Abstract
B7-H2, which is expressed constitutively on B cells and binds the inducible costimulator (ICOS) on antigen-activated T cells, is a member of the B7 family of costimulatory ligands. We have inactivated B7-H2 in the mouse. B7-H2–/– mice generate normal populations of B and T cells in their various lymphoid organs but have lower basal levels of heavy chain class–switched antibodies in their sera. These mice are able to mount normal immune responses to both type I and type II T-cell–independent antigens. However, their pattern of responses to a T-cell–dependent antigen is altered, with greatly reduced production of antigen-specific heavy chain class–switched antibodies, the levels of which could not be elevated even with repeated immunizations. This suggests a critical role for B7-H2 in the recall phases of the immune response. Germinal center formation is also impaired in the mutant mice. While B cells from the mutant mice could response normally to anti-IgM, anti-CD40, and lipopolysaccharide stimulation, the production of T-helper–type II cytokines such as interleukin-4 (IL-4) and IL-10 by primed CD4+ T cells from mutant mice were reduced. This indicated that the defects in humoral responses and germinal center formation in B7-H2–deficient mice are due to the lack of T-cell–mediated help to the B cells. Hence, B7-H2 on B cells is important for recruiting T-cell help via its interaction with ICOS and plays a critical role in costimulating humoral immune responses.
Introduction
Optimal activation of B- and T-lymphocytes requires not only the engagement of their specific antigen receptors but also their accessory costimulatory molecules.1-4 It is widely accepted that B7-1 (CD80) and B7-2 (CD86), which are expressed on activated B and other antigen-presenting cells, play important roles in the adaptive immune responses by binding the costimulatory CD28 and inhibitory cytotoxic T-lymphocyte antigen-4 (CTLA-4) on T cells.5,6 Binding of B7-1 and B7-2 to CD28 on antigen-activated T cells enhances the magnitude and duration of the T-cell responses and leads to the expression of the cell survival protein BclxL5 as well as to the increased secretion of cytokines such as interleukin-2 (IL-2).7,8 Subsequently, activated T cells express CTLA-4, which sequesters B7-1 and B7-2 away from CD28, and at the same time delivers an inhibitory signal to T cells that terminates the immune response.9,10
The importance of B7-1/2–CD28-mediated costimulation pathway in lymphocyte activation has been demonstrated in various model systems whereby their interactions were blocked5 or the genes encoding the B7-1/2 or CD28 molecules were inactivated in the mouse.11,12 Collectively, these studies show that the disruption of B7-1/2–CD28 interaction leads to impairment in germinal center formation, defective switching of immunoglobulin (Ig) heavy chain isotypes, and greatly reduced humoral and cell-mediated immune responses to protein antigens, parasites, and viruses. However, some T-helper (Th) cell functions, in particular those pertaining to the reactivation of antigen-experienced memory and effector cells, remain intact in the absence of B7-1/2–CD28 interaction.11,13 This raises the question as to whether these responses are costimulation-independent or simply that other costimulatory pathways exist.
Recently, a third member of the B7 family was identified and termed B7h,14 B7RP-1,15 GL-50,16 LICOS,17 or B7-H2.18 B7-H2 shares approximately 20% homology to B7-1 and B7-2 and is expressed constitutively on B lymphocytes and at low levels on monocytes.14,15 Its expression level can be increased by interferon (IFN)–γ and may be modulated by LPS treatment.14,18,19 Mice transgenic for B7-H2 provide the first evidence in vivo that this molecule plays an important role in lymphocyte activation as the aberrant expression of B7-H2 leads to T-cell hyperplasia, plasmacytosis, and hypergammaglobulinemia.15
B7-H2 does not contain the SQD'ELY motif necessary for binding to CD28 or CTLA-4.20 Instead, it binds the recently discovered inducible costimulator (ICOS) that is expressed on activated T cells.15,21,22 ICOS shares 30% to 40% sequence similarity to CD28 and CTLA-4 but does not have the conserved MYPPPY motif necessary for binding B7-1 and B7-2.21,22
ICOS is expressed on both activated CD4+ and CD8+ T cells.15,22,23 ICOS engagement in vitro can costimulate the production of cytokines such as IFN-γ, IL-4, and IL-10, but not IL-2.15,21,24 The administration of ICOS-Ig to antigen-immunized mice demonstrated the important contribution of ICOS to T-cell–dependent antibody responses.24 Furthermore, it was shown that the ectopic expression of B7-H2 on tumor cells enhances tumor rejection that could be mediated by CD8+ T cells in the absence of CD4+ T cells.25,26 The immune protection correlated with the priming and expansion of the tumor-specific cytotoxic T lymphocytes (CTLs) and does not appear to regulate the cytolytic activity of the CTLs.25,26 In addition, a recent report indicated involvement of the B7-H2-ICOS interaction in the development of regulatory T cells that is essential for the induction of tolerance in an airway hyper-reactivity model.27
Interestingly, the inhibition of B7-1/2–CD28 interaction can lead to a decrease in ICOS expression, suggesting that some of the effects of costimulation that are attributed to the CD28-mediated pathway may in fact be B7-H2-ICOS–dependent.28 Thus, with the increasing complexity in lymphocyte costimulation, it is of signifi-cant interest to distinguish the relative contribution of each of these costimulatory pathways to the overall activation program of B and T lymphocytes in vivo. To accomplish this goal, we have generated B7-H2–deficient mice to determine the role of this costimulatory ligand in lymphocyte activation and to examine if the phenotype of these mutant mice correlated with that of the ICOS-deficient mice.
Materials and methods
Generation of B7-H2–/– mice
The murine B7-H2 cDNA (accession number AF216747) was used to search the public database to identify genomic DNA sequences that correspond to the gene. A 15-kilobase (kb) b7-h2 genomic locus was assembled by polymerase chain reaction (PCR) and verified by sequencing. A targeting construct was generated as shown in Figure 1 and used to transfect E14.1 embryonic stem (ES) cells. To screen for homologous recombinants, genomic DNA from G418-resistant ES clones was digested with EcoRI and hybridized with a 293–base pair (bp) external probe corresponding to the exon encoding the IgC-like domain of B7-H2. An ES clone bearing the targeted b7-h2 allele was injected into C57BL/6 blastocysts to generate chimeric mice, which subsequently transmitted the mutant locus.
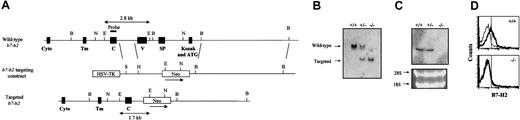
Inactivation of the b7-h2 gene locus. (A) Partial restriction map of the wild-type b7-h2 locus, the targeting construct, and the inactivated locus after homologous recombination (restriction enzymes: B indicates BamHI; E, EcoRI; H, HindIII; N, NcoI; S, SalI). Exons coding for the different domains of the B7-H2 protein (Cyto indicates cytoplasmic tail; Tm, transmembrane; C, Ig constant region–like; V, Ig variable region–like; SP, signal peptide) are indicated together with the external probe used to screen ES cells and mice, which distinguishes the 2.8-kb wild-type and 1.7-kb targeted loci. (B) Southern blot analysis of EcoRI-digested liver DNA from wild-type (+/+), heterozygous (+/–), and homozygous (–/–) B7-H2 knockout mice using the external probe. (C) Northern blot analysis using full-length B7-H2 cDNA as probe of splenic RNA prepared from wild-type, heterozygous, and homozygous B7-H2 knockout mice. (D) Flow cytometry analysis of surface B7-H2 expression on splenocytes of wild-type (top panel) and B7-H2–/– (bottom panel) mice gated on CD19+CD3– cells. The thin line indicates cells that were stained with the isotype-matched control antibody, while the bold line indicates cells that were stained with the PE-conjugated anti–B7-H2 antibody. Vertical line indicates mean fluorescence intensity (MFI).
Inactivation of the b7-h2 gene locus. (A) Partial restriction map of the wild-type b7-h2 locus, the targeting construct, and the inactivated locus after homologous recombination (restriction enzymes: B indicates BamHI; E, EcoRI; H, HindIII; N, NcoI; S, SalI). Exons coding for the different domains of the B7-H2 protein (Cyto indicates cytoplasmic tail; Tm, transmembrane; C, Ig constant region–like; V, Ig variable region–like; SP, signal peptide) are indicated together with the external probe used to screen ES cells and mice, which distinguishes the 2.8-kb wild-type and 1.7-kb targeted loci. (B) Southern blot analysis of EcoRI-digested liver DNA from wild-type (+/+), heterozygous (+/–), and homozygous (–/–) B7-H2 knockout mice using the external probe. (C) Northern blot analysis using full-length B7-H2 cDNA as probe of splenic RNA prepared from wild-type, heterozygous, and homozygous B7-H2 knockout mice. (D) Flow cytometry analysis of surface B7-H2 expression on splenocytes of wild-type (top panel) and B7-H2–/– (bottom panel) mice gated on CD19+CD3– cells. The thin line indicates cells that were stained with the isotype-matched control antibody, while the bold line indicates cells that were stained with the PE-conjugated anti–B7-H2 antibody. Vertical line indicates mean fluorescence intensity (MFI).
Northern blot analyses
Total RNA was prepared from spleens of wild-type and B7-H2–/– mice using TRIZOL reagent (GIBCO BRL, Carlsbad, CA). Northern blot analysis was performed with total RNA and probed using the full-length B7-H2 cDNA sequence.
Flow cytometry analyses
Single-cell suspensions were obtained from the spleens, lymph nodes, bone marrow, and thymus of mice and treated with red blood cell lysis solution (0.15 M NH4Cl and 0.1 mM Na2 EDTA [ethylenediaminetetraacetic acid]) for 5 minutes at 4°C to eliminate erythrocytes. For flow cytometric analyses, cells were stained with optimal amounts of fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, and biotin-conjugated antibodies to cell surface antigens. Streptavidin-CyChrome was used in the second step staining to reveal biotinylated antibodies. Flow cytometry was performed on a FACScan (Becton Dickinson, Mountain View, CA) using CellQuest software. The following antibodies, obtained from BD Pharmingen (San Diego, CA) were used in the flow cytometry analyses: anti-IgM, anti-B220, anti-CD3, anti-CD4, anti-CD5, anti-CD8, and anti-CD19. The anti–B7-H2 antibody was obtained from eBioScience, San Diego, CA.
Detection of basal immunoglobulin levels
To measure basal levels of serum antibodies, ELISA [enzyme-linked immunosorbent assay] plates were coated with 100 μL rat anti–mouse Igκ antibodies (5 μg/mL). Sera obtained from mice were serially diluted and added to each well. After the washing and blocking steps, horseradish peroxidase–coupled rat anti–mouse IgM, IgA, IgG1, IgG2a, IgG2b, or IgG3 antibodies were added, and this was followed by the addition of the substrate tetramethylbenzidine (Pierce, Rockford, IL). Levels of the various serum antibodies were quantified against known standards that were also included in the assays.
In vivo immunizations
Mice were immunized intraperitoneally with 5 μg of the hapten, 4-hydroxy-3-nitrophenyl acetyl (NP) coupled to lipopolysaccharide (NP1-LPS) for the T-cell–independent type I immune response, and with 10 μgNP25-Ficoll for the T-cell–independent type II immune response. Sera were collected one week after immunization to determine the presence of NP-specific IgM and IgG3 antibodies using ELISA plates that were coated with NP2-BSA (bovine serum albumin). For T-cell–dependent immune responses, mice were immunized intraperitoneally with 5 or 100 μg of the alum-precipitated NP21-CG (NP coupled to chicken globulin) in a low- and high-dose immune response, respectively. NP21-CG–primed mice were rechallenged 30 days later with 5 μg of the antigen for the secondary response and again, 30 days later, with 5 μg of the antigen for the tertiary response. Sera were collected at days 7 and 14 after each antigenic challenge to examine for the presence of NP-specific IgM, IgG1, and IgG2a antibodies using ELISA plates that were coated with NP2-BSA.
Histology
Mice were killed 8 days after the secondary immunization with NP21-CG, and cryosections of the spleens were prepared. The sections were fixed in 4% paraformaldehyde in phosphate-buffered saline, followed by quenching of endogenous peroxidase activity with 0.3%(vol/vol) H2O2 and blocking of nonspecific binding with 4%(wt/vol) BSA. Germinal center B cells were stained using biotinylated peanut agglutinin (PNA) (Sigma, St Louis, MO) and detected with VECTASTAIN ABC Reagent (Vector Labs, Burlingame, CA). Finally, the sections were counterstained with 1% hematoxylin.
In vitro proliferation assays
Splenic B cells were isolated from wild-type and B7-H2–/– mice by negative selection using anti-CD43 monoclonal antibody (mAb)–coupled magnetic cell-sorting (MACS) beads (Miltenyi Biotech, Bergisch Gladbach, Germany). The purity of the B cells recovered is more than 90% as assessed by fluorescence-activated cell sorter (FACS) staining. Purified B cells (1 × 105) were either untreated or stimulated with varying concentrations of F(ab)′2 goat anti–mouse IgM (Jackson ImmunoResearch, West Grove, PA) or anti-CD40 (BD Pharmingen) antibody or LPS (Sigma-Aldrich, St Louis, MO) in 96-well flat-bottom plates. After 48 hours, the cells were pulsed with 0.5 μCi (0.0185 MBq) [3H]thymidine (Amersham Biosciences, Freiburg, Germany) for another 16 hours before they were harvested using a Tomtec Harvester 96 MACH III, and the incorporated radioactivity was measured using a MicroBeta TriLux Scintillation Counter.
Analyses of cytokine production
Mice were injected subcutaneously with 100 μL of a 500 μg/mL solution of Keyhole limpet hemocyanin (KLH) emulsified in complete freund adjuvant (CFA). The immunized animals were killed 14 days later, and their draining lymph nodes were isolated. CD4+ T cells (3 × 106), isolated to more than 90% purity as analyzed by FACS staining, were cultured in 24-well tissue culture plates in the presence of 100 μg/mL soluble KLH. Supernatants were collected daily from day 2 to day 5, and the levels of IL-2, IL-4, IL-10, and IFN-γ were quantified using the BD Cytokine OptEIA ELISA Kits (BD BioSciences Pharmingen, San Diego, CA) according to the manufacturer's instructions.
Results
Generation of mice lacking B7-H2
We have generated B7-H2–deficient mice by deleting the DNA segment that codes for part of the promoter, the exon containing the Kozak sequence and starting ATG, and the exons that encode the signal peptide and IgV-like domain (Figure 1A). This targeting strategy should effectively disrupt the transcription of the gene and prevent the generation of alternatively spliced transcripts. The latter is of particular relevance as alternative spliced variants have been documented for the various members of the B7 family of costimulatory ligands, in particular B7-1, B7-2, and B7-H2.29-31 Furthermore, the removal of the IgV-like domain in our inactivation of B7-H2 will ensure that in the unlikely situation whereby a truncated protein is being produced, it will not interact with its counter-receptor ICOS.
The gene targeting of b7-h2 in mice was verified by Southern blotting (Figure 1B). Homozygous mutant mice were viable and morphologically indistinguishable from wild-type littermates. Northern blot (Figure 1C) and reverse transcription (RT)–PCR (data not shown) analyses of spleen samples indicated that neither the full-length nor a truncated version of the B7-H2 message was produced. Furthermore, flow cytometry analysis using an anti–B7-H2 antibody indicated that in contrast to wild-type B cells that constitutively express B7-H2, the mutant B cells lack the surface expression of this molecule (Figure 1D). Thus, the data suggest that the b7-h2 loci have been successfully disrupted in the mutant mice.
Normal B- and T-cell development in B7-H2–/– mice
Since B7-H2 is constitutively expressed on B cells14-16 and its counter-receptor ICOS is inducibly expressed on T cells,15,21 we examined whether the absence of B7-H2 would affect the development and composition of B and T lymphocytes in the various lymphoid organs of mutant mice. Flow cytometry analysis of the thymus indicated that both CD4+ and CD8+ T cells developed normally in the mutant mice, even though B7-H2 expression has been detected in this organ in normal mice (Figure 2A). Likewise, B-cell development was intact in the bone marrow of mutant mice (Figure 2B). Further comprehensive flow cytometric analyses of the spleen (Figure 2C) and lymph nodes (data not shown) revealed that equivalent proportion of IgM+B220+ B cells and CD4+ and CD8+ T cells also were found in the peripheral lymphoid tissues of wild-type and B7-H2–/– mice. The composition of follicular and marginal zone B cells in the spleen (data not shown) or CD5+ B-1 cells in the peritoneal cavity (Figure 2D) also were unchanged in the mutant animals compared with their wild-type counterparts. In addition, the absolute numbers of total cells, T cells (CD3+), and B cells (IgM+B220+) in the various lymphoid tissues did not differ significantly between wild-type and mutant mice (Table 1). Taken together, the data indicate that B7-H2 is not required for B- and T-cell development.
Normal B- and T-cell development in B7-H2–/– mice. Flow cytometry analyses of B- and/or T-cell populations in the (A) thymus, (B) bone marrow, (C) spleen, and (D) peritoneal cavity of wild-type, heterozygous, and homozygous B7-H2 knockout mice. Numbers indicate the percent of lymphocytes. Data shown are representative of 5 independent experiments.
Normal B- and T-cell development in B7-H2–/– mice. Flow cytometry analyses of B- and/or T-cell populations in the (A) thymus, (B) bone marrow, (C) spleen, and (D) peritoneal cavity of wild-type, heterozygous, and homozygous B7-H2 knockout mice. Numbers indicate the percent of lymphocytes. Data shown are representative of 5 independent experiments.
Enumeration of total, T, and B cells in the various organs of wild-type and B7-H2–deficient mice
. | +/+ . | . | . | -/- . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cell type . | Total cells, × 107 . | CD3+, × 107 . | IgM+ B220+, × 107 . | Total cells, × 107 . | CD3+, × 107 . | IgM+B220+, × 107 . | ||||
| Thymus | 7.05 ± 1.61 | 6.04 ± 2.31 | NA | 9.23 ± 1.69 | 7.74 ± 2.84 | NA | ||||
| Spleen | 10.20 ± 0.56 | 2.18 ± 0.12 | 5.35 ± 0.59 | 13.46 ± 2.03 | 1.96 ± 0.42 | 5.37 ± 1.16 | ||||
| Lymph node | 1.78 ± 0.31 | 1.14 ± 0.23 | 0.38 ± 0.11 | 1.36 ± 0.23 | 0.74 ± 0.04 | 0.26 ± 0.07 | ||||
| Bone marrow | 2.22 ± 0.22 | NA | 0.52 ± 0.08 | 2.50 ± 0.24 | NA | 0.42 ± 0.11 | ||||
| Peritoneum | 0.67 ± 0.11 | 0.03 ± 0.01 | 0.20 ± 0.04 | 0.75 ± 0.05 | 0.03 ± 0.01 | 0.21 ± 0.06 | ||||
. | +/+ . | . | . | -/- . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cell type . | Total cells, × 107 . | CD3+, × 107 . | IgM+ B220+, × 107 . | Total cells, × 107 . | CD3+, × 107 . | IgM+B220+, × 107 . | ||||
| Thymus | 7.05 ± 1.61 | 6.04 ± 2.31 | NA | 9.23 ± 1.69 | 7.74 ± 2.84 | NA | ||||
| Spleen | 10.20 ± 0.56 | 2.18 ± 0.12 | 5.35 ± 0.59 | 13.46 ± 2.03 | 1.96 ± 0.42 | 5.37 ± 1.16 | ||||
| Lymph node | 1.78 ± 0.31 | 1.14 ± 0.23 | 0.38 ± 0.11 | 1.36 ± 0.23 | 0.74 ± 0.04 | 0.26 ± 0.07 | ||||
| Bone marrow | 2.22 ± 0.22 | NA | 0.52 ± 0.08 | 2.50 ± 0.24 | NA | 0.42 ± 0.11 | ||||
| Peritoneum | 0.67 ± 0.11 | 0.03 ± 0.01 | 0.20 ± 0.04 | 0.75 ± 0.05 | 0.03 ± 0.01 | 0.21 ± 0.06 | ||||
Calculations of T and B cells are based on the total cell count and the percentage of CD3+ and IgM+B220+ cells as assayed by FACS staining. Cell counts are presented as mean ± SEM. Sample size in each group is at least 3. NA indicates not applicable.
Altered basal immunoglobulin levels in the sera of B7-H2–/– mice
In the absence of any gross alteration of lymphocyte populations in the mutant mice, we next determined the basal levels of the various classes of immunoglobulins in the sera of B7-H2–/– mice. Examination of serum antibodies by ELISA (Figure 3) revealed that B7-H2–/– mice have equivalent amounts of IgM and IgG3 compared with wild-type littermates. However, the levels of IgG2a, IgG2b, and IgA were significantly reduced in the sera of mutant animals. The level of IgG1 also was lower in the mutant mice, although not as statistically significant. These data suggest that B7-H2 costimulation may play a role in B-cell–humoral immune responses that required heavy chain class–switched antibodies.
Basal serum immunoglobulin levels in wild-type and B7-H2–/– mice. The serum immunoglobulin levels of various isotypes for each individual wild-type (•) and mutant (○) mouse were measured via ELISA using known standards and plotted. Statistical signifi-cance was established using a paired 2-tailed Student t test, and the P values are indicated. Horizontal bars indicate the mean.
Basal serum immunoglobulin levels in wild-type and B7-H2–/– mice. The serum immunoglobulin levels of various isotypes for each individual wild-type (•) and mutant (○) mouse were measured via ELISA using known standards and plotted. Statistical signifi-cance was established using a paired 2-tailed Student t test, and the P values are indicated. Horizontal bars indicate the mean.
Intact T-cell–independent immune responses in B7-H2–/– mice
To determine if B7-H2 costimulation had a role in T-cell–independent immune responses, we challenged the mutant mice with either a T-cell–independent type I antigen, NP-LPS, or type II antigen, NP-Ficoll. The antibody response to NP-LPS is mainly of the IgM class, while the response to NP-Ficoll is mainly of the IgM and IgG3 classes. As shown in Figure 4A, B7-H2–/– mice can mount an efficient, if not more enhanced, immune response to NP-LPS a week after the initial immunization. Likewise, the mutant mice also secrete equivalent, if not higher, IgM and IgG3 antibody titers against NP-Ficoll (Figure 4B) compared with the control animals. Thus, B7-H2–/– mice have no defect in their immune responses to T-cell–independent antigens.
Intact T-cell–independent immune responses in B7-H2–/– mice. Wild-type (•) and mutant (○) mice were immunized with (A) NP-LPS for the T-cell–independent type I and (B) NP-Ficoll for the T-cell–independent type II immune responses. Sera were collected one week after immunization, and the presence of NP-specific antibodies was quantified via ELISA. The value for each mouse was plotted, and the dilution of the immune sera is indicated. Preimmune sera were negative for the antigen-specific antibodies and were not shown. Statistical signifi-cance was determined using a paired 2-tailed Student t test, and the P values are indicated. Horizontal bars indicate the mean.
Intact T-cell–independent immune responses in B7-H2–/– mice. Wild-type (•) and mutant (○) mice were immunized with (A) NP-LPS for the T-cell–independent type I and (B) NP-Ficoll for the T-cell–independent type II immune responses. Sera were collected one week after immunization, and the presence of NP-specific antibodies was quantified via ELISA. The value for each mouse was plotted, and the dilution of the immune sera is indicated. Preimmune sera were negative for the antigen-specific antibodies and were not shown. Statistical signifi-cance was determined using a paired 2-tailed Student t test, and the P values are indicated. Horizontal bars indicate the mean.
Impaired T-cell–dependent immune responses in B7-H2–/– mice
To gain better insight into the costimulatory defect in B7-H2–/– mice, we next examined the ability of these mice to respond to a T-cell–dependent antigen. Wild-type and mutant mice were challenged with a high dose of alum-precipitated NP21-CG (100 μg/mouse), and antigen-specific antibodies were measured at various time-points after the primary immunization. As seen in Figure 5A, wild-type and B7-H2–/– mice have comparable level of antigen-specific IgM in their sera 7 days after the initial antigenic challenge. In contrast, the levels of antigen-specific IgG1 and IgG2a were reduced in the mutant mice at this time point.
Impaired secondary and tertiary immune responses to a T-cell–dependent antigen in B7-H2–/– mice. Wild-type (•) and mutant (○) mice were immunized with 100 μg alum-precipitated T-cell–dependent antigen NP-CG for the primary response (A), reboosted 30 days later with 5 μg of the antigen for the secondary response (B), and reboosted 60 days after the initial immunization for the tertiary response (C). Sera were collected from the mice 7 days after the primary immunization, 7 and 14 days after the secondary, and 5 days after the tertiary challenges. NP-specific antibodies of the IgM, IgG1, and IgG2a classes were quantified. The value for each mouse was plotted, and the dilution of the immune sera was indicated. Preimmune sera were negative for the antigen-specific antibodies and were not shown. Statistical significance was established using a paired 2-tailed Student t test, * indicates P < .1 and ** indicates P < .01. The asterisks for the tertiary response are applicable only to the 3 lowest serum dilutions in each graph. Horizontal bars indicate the mean.
Impaired secondary and tertiary immune responses to a T-cell–dependent antigen in B7-H2–/– mice. Wild-type (•) and mutant (○) mice were immunized with 100 μg alum-precipitated T-cell–dependent antigen NP-CG for the primary response (A), reboosted 30 days later with 5 μg of the antigen for the secondary response (B), and reboosted 60 days after the initial immunization for the tertiary response (C). Sera were collected from the mice 7 days after the primary immunization, 7 and 14 days after the secondary, and 5 days after the tertiary challenges. NP-specific antibodies of the IgM, IgG1, and IgG2a classes were quantified. The value for each mouse was plotted, and the dilution of the immune sera was indicated. Preimmune sera were negative for the antigen-specific antibodies and were not shown. Statistical significance was established using a paired 2-tailed Student t test, * indicates P < .1 and ** indicates P < .01. The asterisks for the tertiary response are applicable only to the 3 lowest serum dilutions in each graph. Horizontal bars indicate the mean.
We next examined the ability of B7-H2–/– mice to mount a secondary immune response to the same antigen. Previously immunized wild-type and B7H-2–/– mice were rechallenged 30 days after the initial encounter, and their antigen-specific antibodies were quantified 7 and 14 days after the boost. As shown in Figure 5B, the level of antigen-specific IgM was much higher, while the levels of IgG1 and IgG2a remained greatly reduced in the mutant mice compared with the wild-type controls 7 days after the rechallenge. Interestingly, while the antigen-specific IgM antibodies continued to be greatly enhanced, the antigen-specific IgG1 and IgG2a in the B7-H2–/– mice failed to reach levels comparable to wild-type mice when assessed 14 days after the secondary challenge. This observation suggests that B7-H2 plays an important role in costimulating the recall phase of the immune response, particularly in inducing the production of antigen-specific heavy chain class–switched antibodies. Indeed, as shown in Figure 5C, antigen-specific IgG1 and IgG2a antibodies remained significantly reduced in B7-H2–/– mice during an ongoing tertiary immune response. Similar results were obtained when the mutant mice were immunized with a low dose (5 μg/mouse) of the antigen (data not shown).
Absence of germinal center formation in immunized B7-H2–/– mice
Germinal centers are lymphoid microenvironments that are formed at the height of an ongoing immune response and are critical for the optimization of T-cell–dependent humoral immune responses.32,33 During germinal center reactions, activated B cells undergo antigen-driven changes such as Ig heavy chain class switching and affinity maturation and ultimately leading to the generation of memory B and antibody-producing plasma cells.
To determine if the impairment in the humoral immune response in B7-H2–/– mice can be correlated with defects (if any) in germinal center formation, immunohistological staining of spleen sections, taken at the height of a secondary immune response, were performed. Antigen-specific B cells within germinal centers can be identified by PNA staining.34 As shown in Figure 6A, B-cell follicles can be found in both immunized wild-type and mutant mice. However, no positive PNA staining was observed within the splenic follicles of immunized B7-H2–/– mice, indicating the lack of germinal center formation in these mice. This result is similar to that obtained with immunized CD28–/– mice, which had been reported previously also to be defective in germinal center formation.35 In contrast, distinct germinal centers were observed in the splenic follicles of immunized wild-type mice as depicted by the strong positive staining for PNA.
Impaired germinal center formation in immunized B7-H2–/– mice. (A) Cryosections of spleens from wild-type, CD28–/–, and B7-H2–/– mice were stained with biotin-conjugated PNA and counterstained with 1% hematoxylin to identify germinal center and follicular B cells 8 days after the secondary immunization. Original magnification, × 100. (B) Flow cytometry analysis of PNA-binding germinal center B cells in the spleens of wild-type and B7-H2–/– mice 7 days after the secondary antigenic challenge. Cell sample from similarly immunized CD28–/– mice is included as a control. Numbers indicate the percent of lymphocytes.
Impaired germinal center formation in immunized B7-H2–/– mice. (A) Cryosections of spleens from wild-type, CD28–/–, and B7-H2–/– mice were stained with biotin-conjugated PNA and counterstained with 1% hematoxylin to identify germinal center and follicular B cells 8 days after the secondary immunization. Original magnification, × 100. (B) Flow cytometry analysis of PNA-binding germinal center B cells in the spleens of wild-type and B7-H2–/– mice 7 days after the secondary antigenic challenge. Cell sample from similarly immunized CD28–/– mice is included as a control. Numbers indicate the percent of lymphocytes.
In addition, flow cytometric analysis also showed that there are considerably fewer PNA+ B cells from the spleens of immunized B7-H2–/– and CD28–/– mice as compared with wild-type controls (Figure 6B). Thus, the data indicate that B7-H2 is required for the formation of antigen-specific germinal centers during an ongoing T-cell–dependent immune response.
Normal B-cell responses in B7-H2–/– mice
Since B7-H2 is expressed constitutively on B cells, we next examined if there would be a defect in B-cell activation per se. The proliferation of B cells appeared to be equivalent with or without B7-H2 after anti-IgM or anti-CD40 antibody or LPS stimulation (Figure 7). Since CD28 signaling had been shown to affect ICOS expression on T cells,28 we checked if the absence of B7-H2 could affect the induction of B7-1 and B7-2. Our data indicate that the up-regulation of B7-1 and B7-2 occurred to the same extent on both wild-type and mutant B cells after activation with either anti-IgM or LPS (data not shown), suggesting that there is no compensatory mechanism between the costimulatory molecules. These data indicate that the B cells without B7-H2 are intrinsically normal in their ability to be activated, and this is consistent with the ability of the B7-H2–/– mice to respond to T-cell–independent antigens.
Normal B-cell proliferation in B7-H2–/– mice. Splenic B cells isolated from wild-type (▪) and B7-H2–/– (□) mice were stimulated in vitro with varying concentrations of anti-IgM (left panel), anti-CD40 (middle panel), or LPS (right panel). Proliferation was assessed by [3H]thymidine incorporation after 64 hours. The results represent the average of triplicate wells, and the error bars depict the SDs. Data shown are representative of 2 independent experiments performed.
Normal B-cell proliferation in B7-H2–/– mice. Splenic B cells isolated from wild-type (▪) and B7-H2–/– (□) mice were stimulated in vitro with varying concentrations of anti-IgM (left panel), anti-CD40 (middle panel), or LPS (right panel). Proliferation was assessed by [3H]thymidine incorporation after 64 hours. The results represent the average of triplicate wells, and the error bars depict the SDs. Data shown are representative of 2 independent experiments performed.
Altered pattern of Th1 and Th2 cytokine production in the absence of B7-H2 costimulation
T- and B-cell interaction is obligatory for triggering further B-cell differentiation that leads to Ig class switching, somatic hypermutation, and plasma cell formation. Our analyses so far indicate that B7-H2–/– mice do not generate germinal centers and have a defect in producing heavy chain class–switched antibodies. Since the B cells from mutant mice appear to be intrinsically normal, we then examined if the defect in T-cell–dependent B-cell immune response in B7-H2–/– mice is due to a defect in T-cell–mediated help. T-cell help to B cells can be delivered via both the secretion of cytokines and direct cell-cell contact.
First, to assess the ability of T cells to be activated and up-regulate the expression of activation markers that are required for T cells' cognate interaction with B cells, we individually purified CD4+ T cells from either wild-type or mutant mice and stimulated them with plate-bound anti-CD3 antibody in the presence of equal number of purified syngeneic B cells from either wild-type or mutant animals. Such a stimulation condition can efficiently induce the expression of ICOS on T cells (McAdam et al28 and data not shown). In the wild-type context, the ICOS that is induced on the surface of T cells will then recognize the constitutively expressed B7-H2 on B cells. However, such an interaction will be lacking with B7-H2–deficient B cells. Our flow cytometric analyses revealed that the activated T cells, in the presence or absence of B7-H2 on the accompanying B cells, can up-regulate equivalent levels of CD25, CD40L, CD44, CD69, CD95, and CD95L (data not shown).
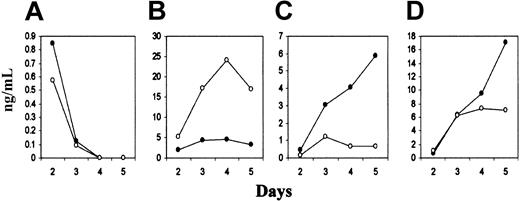
Signals provided by the various costimulatory molecules may be critical for the polarization of Th cell differentiation into the Th1 or Th2 cells that secrete specific sets of cytokines and provide help for cell-mediated or humoral immune responses, respectively.8,13 Thus, we determined the cytokine profiles secreted by activated Th cells from wild-type and B7-H2–/– mice. We isolated CD4+ T cells from the draining lymph nodes of KLH/CFA-immunized wild-type and B7-H2–/– mice and restimulated them in vitro with soluble KLH. A hallmark of T-cell activation is the secretion of IL-2, and this cytokine was produced at similar levels by the activation of previously primed T cells with or without the initial B7-H2 costimulation (Figure 8). However, T cells from B7-H2–/– mice secreted very little IL-4 as compared to that from wild-type mice, and furthermore, they were unable to sustain the production of IL-10 (Figure 8). Similar to that reported by McAdam et al36 for ICOS–/– mice, CD4+ T cells from B7-H2–/– mice produced much more IFN-γ as compared to CD4+ T cells from wild-type animals (Figure 8). Thus, the absence of B7-H2 costimulation appears to result in the specific drop in the production of Th2 cytokines such as IL-4 and IL-10 by activated CD4+ T cells.
Perturbed cytokine production by primed CD4+ T cells in the absence of B7-H2 costimulation. CD4+ T cells from the draining lymph nodes of KLH/CFA-immunized wild-type (•) and B7-H2–/– (○) mice were isolated and cultured in vitro in the presence of soluble KLH for 2 to 5 days. The production of various cytokines was quantified via ELISA using known standards. IL-2 (A), IFN-γ (B), IL-4 (C), and IL-10 (D) were measured from day 2 to day 5 after restimulation. Data shown are representative of 2 independent experiments, each performed with 3 mice per group.
Perturbed cytokine production by primed CD4+ T cells in the absence of B7-H2 costimulation. CD4+ T cells from the draining lymph nodes of KLH/CFA-immunized wild-type (•) and B7-H2–/– (○) mice were isolated and cultured in vitro in the presence of soluble KLH for 2 to 5 days. The production of various cytokines was quantified via ELISA using known standards. IL-2 (A), IFN-γ (B), IL-4 (C), and IL-10 (D) were measured from day 2 to day 5 after restimulation. Data shown are representative of 2 independent experiments, each performed with 3 mice per group.
Discussion
The roles of B7-1 and B7-2, which are B-cell molecules that bind CD28 and are involved in the costimulation of T cells, are well known.5,6 Recently, several other molecules related to B7-1 and B7-2 and possibly involved in lymphocyte costimulation were discovered, and these include B7-H2,14-16 B7-H1/PD-L1,37,38 B7-DC/PD-L2,39 and B7-H3.40 This immediately raises the question of the specific contribution of each of these molecules in the overall program of lymphocyte activation. In an attempt to dissect the roles of these various costimulatory pathways, we have now generated mice deficient in B7-H2, which is constitutively expressed on B cells and has been shown to bind ICOS on activated T cells.14,15,21
Our data indicate that B7-H2–deficient mice have normal T- and B-cell development, even though B7-H2 is expressed in the thymus where T cells develop and is also expressed constitutively on B lymphocytes14-16 in wild-type mice. These mice, however, have lower basal levels of IgA, IgG1, IgG2a, and IgG2b antibodies in their sera. Upon immunization with a T-cell–dependent antigen, they exhibit a severe defect in the phases of humoral immune responses that require heavy chain class–switched antibodies. Furthermore, B7-H2–deficient mice have a defect in the generation of germinal centers during an ongoing immune response. The defects in humoral immune response were seen in mutant mice that were challenged with both a low and high dose of the antigen, suggesting that B7-H2–ICOS interaction is not involved in optimizing the response in situations of low antigen concentrations.
The production of antigen-specific IgM antibodies during primary and secondary immune responses was not reduced by the lack of B7-H2 expression, indicating that intrinsic B-cell responsiveness is not dependent on the expression of B7-H2. This is similar to the findings in B7-1/2–deficient mice.12 The absence of B7-H2 also does not appear to affect the proliferation of splenic B cells in response to anti-IgM or anti-CD40 antibody or LPS stimulation in vitro. Finally, B7-H2–/– mice could mount an immune response against T-independent antigens to the same extent, if not better than wild-type mice, and were perfectly competent in generating antigen-specific IgG3 antibodies toward NP-Ficoll, further suggesting that the B cells from these mutant mice are intrinsically normal in their functions. At this point, we do not understand why the B7-H2–/– mice appear to respond better than wild-type mice to T-independent antigens. However, a similar phenomenon is also observed with ICOS–/– mice.23
In both our B7-H2–/– mice and ICOS–/– mice,23 repeated immunization with protein antigens cannot elevate the low levels of production of antigen-specific IgG1 and IgG2a antibodies. This indicates that the B7-H2–ICOS costimulatory pathway may play an essential role in the recall phase of the immune response; in particular, in the induction of heavy chain class–switched antibodies. These data appear to be consistent with the in vivo B7-H2–ICOS blockage experiments carried out by several groups. For instance, it was shown that ICOS-Ig treatment could suppress the secondary phase of a T-cell–dependent immune response.24 Furthermore, blockage of ICOS signals during the effector function phase of an allergic lung inflammation model was more efficient at inhibiting airway hyper-responsiveness than when administered at the time of antigen priming.41 In yet another disease model of contact hypersensitivity, B7-H2-Ig exacerbated the disease when given specifically at the time of rechallenge.15,42 Thus, these data together with ours on the B7-H2–/– mice indicate that B7-H2 and ICOS play important roles in the secondary phase of an immune response.
The formation of germinal centers is the anatomic hallmark of Th cell activity33 during a T-cell–dependent B-cell immune response. Activated T cells provide help to B cells through the secretion of cytokines and the engagement of cell-associated molecules.43,44 The defect in germinal center formation in B7-H2–/– mice correlated well with the impairment in the recall phase of the humoral immune responses in these mice. A defect in germinal center formation also was reported for ICOS–/– mice.23,36,45 It is noted that the anatomical localization of B, T, and follicular dendritic cells was normal in immunized ICOS–/– mice.36,45 Thus, the defect in germinal center formation in both B7-H2–/– and ICOS–/– mice is likely due to the inability of T cells to provide help to the B cells in the follicles, and because of that, the mutant B cells are poorly stimulated in the T-cell–rich regions of the secondary lymphoid organs and are unable to migrate to form germinal centers.
Besides B7-H2 and ICOS, other costimulatory molecules such as B7-1/2–CD2835 and CD40-CD40L46 also are critical for the formation of germinal centers. Our preliminary results indicate that the up-regulation of B7-1 and B7-2 occurred to the same extent on wild-type and B7-H2–deficient B cells after activation (data not shown). Furthermore, T cells in B7-H2–deficient mice also express normal levels of CD28 and can be induced to express CD40L (data not shown). Thus, it seems that B7-1/2–CD28 and CD40-CD154 (CD40L) interactions are not by themselves sufficient for germinal centers to form and that B7-H2–ICOS interaction between B and T cells also is required and necessary.
The importance of B7-H2–ICOS interaction in T-cell–dependent isotype switching and recall antibody response is further highlighted by the defect in isotype switching to antigen-specific IgG1 and IgG2a antibodies in both B7-H2–/– and ICOS–/–23,36 mice that were challenged with a T-cell–dependent protein antigen. B-cell antibody isotype class switching is modulated by cytokines, for example, IL-4 and IL-10, which can stimulate the production of IgG1.47,48 Our data indicate that the production of IL-4 was profoundly reduced, while that of IL-10 could not be sustained in B7-H2–/– mice that were challenged with a T-dependent antigen. This would possibly explain the lack of antigen-specific IgG1 antibodies in the immunized mutant mice.
Various reports had indicated that ICOS engagement in vitro can enhance the production of IL-4, IL-5, and IL-10.21,28 It had been shown that the in vitro blockage of ICOS could inhibit the secretion of IL-4 and IL-10.28 In contrast, the in vitro stimulation of CD4+ ICOS–/– T cells had led to the normal production of IL-10,45 although little IL-423,45 was being produced. Our data, showing a reduction in IL-4 production in B7-H2–deficient mice, are consistent with a role for B7-H2–ICOS interaction in the secretion of this cytokine. In addition, our data appear to reconcile the differences in the production of IL-10 reported by other groups.28,45 Our data indicate that IL-10 can be produced in the absence of B7-H2–ICOS interaction but could not be sustained. Thus, the discrepancy in the IL-10 data could be attributed to the time point at which the cytokine was assayed, since we only observed a reduction in IL-10 secretion 3 days after the cells were restimulated.
Similar to that reported by others,23,36 the secretion of Th1 cytokines, that is, IL-2 and IFN-γ, were not compromised in the absence of B7-H2–ICOS costimulation. In fact, an increase in the level of IFN-γ production was observed in our B7-H2–/– mice. Similarly, ICOS-deficient mice also exhibited a higher secretion of IFN-γ.36 This may well be explained by the use of CFA as an adjuvant in the immunization of mice in both cases. CFA contains bacterial cell wall products that promote stronger innate immune activation, hence primarily enhancing a Th1 response.49 In addition, B7-H2–ICOS interaction is generally more effective in costimulating Th2 responses,24,28 and the absence of B7-H2 may alter the polarization of T cells leading to the selective increase in the generation of Th1 cells.
Our data showed that the B7-H2–deficient mice have similarities to ICOS-deficient mice.23,36,45 Both mutant mice have a decreased basal level of the different immunoglobulin isotypes in their sera.23,36 Furthermore, both mutant mice have defects in germinal center formation and in the generation of efficient humoral immune responses.23,36 Although it is not known at present if B7-H2 interaction with ICOS is unique, our finding indicates that their mutual interaction does play an important and nonredundant role in mediating CD4+ T-cell and B-cell functions.15,21,24 It will be of interest to examine the signaling pathways activated by the engagement of B7-H2–ICOS since they play an important role in priming the recall phase of the immune response, and how these signals differ from those activated by B7-1/2–CD28 engagement. Furthermore, it will be of medical relevance to determine if blockage of B7-H2–ICOS interaction may be therapeutic in autoimmune diseases characterized by high titers of Ig heavy chain class–switched antibodies such as in lupus.
Finally, given the complex interplay of the various costimulatory molecules, the availability of B7-H2–/– mice will greatly facilitate the generation and study of mice lacking both the B7-H2–ICOS and B7-1/2–CD28 costimulation pathways. This is especially so since ICOS, CD28, and CTLA-4 are closely linked on the same chromosome.50,51 Hence, the generation of double-deficient mutant mice simply by crossing the single mutants would not be possible. However, by crossing B7-H2–/– mice with either B7-1/2–/– or CD28–/– mice, this problem can be circumvented.
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2002-08-2416.
Supported by grants from the Biomedical Research Council (BMRC) of the Agency for Science, Technology and Research (A*STAR).
S.-C.W. and E.O. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Esther Wong for blastocyst injection, the IMCB In Vivo Model Unit for the care and maintenance of mice, Weng-Keong Chew for technical assistance, and Guo-ke and Li Jie for their help with immunohistological staining. We also thank Dr Le-Ann Hwang for her helpful discussion and technical advice on the cytokine secretion assay, and Professor Thomas Waldschmidt for critical reading of the manuscript.






![Figure 7. Normal B-cell proliferation in B7-H2–/– mice. Splenic B cells isolated from wild-type (▪) and B7-H2–/– (□) mice were stimulated in vitro with varying concentrations of anti-IgM (left panel), anti-CD40 (middle panel), or LPS (right panel). Proliferation was assessed by [3H]thymidine incorporation after 64 hours. The results represent the average of triplicate wells, and the error bars depict the SDs. Data shown are representative of 2 independent experiments performed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/4/10.1182_blood-2002-08-2416/6/m_h81634758007.jpeg?Expires=1765931158&Signature=zp-teI0idM0poxq7bA39BSfk5p15xSq4NulG99JOxq4vMOFjZeZ2e9wClWKxTKLc2lFZaj~EHStpGoJM7eO3uUM0qA3QGOMD4Oni33BDwdbL5i-kLqrbhsU8fJbWcX-L7lVLIPXztmH8sIYNZOYItvqeK-DM~UATK74S4Q8W5KpZBwwUFrYkVbtA513zLbj8BhfiEQyUn4A1IvDEpRC86~9oIfOGSvGxbEyv7siWQykLCKrSaEWZeBTfnpIJ-sojSEhHCyWMmAn4uG4NYSeLRNWQTDA3nCIuHiucmec~h4tCW7DsldPd2cq84qh3QiPr6dasyrcl0ftT-WPm2ASXpw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal