Abstract
The α2β1 integrin is a major collagen receptor on platelets. Although it has been proposed that α2β1, like αIIbβ3, undergoes agonist-induced activation, neither the potential contributions of α2β1 receptor/ligand internalization to the increase in ligand binding nor the roles of the α2 and β1 cytoplasmic domains in activation of this integrin have been previously explored. Activation of α2β1 was assessed with fluorescein isothiocyanate–labeled soluble type I collagen binding to platelets by flow cytometry. Although collagen internalization in response to agonist activation of platelets was significant, agonist-induced collagen binding still occurred under conditions that block internalization, with minimal changes in cell surface α2β1 expression. Introduction of cell-permeable peptides containing the α2 cytoplasmic tail, and especially the membrane proximal KLGFFKR domain, induced α2β1 activation in resting platelets, whereas a cell-permeable peptide containing the β1 cytoplasmic tail was without effect. Thus, collagen binding to stimulated platelets is increased due to α2β1 activation, in addition to internalization, and the GFFKR motif appears to play an important role in the activation process.
Introduction
Collagen is the major matrix protein of the blood vessel wall, and its exposure in ruptured atherosclerotic plaques activates platelets via the platelet collagen receptors, α2β1 and glycoprotein (GP) VI, thereby contributing to thrombosis.1 The importance of the α2β1 integrin in thrombosis was initially recognized from studies of patients who lack α2β1 expression or who have antibodies against this receptor, because these patients have a mild bleeding disorder and their platelets exhibit defective aggregation in response to collagen.2 In recent studies it was determined that an α2 gene polymorphism is associated with several cardiovascular disorders as reviewed by Kunicki,3 including myocardial infarction,4 vascular mortality in women,5 and stroke in younger patients.6
Although numerous studies have focused on the role of α2β1 in the process of collagen-stimulated platelet activation, only recently has any attention been given to the platelet-mediated activation of α2β1. Intracellular signals provoke changes in the integrin extracellular domain, resulting in integrin activation, as manifested by an increase in affinity of the integrin for its ligands, an important control mechanism for integrin function.7 This regulation is potentially critical for both normal hemostasis and thrombosis. For example, Wen et al8 showed that gangliosides from atherosclerotic plaques enhance platelet adhesion to immobilized soluble collagen via the α2β1 integrin. In addition, recent studies by Jung and Moroi and Moroi et al have provided evidence for conversion of α2β1 to an active conformation on agonist-stimulated platelets, as defined by the ability of these platelets to bind increased amounts of 125I-labeled soluble type III collagen.9-12
However, many questions remain concerning the process and mechanisms of activation of α2β1. For example, αIIbβ3 on activated platelets13 and α2β1 on other cells are known to internalize ligands, and this process may increase the apparent extent of collagen binding to stimulated platelets, independent of significant α2β1 activation per se. Thus, the relative contribution of internalization versus activation of α2β1 to the binding of soluble collagen to activated platelets is unknown. Also unknown are the roles of the α2 and β1 cytoplasmic domains in the process of α2β1 activation on platelets. Studies of the αIIbβ3 integrin have shown that the cytoplasmic domains of each subunit play important roles in the activation process, particularly, the GFFKR motif, which is highly conserved in all known integrin α subunits.
Here we provide evidence that agonist stimulation of platelets activates the collagen-binding capacity of the α2β1 integrin and induces significant collagen internalization. These events occur with minimal changes in α2β1 expression on the cell surface. Furthermore, we provide evidence that the α2 cytoplasmic tail, especially the KLGFFKR domain, plays an important role in α2β1 activation. Therefore, these data further clarify properties of the activation and regulation of α2β1 on platelets.
Materials and methods
Materials
Soluble fluorescein isothiocyanate (FITC)–labeled bovine type I collagen (1 mg/mL in 0.01 M acetic acid, which lacks the telopeptides that are necessary for cross-linking) and unlabled bovine type I collagen (4 mg/mL in 0.01 M acetic acid, also lacking telopeptides) were purchased from Chondrex (Redmond, WA). Mouse anti-human α2β1 integrin monoclonal antibody (mAb; clone BHA 2.1) was from Chemicon International (Temecula, CA). Hamster antimouse α2 mAb, control IgGκ and R-phycoerythrin–conjugated mouse antihamster IgG were obtained from Pharmingen International (San Diego, CA). Bovine serum albumin (BSA; Pentex fraction V) was from Serologicals Proteins (Kankakee, IL). Adenosine 5′-diphosphate (ADP), heparin, apyrase, prostaglandin I2 (PGI2), and indomethacin were purchased from Sigma (St Louis, MO). Trypan blue was purchased from EM Diagnostic Systems (Gibbstown, NJ). Convulxin was a kind gift from Dr Thomas J. Kunicki (Scripps Research Institute, La Jolla, CA). Thrombin receptor activation peptide (TRAP) and cytoplasmic domain peptides were synthesized by solid-phase techniques, purified by high-performance liquid chromatography, and analyzed by the University of North Carolina-Chapel Hill Protein Chemistry Laboratory.
Peptide design
Cell-permeable chimeric peptides were designed by placing a hydrophobic delivery sequence N-terminal to the human α2 or β1 integrin cytoplasmic tail sequence (Table 1). The hydrophobic delivery sequence (VTVLALGALAGVGVG), is derived from the signal peptide sequence of the human β3 integrin.14 Peptides without the cell signal sequence or peptides with a scrambled cytoplasmic tail sequence were designed as negative controls. Mutant α2 cytoplasmic tail peptides with either a deletion or truncation after the GFFKR motif were also synthesized. Peptides synthesized and purified as in the previous paragraph were further purified by exchange of trifluoroacetic acid for HCl. Peptide amino acid content was confirmed by amino acid analysis.
Sequences of cell-permeable α2 or β1 cytoplasmic tail peptides or control peptides
. | Signal sequence . | Cytoplasmic tail sequence . |
|---|---|---|
| Signal-α2 cytoplasmic tail | VTVLALGALAGVGVG | KLGFFKRKYEKMTKNPDEIDETTELSS |
| α2 cytoplasmic tail | KLGFFKRKYEKMTKNPDEIDETTELSS | |
| Signal-α2 without GFFKR | VTVLALGALAGVGVG | KL_____KYEKMTKNPDEIDETTELSS |
| Signal-KLGFFKR | VTVLALGALAGVGVG | KLGFFKR |
| Signal-scrambled α2 tail | VTVLALGALAGVGVG | TKSKYNEGSELIKTLKMTDFFREDKEP |
| Signal-β1 cytoplasmic tail | VTVLALGALAGVGVG | NPIYKSAVTTVVNPKYEGK |
| β1 cytoplasmic tail | NPIYKSAVTTVVNPKYEGK |
. | Signal sequence . | Cytoplasmic tail sequence . |
|---|---|---|
| Signal-α2 cytoplasmic tail | VTVLALGALAGVGVG | KLGFFKRKYEKMTKNPDEIDETTELSS |
| α2 cytoplasmic tail | KLGFFKRKYEKMTKNPDEIDETTELSS | |
| Signal-α2 without GFFKR | VTVLALGALAGVGVG | KL_____KYEKMTKNPDEIDETTELSS |
| Signal-KLGFFKR | VTVLALGALAGVGVG | KLGFFKR |
| Signal-scrambled α2 tail | VTVLALGALAGVGVG | TKSKYNEGSELIKTLKMTDFFREDKEP |
| Signal-β1 cytoplasmic tail | VTVLALGALAGVGVG | NPIYKSAVTTVVNPKYEGK |
| β1 cytoplasmic tail | NPIYKSAVTTVVNPKYEGK |
Sequences are given in the single-letter amino acid code. The cell membrane—permeable signal sequence (italicized) was derived from the hydrophobic domain of the human β3 integrin signal sequence.24 Peptides were synthesized and purified as described in “Materials and methods.” Underscore indicates deleted amino acids.
Platelet preparation
Approval for this study was obtained from the University of North Carolina-Chapel Hill institutional review board, with informed consent provided according to the Declaration of Helsinki. Whole blood was drawn from healthy volunteers by venipuncture and collected into 0.14 volume of acid-citrate-dextrose anticoagulant. Platelet-rich plasma (PRP) was isolated by centrifugation using a Beckman GP tabletop centrifuge at 200g for 20 minutes at room temperature. PRP was treated with PGI2 (5 ng/mL) and centrifuged at 800g for 10 minutes. Platelet pellets were resuspended in modified Tyrode buffer (5 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 6.5, 135 mM NaCl, 2.7 mM KCl, 11.9 mM NaHCO3, 0.42 mM NaH2PO4, 1 mM MgCl2, 1 mg/mL dextrose, 0.1% BSA) and incubated at 37°C with 50 U/mL heparin and 1 U/mL apyrase for 20 minutes and supplemented with 5 ng/mL PGI2 immediately prior to centrifugation at 800g for 10 minutes. In most experiments the resulting pellet was resuspended in Tyrode buffer, pH 6.5, containing 0.5 U/mL apyrase and incubated for 15 minutes at 37°C. PGI2 (5 ng/mL) was added prior to centrifugation (800g, 10 minutes). Washed platelet pellets were resuspended at 2 × 108/mL in pH 7.4 Tyrode buffer containing 1% BSA and 2 mM MgCl2, and incubated at 37°C for 30 to 60 minutes before use in experiments. The platelet concentration was determined with a Thrombo-counter (Coulter, Hialeah, FL) or Z1 Coulter particle counter (Coulter, Miami, FL). All experiments were performed with human platelets except where the use of mouse platelets is indicated. Murine PRP was isolated as described.15 Briefly, murine blood was obtained by cardiac puncture and collected into a 0.1 volume of 3.8% sodium citrate. Murine PRP was isolated by centrifugation at 90g for 10 minutes at room temperature.
Peptide labeling and confocal microscopy
Peptides with or without the signal delivery sequence were labeled by FITC isomer I on Celite 10% (Calbiochem, San Diego, CA) at a 1:2 molar ratio in 100 mM NaHCO3 buffer (pH 9.0) for 30 minutes at room temperature. Excessive FITC dye was removed by dialysis overnight in NaHCO3 buffer followed by phosphate-buffered saline (PBS) for 8 hours at 4°C. Platelets were incubated at room temperature for 20 minutes with 120 μM FITC-labeled peptide at a final concentration of 1 × 108 platelets/mL. The platelet suspension was applied to coverslips that were preincubated with poly l-lysine (Sigma). Adhesion was conducted in the dark at room temperature for 20 minutes and coverslips were washed once with HEPES-PBS (pH 7.4). Platelets were fixed with 1% paraformaldehyde (PFA) for 15 minutes at room temperature then washed with NH4Cl2, followed by ice-cold 1% BSA in PBS. Coverslips were mounted on slides with FluorSave Reagent (Calbiochem) and the margins sealed with nail polish. Confocal images were obtained on an Olympus IX 70 confocal microscope.
Binding of soluble collagen to live platelets
Equal volumes of platelet suspensions were usually added last at a final concentration of 1 × 108/mL to polypropylene tubes containing 25 to 50 μL soluble FITC-labeled collagen (25-50 μg/mL final concentration) and various agonists in Tyrode buffer (pH 7.4, containing 2 mM MgCl2 and 1% BSA). Incubations were performed at room temperature for 30 minutes and stopped by adding a 10 × volume of ice-cold PBS. Tubes were kept on ice in the dark and were evaluated by flow cytometry within 2 hours.
Peptide incubation and collagen binding to fixed platelets
Peptides were dissolved in PBS and diluted with Tyrode buffer (pH 7.4). Platelets (1 × 108/mL) were incubated with 200 μM peptide at room temperature for 30 minutes and treated with agonist or control buffer for 2 to 5 minutes before fixation with 0.7% PFA (1 hour at room temperature). Fixed platelets were washed once with PBS, centrifuged, and the platelet pellet was resuspended in Tyrode buffer (pH 7.4, containing 1% BSA, 2 mM MgCl2), and incubated with FITC-labeled collagen (10-25 μg/mL) for 30 minutes at room temperature. The reaction was stopped as described for analysis by flow cytometry. Most experiments were performed with triplicate or duplicate samples.
Flow cytometry and data analysis
Cells were individually analyzed on a FACStar Plus flow cytometer (Becton Dickinson, San Jose, CA). Association of FITC-collagen or FITC-labeled peptides with platelets was monitored in the FL1 channel on the gated population of platelets from forward-scatter, side-scatter histograms. Data were collected as fluorescence mean values or as percentages of the positive population. The mean value of the fluorescence intensity of basal binding (ie, FITC-collagen binding to resting platelets) as measured by flow cytometry was normalized to 100 arbitrary units. All mean values of other data points within each experiment were normalized to arbitrary units relative to basal binding. Therefore, basal binding from all experiments is equal to 100. The Student t test was used in statistical analyses.
Results
FITC-collagen association with live, agonist-stimulated platelets is dependent on α2β1 integrin and divalent cation
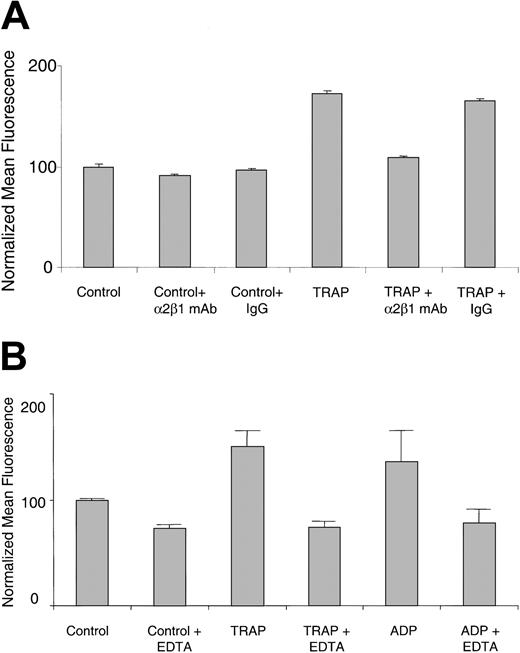
It was recently reported that collagen binding to the α2β1 integrin on platelets increases on agonist stimulation, as detected by 125I-labeled soluble type III collagen.9,16 Using FITC-labeled soluble type I collagen and flow cytometry, we obtained similar results with live platelets. Washed human platelets at room temperature bound significantly more FITC-collagen on TRAP stimulation compared with resting platelets (Figure 1A). Increased binding of FITC-collagen was also observed with other agonists, including ADP (Figure 1B), thrombin, and the GPVI-specific agonist, convulxin (data not shown). The increased binding was inhibited by 10 μg/mL α2β1 blocking mAb, but not by a control IgG (Figure 1A), and by 5 mM EDTA (ethylenediaminetetraacetic acid; Figure 1B). These results indicate that the agonist-stimulated FITC-collagen binding is dependent on both α2β1 integrin and divalent cation. Similar results were also observed with TRAP- or ADP-stimulated human or mouse PRP (data not shown).
FITC-collagen binding is dependent on α2β1 integrin and divalent cation in agonist-stimulated live human platelets. (A) Human platelets were preincubated with an α2β1 function-blocking mAb (BHA 2.1) or an isotype-matched control IgG (10 μg/mL final) as indicated, for 30 minutes. Platelets were simultaneously exposed to FITC-collagen and TRAP (20 μM) or control buffer. Data shown are the normalized mean fluorescence ± SEM from one experiment, representative at least 5 similar experiments. P < .0001 for TRAP compared with control and for α2β1 mAb BHA 2.1 compared with control IgG. (B) Human platelets resuspended in EDTA (5 mM) or control buffer were exposed simultaneously to FITC-collagen and TRAP (50 μM) or ADP (50 μM). Data shown are normalized mean fluorescence ± SEM of one experiment, representative of 3 similar experiments. P < .05 for TRAP plus EDTA compared with TRAP alone.
FITC-collagen binding is dependent on α2β1 integrin and divalent cation in agonist-stimulated live human platelets. (A) Human platelets were preincubated with an α2β1 function-blocking mAb (BHA 2.1) or an isotype-matched control IgG (10 μg/mL final) as indicated, for 30 minutes. Platelets were simultaneously exposed to FITC-collagen and TRAP (20 μM) or control buffer. Data shown are the normalized mean fluorescence ± SEM from one experiment, representative at least 5 similar experiments. P < .0001 for TRAP compared with control and for α2β1 mAb BHA 2.1 compared with control IgG. (B) Human platelets resuspended in EDTA (5 mM) or control buffer were exposed simultaneously to FITC-collagen and TRAP (50 μM) or ADP (50 μM). Data shown are normalized mean fluorescence ± SEM of one experiment, representative of 3 similar experiments. P < .05 for TRAP plus EDTA compared with TRAP alone.
Contribution of collagen internalization to the apparent FITC-collagen binding to platelets
Integrins play a role in fibrinogen internalization in platelets,13 collagen internalization in fibroblasts,17,18 and phagocytosis in macrophages.19 We therefore asked whether the increased collagen binding was caused by an activation of α2β1, an increase in collagen internalization, or both. Trypan blue, which interacts with FITC dye and quenches its fluorescence emission, was used to distinguish internalized from surface-bound FITC-collagen. Trypan blue is expected to quench only surface-bound FITC-collagen molecules on live platelets because it is actively excluded from live cells.20-23 However, trypan blue will quench intracellular fluorescence in dead cells. In live, non-TRAP–activated, control platelets incubated with FITC-collagen, trypan blue reduced the FITC fluorescence by about 15%, indicating that a portion of collagen was on the surface but that the rest was internalized (Figure 2). Upon TRAP activation, a more than 2.5-fold increase in FITC fluorescence was observed within 30 minutes and about 22% of this fluorescence was quenched by trypan blue, indicating that about 78% of the FITC-collagen was protected from trypan blue quenching and therefore likely to be internalized. The amount of internalized FITC-collagen was significantly decreased in platelets pretreated with an α2β1 function–blocking mAb, indicating that collagen internalization is at least partly α2β1 dependent (data not shown). To further confirm that internalization was occurring, we predicted that trypan blue should substantially quench FITC fluorescence if trypan blue was not actively excluded from the cell, thereby allowing access to internalized FITC-collagen. Indeed, when resting or TRAP-activated platelets were incubated with FITC-collagen as described, but subsequently fixed with PFA before addition of trypan blue, thereby abolishing its active extrusion, FITC fluorescence was significantly quenched by about 56% in control platelets and 80% in TRAP-activated platelets (Figure 2). These data indicate that a substantial proportion of FITC-collagen becomes internalized by live, agonist-stimulated platelets.
FITC-collagen is protected from trypan blue quenching in live, TRAP-stimulated platelets. Human platelets were incubated with 25 μg/mL FITC-collagen in the presence or absence of 25 μM TRAP at room temperature for 30 minutes as described in “Materials and methods.” In another group, platelets were fixed with PFA after incubation with FITC-collagen to stop the reaction and to prevent trypan blue exclusion. Platelets were then washed and diluted 1:1 with ice-cold PBS. To compare the fluorescence with or without trypan blue quenching, cell suspensions were mixed 1:1 with trypan blue (400 μg/mL in PBS) or PBS for 1 to 5 minutes before flow cytometry. Data shown are averaged normalized mean fluorescence ± SEM from 2 to 4 experiments. *P < .05, **P > .05, compared with PBS controls.
FITC-collagen is protected from trypan blue quenching in live, TRAP-stimulated platelets. Human platelets were incubated with 25 μg/mL FITC-collagen in the presence or absence of 25 μM TRAP at room temperature for 30 minutes as described in “Materials and methods.” In another group, platelets were fixed with PFA after incubation with FITC-collagen to stop the reaction and to prevent trypan blue exclusion. Platelets were then washed and diluted 1:1 with ice-cold PBS. To compare the fluorescence with or without trypan blue quenching, cell suspensions were mixed 1:1 with trypan blue (400 μg/mL in PBS) or PBS for 1 to 5 minutes before flow cytometry. Data shown are averaged normalized mean fluorescence ± SEM from 2 to 4 experiments. *P < .05, **P > .05, compared with PBS controls.
Specific collagen binding in the absence of receptor recycling
To determine whether agonist-induced increases in collagen binding can occur in the absence of collagen/receptor internalization, platelets were first activated, fixed with PFA in this case to prevent internalization, washed, and incubated with FITC-collagen. We found that increased FITC-collagen binding still occurred to TRAP-stimulated, fixed platelets relative to unstimulated, fixed platelets (Figure 3A). To determine whether agonist-induced increases in FITC-collagen binding were specific, TRAP-activated, fixed platelets were incubated with FITC-collagen with or without 100-fold excess unlabeled collagen (Figure 3A). The excess unlabeled collagen significantly inhibited the agonist-induced FITC-collagen binding to below basal levels (P < .05). Similar results were seen with thrombin or ADP-stimulated murine or human fixed platelets (data not shown), indicating that agonist-induced collagen binding in the absence of receptor-mediated internalization is specific.
Specific FITC-collagen binding still occurs to agonist-stimulated, fixed platelets. (A) Human platelets were treated with control buffer or 25 μM TRAP for 2 minutes, fixed with PFA, washed, and incubated with 10 μg/mL FITC-collagen in the presence of control buffer or 1 mg/mL unlabeled collagen (Col) for 30 minutes at room temperature. Data shown are normalized mean fluorescence ± SEM from one experiment, representative of 4 similar experiments. P < .05 for unlabeled collagen versus TRAP only. (B) Agonist-treated or control, fixed murine platelets were preincubated with hamster antimouse α2-blocking mAb or control IgG (5 μg/mL) before incubation with FITC-collagen. Data are average normalized mean fluorescence ± SEM from 3 independent experiments. P < .05 for α2-blocking mAb compared with control IgG.
Specific FITC-collagen binding still occurs to agonist-stimulated, fixed platelets. (A) Human platelets were treated with control buffer or 25 μM TRAP for 2 minutes, fixed with PFA, washed, and incubated with 10 μg/mL FITC-collagen in the presence of control buffer or 1 mg/mL unlabeled collagen (Col) for 30 minutes at room temperature. Data shown are normalized mean fluorescence ± SEM from one experiment, representative of 4 similar experiments. P < .05 for unlabeled collagen versus TRAP only. (B) Agonist-treated or control, fixed murine platelets were preincubated with hamster antimouse α2-blocking mAb or control IgG (5 μg/mL) before incubation with FITC-collagen. Data are average normalized mean fluorescence ± SEM from 3 independent experiments. P < .05 for α2-blocking mAb compared with control IgG.
To determine whether the increased collagen binding that occurs in the absence of receptor recycling is mediated by α2β1, ADP-stimulated, fixed platelets were incubated with an α2-specific blocking mAb before FITC-collagen addition. This mAb, but not an isotype-matched control IgG, prevented an increase in FITC-collagen binding (Figure 3B; P < .05), indicating that the increased FITC-collagen binding is α2β1 dependent. This experiment was performed with murine instead of human platelets because we were unable to identify a blocking antihuman α2β1 mAb that retained its functional activity to fixed human platelets. These results are also consistent with an internalization-independent component of α2β1-mediated collagen binding.
Lack of apparent change in α2β1 expression on the activated platelet surface
To determine whether the increased collagen binding to platelets on agonist stimulation was simply caused by increased numbers of α2β1 integrin molecules on the activated platelet surface, we evaluated expression of α2β1 on resting and agonist-stimulated, fixed platelets with a mAb against α2β1 not known to be conformationally sensitive. By flow cytometry, there was no significant difference in α2β1 staining intensity between resting or TRAP-activated, fixed human platelets (Figure 4). Similar results were also obtained with ADP as the agonist, with either human or murine platelets (data not shown). Taken together, these results demonstrate that there is a component of agonist-induced collagen binding to platelets through α2β1 that is independent of both internalization and increased numbers of available α2β1 molecules, suggesting an activation of α2β1. Therefore, in most subsequent experiments we fixed resting or activated platelets first, before adding FITC-collagen to measure α2β1 activation only.
Surface expression of α2β1 remains constant on resting versus TRAP-stimulated platelets. Platelets were treated with control buffer (resting) or TRAP for 5 minutes, fixed with 0.7% PFA, and washed as described in “Materials and methods.” Platelets were then incubated with the α2β1 mAb BHA 2.1 (□) or control IgG1 (▦) for 20 minutes and stained with FITC-labeled secondary goat antimouse IgG for 30 minutes. Data are shown as normalized mean fluorescence ± SEM from one experiment, representative of 2 independent experiments.
Surface expression of α2β1 remains constant on resting versus TRAP-stimulated platelets. Platelets were treated with control buffer (resting) or TRAP for 5 minutes, fixed with 0.7% PFA, and washed as described in “Materials and methods.” Platelets were then incubated with the α2β1 mAb BHA 2.1 (□) or control IgG1 (▦) for 20 minutes and stained with FITC-labeled secondary goat antimouse IgG for 30 minutes. Data are shown as normalized mean fluorescence ± SEM from one experiment, representative of 2 independent experiments.
Effect of integrin cytoplasmic tail peptides on activation of α2β1 integrin
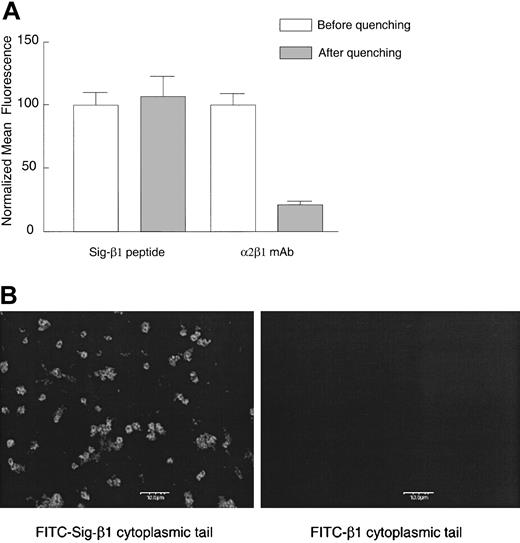
We next examined the potential roles of the α2 and β1 cytoplasmic tails in the activation of this integrin by introducing integrin cytoplasmic tail peptides into platelets. We constructed permeable, chimeric peptides with a hydrophobic delivery signal sequence14 N-terminal to the integrin tail (Table 1). This hydrophobic signal sequence was previously shown to deliver β3 cytoplasmic peptides into human erythroleukemic cells and fibroblasts.24 Delivery was confirmed in 2 ways, first by evaluating live platelets incubated with FITC-labeled signal (Sig)–β1 peptide or β1 peptide without the signal sequence using flow cytometry with trypan blue quenching, and second, by confocal microscopy. The FITC-labeled chimeric peptide appears to enter platelets because it was protected from trypan blue quenching (Figure 5A), whereas the control peptide (β1 peptide without the signal sequence) exhibited only an extremely low level of background fluorescence (data not shown). As a positive control for quenching in this experiment, platelets were incubated with an α2β1 mAb on ice, followed by an FITC-labeled secondary goat antimouse IgG on ice for 30 minutes before flow cytometry. We found extensive quenching of the FITC-conjugated secondary antibody when internalization was inhibited at 4°C. Confocal immunofluorescence microscopy further confirmed that FITC-labeled chimeric peptides were able to fluorescently label platelets, whereas the control peptide lacking the signal sequence did not (Figure 5B). We obtained similar results in platelets incubated with FITC-labeled Sig-α2 as observed by light microscopy (data not shown).
Cytoplasmic domain peptide with an N-terminal signal delivery sequence enters platelets while a peptide lacking the delivery sequence is excluded. Human platelets were incubated with 120 μM FITC-labeled Sig-β1 peptide or β1 peptide without the signal delivery sequence for 20 minutes at room temperature. (A) As a positive control for trypan blue quenching, platelets were incubated with the α2β1 mAb BHA 2.1 (5 μg/mL) on ice for 10 minutes, followed by an FITC-labeled secondary goat antimouse IgG (10 μg/mL) for 30 minutes, and cell fluorescence was evaluated by flow cytometry before or after addition of trypan blue (2 mg/mL). Data are shown as the normalized mean fluorescence ± SEM from one experiment, representative of 3 similar experiments. (B) Platelet suspensions treated as in panel A were applied to coverslips and visualized by confocal microscopy. Bar represents 10 μm. One of 2 similar experiments is shown.
Cytoplasmic domain peptide with an N-terminal signal delivery sequence enters platelets while a peptide lacking the delivery sequence is excluded. Human platelets were incubated with 120 μM FITC-labeled Sig-β1 peptide or β1 peptide without the signal delivery sequence for 20 minutes at room temperature. (A) As a positive control for trypan blue quenching, platelets were incubated with the α2β1 mAb BHA 2.1 (5 μg/mL) on ice for 10 minutes, followed by an FITC-labeled secondary goat antimouse IgG (10 μg/mL) for 30 minutes, and cell fluorescence was evaluated by flow cytometry before or after addition of trypan blue (2 mg/mL). Data are shown as the normalized mean fluorescence ± SEM from one experiment, representative of 3 similar experiments. (B) Platelet suspensions treated as in panel A were applied to coverslips and visualized by confocal microscopy. Bar represents 10 μm. One of 2 similar experiments is shown.
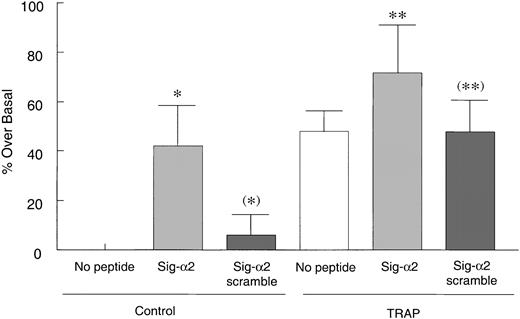
To determine how the α2 or β1 cytoplasmic tails affected collagen binding to platelets, resting platelets were incubated with the native α2 sequence (Sig-α2) chimeric peptide followed by fixation and FITC-collagen addition. The Sig-α2 peptide significantly increased collagen binding to these nonagonist-treated platelets (P < .05; Figure 6), whereas an α2 cytoplasmic peptide without the signal sequence (data not shown) or a Sig-α2 scrambled chimeric peptide had no effect (P > .1; Figure 6). In addition, platelets pretreated with the α2 chimeric peptide before TRAP addition, exhibited only a slight, but not statistically significant, increase in collagen binding above collagen binding observed with TRAP alone, perhaps because this binding was approaching a maximum. A scrambled peptide had no apparent effect on TRAP-induced collagen binding. Thus, these results suggest that the α2 tail may activate α2β1 in resting platelets. In contrast, the β1 chimeric peptide had no effect on collagen binding to resting or activated platelets (data not shown).
Sig-α2 peptide increases collagen binding to resting platelets. Human platelets were incubated with 200 μM Sig-α2 peptide or Sig-scrambled α2 peptide at room temperature for 30 minutes before a 2-minute treatment with control buffer or 50 μM TRAP. Platelets were then fixed with PFA, washed, incubated with 25 μg/mL FITC-collagen for 30 minutes, and evaluated by flow cytometry. Data are shown as averaged normalized percent fluorescence over basal binding in the absence of peptide (set to zero) ± SEM from 5 independent experiments. *P < .05 and (*)P > .5 compared with no peptide control; ** P > .1 (ie, not significantly different) compared with Sig-α2 scramble peptide in TRAP-stimulated group; (**)P > .5 (not significantly different) versus TRAP-stimulated control.
Sig-α2 peptide increases collagen binding to resting platelets. Human platelets were incubated with 200 μM Sig-α2 peptide or Sig-scrambled α2 peptide at room temperature for 30 minutes before a 2-minute treatment with control buffer or 50 μM TRAP. Platelets were then fixed with PFA, washed, incubated with 25 μg/mL FITC-collagen for 30 minutes, and evaluated by flow cytometry. Data are shown as averaged normalized percent fluorescence over basal binding in the absence of peptide (set to zero) ± SEM from 5 independent experiments. *P < .05 and (*)P > .5 compared with no peptide control; ** P > .1 (ie, not significantly different) compared with Sig-α2 scramble peptide in TRAP-stimulated group; (**)P > .5 (not significantly different) versus TRAP-stimulated control.
GFFKR is required for increased collagen binding
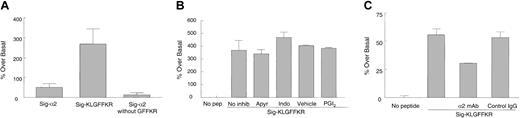
We next asked which domain of the α2 tail is required for the increase in collagen binding. Because the GFFKR motif is important in αIIbβ3 activation and mutation of this conserved region alters the affinity of αIIbβ3 for ligands,25,26 we designed 2 mutant permeable peptides, one lacking GFFKR, called Sig-α2 without GFFKR, and another containing GFFKR but truncated C-terminal to it, called Sig-KLGFFKR (Table 1). We found that when resting platelets were treated with Sig-KLGFFKR and subsequently fixed before FITC-collagen addition, even more collagen binding occurred than with the full-length α2 tail (P < .05; Figure 7A). However, Sig-α2 without GFFKR was unable to induce collagen binding beyond basal levels (P > .1).
Signal-KLGFFKR peptide increases collagen binding to resting platelets independent of platelet activation. (A) Resting human platelets were incubated with 200 μM Sig-α2 peptide, Sig-KLGFFKR peptide, or Sig-α2 peptide without GFFKR for 30 minutes at room temperature, fixed, washed, and incubated with FITC-collagen as in Figure 6. Data are shown as average normalized percent fluorescence over basal binding in the absence of peptide (set to zero) ± SEM from 3 to 4 independent experiments. (B) Resting platelets were preincubated with inhibitors (2 U/mL apyrase, 25 μM indomethacin, or 5 ng/mL PGI2) or vehicle control for indomethacin (ethanol) for 5 to 10 minutes before incubation with 200 μM Sig-KLGFFKR peptide. Data are shown as in panel A, except from 2 to 4 independent experiments. P > .1 for all inhibitors compared with the no inhibitor or vehicle control. (C) Resting live murine platelets were preincubated with hamster antimouse α2-blocking mAb or control IgG (10 μg/mL final) for 1 hour before incubation with 50 μg/mL FITC-collagen in the presence or absence of 200 μM Sig-KLGFFKR peptide for 8 to 10 minutes at room temperature. Data are shown as in panel A, except from one experiment, representative of 3 separate experiments. P < .05 for the α2-blocking mAb compared with control IgG.
Signal-KLGFFKR peptide increases collagen binding to resting platelets independent of platelet activation. (A) Resting human platelets were incubated with 200 μM Sig-α2 peptide, Sig-KLGFFKR peptide, or Sig-α2 peptide without GFFKR for 30 minutes at room temperature, fixed, washed, and incubated with FITC-collagen as in Figure 6. Data are shown as average normalized percent fluorescence over basal binding in the absence of peptide (set to zero) ± SEM from 3 to 4 independent experiments. (B) Resting platelets were preincubated with inhibitors (2 U/mL apyrase, 25 μM indomethacin, or 5 ng/mL PGI2) or vehicle control for indomethacin (ethanol) for 5 to 10 minutes before incubation with 200 μM Sig-KLGFFKR peptide. Data are shown as in panel A, except from 2 to 4 independent experiments. P > .1 for all inhibitors compared with the no inhibitor or vehicle control. (C) Resting live murine platelets were preincubated with hamster antimouse α2-blocking mAb or control IgG (10 μg/mL final) for 1 hour before incubation with 50 μg/mL FITC-collagen in the presence or absence of 200 μM Sig-KLGFFKR peptide for 8 to 10 minutes at room temperature. Data are shown as in panel A, except from one experiment, representative of 3 separate experiments. P < .05 for the α2-blocking mAb compared with control IgG.
Platelet inhibitors do not significantly affect collagen binding induced by the Sig-KLGFFKR peptide
A previous study showed that a lipid-modified peptide corresponding to the cytoplasmic region of integrin αIIb induced platelet activation and aggregation in part via thromboxane A2 synthesis.27 We therefore asked whether the chimeric Sig-KLGFFKR peptide induced collagen binding to platelets by activating platelets via signal transduction events. Various platelet inhibitors including apyrase, which scavenges released ADP, indomethacin, which inhibits cyclooxygenase and therefore thromboxane A2 production, and PGI2, which increases cyclic adenosine monophosphate (cAMP) formation, were added to live platelets before incubation with Sig-KLGFFKR, fixation, and FITC-collagen addition. These inhibitors had no significant effect on Sig-KLGFFKR–induced collagen binding to platelets (Figure 7B), suggesting that this peptide may affect α2β1 directly to increase platelet binding to collagen.
Sig-KLGFFKR–induced collagen binding is α2β1 dependent
To determine whether the Sig-KLGFFKR peptide induces collagen binding specifically through the α2β1 integrin, we preincubated live murine platelets with an α2-blocking mAb or control IgG before incubation with the peptide and FITC-collagen. Collagen binding induced by Sig-KLGFFKR was partly inhibited by the α2-blocking mAb (P < .05), but not by control IgG (P > .1; Figure 7C). Similar results were seen with live human platelets (data not shown). These results suggest that at least 40% of the Sig-KLGFFKR–induced collagen binding occurs through α2β1, but that other collagen binding sites may also contribute, unlike thrombin stimulation of platelets, which appeared to increase collagen binding largely through α2β1 (Figure 1A). To determine if the most abundant platelet integrin, αIIbβ3, also contributed to this increased collagen binding, platelets were preincubated with 1B5, a function-blocking mAb against the murine αIIbβ3 complex. However, this mAb had no effect on Sig-KLGFFKR–induced collagen binding (data not shown).
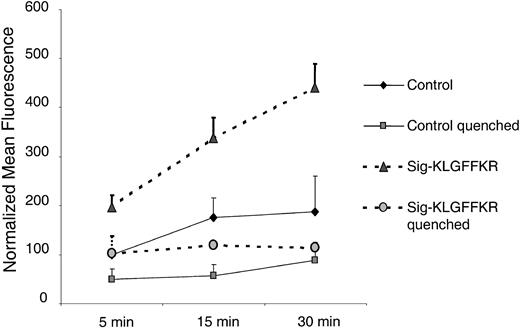
To test whether the Sig-KLGFFKR peptide also induces collagen internalization into platelets, we preincubated live platelets with this peptide or control buffer. We then added FITC-collagen for various times and measured internalization as protection from trypan blue quenching. These time-course experiments indicated that the majority of Sig-KLGFFKR–induced FITC-collagen binding to platelets was extracellular and quenched by trypan blue (Figure 8). However, when platelets were treated simultaneously with Sig-KLGFFKR and ADP, most of the FITC-collagen was protected from trypan blue quenching and therefore likely internalized (data not shown).
FITC-collagen binding induced by the Sig-KLGFFKR peptide is largely surface associated. Human platelets were incubated with control buffer or the Sig-KLGFFKR peptide at a final concentration of 200 μM and exposed to 25 μg/mL FITC-collagen. The reactions were stopped by the addition of 10 × ice-cold PBS at the indicated time points. For quenched groups, trypan blue was added at a final concentration of 200 μg/mL. Data shown are the normalized mean fluorescence ± SEM from 2 independent experiments, representing 3 similar experiments.
FITC-collagen binding induced by the Sig-KLGFFKR peptide is largely surface associated. Human platelets were incubated with control buffer or the Sig-KLGFFKR peptide at a final concentration of 200 μM and exposed to 25 μg/mL FITC-collagen. The reactions were stopped by the addition of 10 × ice-cold PBS at the indicated time points. For quenched groups, trypan blue was added at a final concentration of 200 μg/mL. Data shown are the normalized mean fluorescence ± SEM from 2 independent experiments, representing 3 similar experiments.
Discussion
Although activation of the αIIbβ3 integrin on platelets has been extensively studied, the activation properties of other platelet integrins are not well understood. Another important integrin on platelets is the collagen receptor, α2β1. This integrin is critical for collagen-induced platelet activation and platelet adhesion to collagen, because human platelets lacking this integrin or blockage of this integrin with function-blocking mAbs results in a loss of collagen-mediated platelet activation and adhesion to collagen.2,28-30 However, agonist-induced activation of α2β1 on platelets has not been extensively studied. Intriguing reports from Jung and Moroi9,16 provide evidence for α2β1 integrin activation on platelets as defined by an enhanced binding capacity of α2β1 on live, agonist-stimulated platelets for the soluble ligand 125I-type III collagen. In the present study, we further analyzed some fundamental properties of α2β1 integrin activation, as measured with soluble FITC-labeled type I collagen and flow cytometry. The FITC-labeled type I collagen used in our study was native bovine collagen type I that lacks the telopeptide, which is essential for collagen molecules to cross-link into fibrillar collagen. We found that type I collagen binding to platelets increases with agonist stimulation in a divalent cation- and α2β1-dependent manner. Collagen internalization accounts for a significant part of this increase; however, specific collagen binding still occurs under conditions known to block internalization and is not dependent on increased α2β1 expression on the platelet surface. These results suggest that a significant component of soluble FITC-collagen binding to platelets is due to activation of α2β1, independent of internalization or increases in α2β1 expression levels in the platelet surface. Our results also indicate that increased collagen binding to α2β1 may be mediated by the GFFKR motif of the α2 cytoplasmic tail.
It is important to explore the contribution of collagen internalization to α2β1-mediated collagen binding because the increased association of soluble ligand with platelets may not be exclusively due to an increased binding capacity of α2β1. Because most studies on integrin-ligand interactions are performed in live platelets at room temperature or 37°C, it is possible that the ligand is internalized through an agonist-induced increase in the rate of integrin-mediated receptor recycling. In fact, we previously found that fibrinogen is rapidly taken up via αIIbβ3 into activated platelets.13 This suggests that the increase in ligand binding to activated platelets could result from a few activated integrin molecules that exhibit an accelerated uptake of ligand, or from activation of a large proportion of integrin molecules that either remain on the surface or are internalized. Ligand-binding studies per se do not necessarily distinguish between these possibilities. By distinguishing internalized from surface-bound FITC-collagen using trypan blue quenching, we demonstrate that internalization contributes to a significant proportion of the increased association of FITC-collagen with platelets upon agonist stimulation (Figure 2), and that internalization is inhibited by an α2β1 function-blocking mAb (data not shown). This is consistent with a previous study of human fibroblasts showing that internalization of type I collagen–coated beads is α2β1 dependent and that the α2 integrin subunit is rapidly recycled or resynthesized following a phagocytic load.31 The increased association of soluble type I collagen to activated platelets is decreased by an α2β1-blocking mAb, which is in agreement with the findings that soluble collagen interacts largely through the α2β1 integrin and minimally through GPVI.9,32 Interestingly, it was found in a previous study that fibrillar collagen could be internalized by platelets, although the receptor mediating internalization was not described.33 Because GPVI is a platelet collagen receptor that is most responsible for fibrillar collagen binding to platelets,32 it would be interesting to determine whether fibrillar collagen internalization occurs in platelets via this receptor. One functional consequence of collagen internalization by platelets could be lysosomal degradation, because this is an important mechanism for collagen clearance in other cells.34,35 The dynamic regulation of collagen internalization by agonist stimulation suggests its potential significance in wound healing, inflammation, and vascular wall remodeling.
It is most likely that the α2β1 integrin is activated by agonist stimulation via inside-out signaling, because FITC-collagen binding to agonist stimulated, fixed platelets was still increased independent of internalization, and there was no significant change in α2β1 expression on the platelet surface before or after agonist stimulation. Experiments by Jung and Moroi suggest that the α2β1 integrin becomes activated when platelets are flowed over collagen, resulting in a firm adhesion to the immobilized collagen.10 The activation of α2β1 by agonist stimulation supports a modified 2-step, 2-site model in which collagen binds initially to α2β1 or GPVI or both, and signals from GPVI increase the affinity of α2β1 for collagen.36 Mapping the signaling pathways from different agonist receptors such as GPVI or the thrombin or ADP receptor to α2β1 is an important next step in understanding the mechanism of platelet adhesion to collagen.
In addition to strengthening an adhesion to collagen, the activation of α2β1 on platelets may also contribute to the extracellular collagen degradation pathway by matrix metalloproteinases (MMPs). Upon activation, platelets release pro-MMP-1 and -2 as well as other MMPs37,38 and MMP-1 activity increases both intracellularly and extracellularly.39 Recent studies demonstrated that pro-MMP-1 complexes with α2β1 on keratinocytes and platelets and that α2β1 can bind the pro or active form of this protease via the I domain of α2 and the linker and hemopexin motifs of MMP-1.40,41 The possibility exists that activation of α2β1 on platelets may also regulate MMP activity or release, which is important in inflammation, matrix remodeling, and metastasis.
To further understand how α2β1 activation is regulated, we explored the roles of the α2 and β1 cytoplasmic tails in this process. Although the roles of integrin cytoplasmic domains in αIIbβ3 activation have been explored, only a few studies have examined the roles of these domains in α2β1-mediated processes. For example, several groups have shown that the α subunit cytoplasmic domain contributes to the regulation of adhesion, morphology, and motility mediated by α2β1 in K562, T47D, and RD cells.42-44 Because platelets are anucleate and difficult to genetically manipulate, we introduced excess integrin cytoplasmic tails as cell-permeable chimeric peptides. Previous work from Hawiger and Liu et al has established that cell-permeable chimeric peptides of various β3 cytoplasmic sequences complexed to a hydrophobic delivery sequence enter human erythroleukemic cells or human fibroblasts and regulate the function of αIIbβ3.14,24 Using this approach, we successfully delivered the α2 and β1 cytoplasmic tails into platelets. Although we found no effect of the β1 tail, excess α2 cytoplasmic peptide increased FITC-collagen binding in resting but not TRAP-activated platelets. Our data are in agreement with Stephens et al,27 who observed that introduction of a portion of the αIIb cytoplasmic domain increased fibrinogen binding to platelets via αIIbβ3. However, our data differ from Vinogradova and coworkers, where myristoylated αIIb peptides were found to inhibit fibrinogen binding to platelets upon ADP or thrombin stimulation.45 Possibilities that may contribute to this difference are that our study involved a different integrin and different peptide modification, which may affect peptide location and function in cells.
We also performed experiments using live platelets preincubated with the full-length α2 cytoplasmic tail (Sig-α2), as well as the Sig-α2 scramble, and an α2 peptide lacking the signal sequence, before a 30-minute incubation with FITC-collagen. However, we did not find a significant difference in FITC-collagen binding between these peptides (data not shown). Although we believe that Sig-α2 does activate collagen binding in live platelets, we speculate that these results are more difficult to observe in live platelets, because FITC-collagen itself is a platelet agonist with or without the Sig-α2 peptide. Therefore, in live, unfixed platelets, any increase in FITC-collagen binding by the Sig-α2 peptide may be masked by the effects of agonist stimulation by FITC-collagen. This is one of the reasons that agonist-stimulated, fixed platelets were used in many of our studies, such that FITC-collagen would serve only as a readout for active α2β1 and not as an agonist.
To further localize the portion of the integrin tail responsible for α2β1 activation, we introduced mutant peptides into platelets and found that an excess of peptide containing only the membrane-proximal KLGFFKR motif of α2 induced substantial collagen binding to resting platelets. The membrane proximal region is highly conserved among α subunits. These results are consistent with previous studies of the αIIbβ3 integrin, which showed that a lipid-soluble peptide, palmitoyl-KVGFFKR, induces platelet αIIbβ3 activation. However, activation in that case was partly dependent on thromboxane formation,27 whereas we observed that α2β1 integrin activation was independent of multiple intracellular signaling events. Our results are also in agreement with another study showing that deletion of most of the αIIb cytoplasmic tail downstream of KVG, resulted in the expression of constitutively active αIIbβ3 in CHO cells,25 suggesting that the distal cytoplasmic tail of αIIb (FFKRNRPPLEEDDEEGE) exerts a negative regulatory function and locks αIIbβ3 in a low-affinity state. Consistent with the importance of the GFFKR motif, we also found that the Sig-α2 cytoplasmic domain peptide lacking this motif did not affect FITC-collagen binding in either resting or TRAP-activated platelets. Interestingly, collagen binding induced by the Sig-KLGFFKR peptide was not completely blocked by an α2β1 function–blocking mAb, suggesting either the contribution of other activated integrin or nonintegrin receptors, or a peptide-induced conformational change in α2β1 such that it cannot be blocked by this mAb. However, this peptide-induced collagen binding was not affected by an αIIbβ3 function–blocking mAb, suggesting a lack of contribution of this particular integrin to the non–α2β1-mediated portion of this increased binding (data not shown).
We noted that while Sig-KLGFFKR also induced a slight increase in collagen internalization as determined by trypan blue quenching, the majority of Sig-KLGFFKR–induced FITC-collagen binding remained on the surface of resting platelets. Interestingly, internalization induced by Sig-KLGFFKR was greatly increased in ADP-activated platelets (data not shown). This enhanced effect may be due to a synergy between the peptide and ADP, that is, a combination of a Sig-KLGFFKR–induced increase in collagen binding and an ADP-induced acceleration of both internalization and collagen binding. These results suggest that this peptide may directly activate the α2β1 integrin.
The activation of α2β1 on resting platelets by Sig-KLGFFKR may be explained in 2 ways. One possibility is that the KLGFFKR sequence may compete for a molecule bound to α2 that is necessary to maintain α2β1 in a resting conformation. Removal of this molecule may result in integrin activation. This may explain why excess Sig-β1 peptide did not alter FITC-collagen binding in our study. Although the KLGFFKR peptide has been shown to interact with the 60-kD protein, calreticulin,46 calreticulin binding to α2β1 is induced by phorbol myristate acetate, suggesting that its binding to α2β1 is more likely to stabilize a high-affinity state rather than maintain a resting conformation of this integrin.47 The identities of other molecules that bind to the α2 subunit are currently unknown but their discovery will lead to a better understanding of the regulation of α2β1 activation.
A second explanation of our results is that the α2 and β1 subunits may interact to maintain α2β1 in a resting conformation. The KLGFFKR motif may disrupt the resting conformation by competing for an α2-binding site on β1, thereby activating this integrin, consistent with previous studies of αIIbβ348 or αLβ249 in which the conserved GFFKR motif in the integrin α cytoplasmic domain helps to maintain the integrin in a low-affinity, resting state. The reason that the full-length Sig-α2 peptide was less active than Sig-KLGFFKR may be due to decreased accessibility of this peptide to the sites of α2 and β1 interaction or adoption of a secondary conformation that masks the KLGFFKR motif. However, this model is inconsistent with the inability of Sig-β1 to activate α2β1, suggesting that the first model may be more likely.
In summary, our results suggest that the α2β1 integrin plays a role in both platelet-collagen binding and collagen internalization in agonist-stimulated platelets. Activation of α2β1 most likely occurs because increased collagen binding to α2β1 was observed under conditions where internalization was prevented and because no significant increase in α2β1 surface expression was observed on activated platelets. We have further determined that the proximal KLGFFKR motif on the α2 cytoplasmic tail may play an important role in modulating the activation state of α2β1 on platelets. Our data provide further information for understanding the function and regulation of α2β1 on platelets and potentially on other cell types such as fibroblasts, osteoblasts, and macrophages where this integrin mediates cell migration, invasion, and phagocytosis.
Prepublished online as Blood First Edition Paper, May 8, 2003; DOI 10.1182/blood-2002-09-2753.
Supported by National Institutes of Health grants 2-P01-HL06350 and 2-P01-HL45100 (L.V.P.) and Lineberger Comprehensive Cancer Center postdoctoral training grant CA09156 (T.M.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Russ Henry for peptide synthesis, purification, and analysis, and Weiping Yuan for help with mouse platelet experiments.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal