recognition receptor TLR9 is intriguing because it denotes a positive feedback loop in which CpG can potentiate its own effects. This is, however, contradictory to the results obtained in a recent study by Hornung et al28 in which CpG DNA was shown to down-regulate the RNA expression of TLR9. Augmentation of TLR9 expression by CpG was also observed in a recent study45 ; the effect of CpG 2006 was lower than that of anti-Ig and variable between donors.45 The lower levels of induction and donor variability may explain the previous lack of stimulation reported by Hornung et al.28
TLR9 and/or TLR10 were found to be highly expressed in EBV-transformed B-cell lines and in cell lines established from Burkitt lymphoma, follicular lymphoma, and multiple myeloma. In contrast, 2 pre-B cell lines were negative for TLR9 and TLR10, consistent with the hypothesis that expression of TLR9 and TLR10 parallels B-cell differentiation and maturation. In addition, the neoplastic cells tested were found to be fully responsive to the ligand for TLR9, demonstrating the functionality of the high levels of receptor found on these cells. Direct growth stimulation via TLR may provide a link between chronic infection and pathogenesis of lymphomas, for instance in mucosal tissues. Moreover, TLR-induced chemokine production may amplify inflammation and, indirectly, promote tumor growth.47-49
TLR expression profile analysis revealed TLR9 and TLR10 to be the main TLRs expressed on human B lymphocytes. Expression of both receptors is up-regulated as B cells progress from resting to activated mature B cells. Increased TLR9 expression is associated with increased responsiveness to the ligand. Thus, in addition to their role as antibody-producing cells during the adaptive immune response, B cells may also respond to pathogens in a nonspecific manner associated with the innate branch of the response. This is corroborated by our finding that B cells can be induced to produce chemokines, molecules central to the innate immune response, through stimulation of TLR9. Furthermore, B cells can also present antigen50 and express antimicrobial activity by producing reactive oxygen intermediates and other inflammatory cytokines.51,52 Inducible expression of TLRs in B lymphocytes may provide a link between the innate and adaptive branches of the immune system giving a clue as to how both systems cooperate in order to eliminate invading pathogenic microorganisms.
Prepublished online as Blood First Edition Paper, April 10, 2003; DOI 10.1182/blood-2002-11-3355.
Supported by a Marie Curie research training grant from the European Community (E.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 2. Regulation of TLR9/10 expression by the mitogens anti-μ and anti-CD40. (A) Purified resting tonsillar B lymphocytes were stimulated in vitro for 24 hours and 48 hours (2 ×109 cells/L) with or without anti-μ (10.5 μg/mL) + CD40 Ab (0.5 μg/mL). Total RNA was prepared from control resting B cells (R), buoyant in vivo–activated B cells (GC), and αμ/CD40-stimulated B cells.24,48 (B) Purified resting tonsillar B lymphocytes were stimulated in vitro for the indicated time periods (2 × 109 cells/L) with anti-μ (10.5 μg/mL), anti-CD40 Ab (0.5 μg/mL), or anti-μ in combination with CD40 Ab (0.5 μg/mL). Total RNA was prepared and cDNA synthesized and analyzed for expression of TLR9 and TLR10 by real-time PCR as outlined in “Materials and methods.” β-Actin expression was used to standardize all results. Results are expressed as copy number × 106/μg RNA added to the cDNA synthesis reaction (mean ± SEM [n = 3] for a representative experiment). These results are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/3/10.1182_blood-2002-11-3355/6/m_h81534699002.jpeg?Expires=1766050085&Signature=KD4hRS1PhR-GS10Geu7m34seDL4zGr96ay5jKzXfmqH~GpyIXbMmoHOGeE9VFop01WgAcco1I6zuL3bMsashCAFmU~1qckBHMN8pEpyX5xIbtiywOlVgHfle4IeXRxh34UmvLy2ajXm5dclZN1fC5LAfL3p5bkrf6XqV3OGymyfLRXRa9tKyBfcAYHagB4rP63B-h-gw7zyIXRaM4Rncn3h1xaRmaGwLqfppJsHt~f6TG59TafqxYz9bV7JllKKVETg4zDa1oqUY-aYSKLcQFn5Zjgl-5UoBHZRRTB5uCEZH3bd01o-~Taz90txyF9xQPXvEii8zu57jMZ2wf6FbiA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Augmented TLR9 expression correlates with increased responsiveness to CpG DNA. (A) Resting B lymphocytes were seeded in a 96-well plate (1 × 109 cells/L) in complete RPMI. Cells were incubated in medium or prestimulated with anti-μ (10.5 μg/mL) for 12 hours. Cells were then stimulated for a further 48 hours with the indicated concentrations of CpG DNA (2006). Proliferation was determined by measuring the uptake of [3H]-thymidine (0.5 μCi/well [0.0185 MBq/well]) into the cells. Control cells were incubated in medium alone for the duration of the experiment. Results are expressed as cpm × 103 ± SEM and are representative of 3 independent experiments. (B-C) Resting B lymphocytes were seeded in a 24-well plate (1 × 109 cells/L) in complete RPMI. Cells were incubated in medium or prestimulated with anti-μ (10.5 μg/mL) or anti-CD40 (0.5 μg/mL) for 12 hours. Cells were then stimulated for a further 24 hours with CpG DNA (2006; 0.6 μg/mL). Supernatants were removed from cells and analyzed for CCL3 (B) and CCL22 (C) production by ELISA. Results are expressed in pg/mL (mean ± SEM) and are representative of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/3/10.1182_blood-2002-11-3355/6/m_h81534699006.jpeg?Expires=1766050086&Signature=mnub~q62rSCN-sXEVtCD~VHF7qvML1yqEXxzsoFnaOXnLwgVCQUGBeX4RXgtHgVEpU6HiOTvuME8K36IT7XcrKz-VjwX8rYPqRer9m~xtTRQqLeuSK~CCwYvWjHvXeK3Ni9OcY3~PiamahxhQitYC-p4JVdFpuNhWzeWxjuAPAiw6qFzPAFxeO2rt70-VIMJ9nVVp3ihgfDMLmhf-PDH6gC0JYBiWeN-pOHaG1VR5XcZb0oHwv4c6u~l7MeMCKIGppHDDT-t6NHBG1F2Fti~xP2M7Vh-mL3yDqoo4~27bwHc7xH~pXskZQ~LloblF0fAzfjTJRufloZXbhlxK7fUbw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal