Abstract
On encountering a danger signal, dendritic cells (DCs) undergo a complex maturation process and become specialized in antigen presentation. We previously reported that engagement of major histocompatibility complex (MHC) class II molecules located on immature DCs in membrane rafts by lymphocyte activation gene-3 (LAG-3; CD223) leads to DC maturation. In contrast, exposure of DCs to class II–specific monoclonal antibodies (mAbs) did not lead to maturation. Here, we have investigated the signal transduction pathways involved in the LAG-3–induced maturation of human monocyte-derived DCs. We first show that areas of raft aggregation (both cholesterol rich and CDw78 microdomains) could be visualized using a soluble LAG-3 protein and confocal microscopy. Engagement of class II molecules by both its natural ligand LAG-3 and class II mAb induces rapid protein phosphorylation of phospholipase Cγ2 (PLCγ2) and p72syk as well as activation of phosphatidyl inositol 3-kinase/Akt, p42/44 extracellular signal-regulated protein kinase, and p38 mitogen-activated protein kinase pathways. Studies using inhibitors demonstrate that these 3 pathways are all important in inducing the maturation process of LAG-3–stimulated DCs. When class II molecules were ligated with LAG-3 versus specific antibody, differences in the phosphorylation pattern of c-Akt were observed. Thus, MHC class II signaling in DCs involves several pathways that have to be finely regulated to lead to cell activation and maturation.
Introduction
Dendritic cells (DCs) are considered to be the most powerful antigen-presenting cells (APCs) because these are the only cells that are able to induce primary immune responses. DCs reside in tissues as immature sentinel cells and have a high capacity for antigen processing.1 Following uptake of antigen in the context of a “danger” signal, DCs migrate to stimulate antigen-specific T cells in the regional lymph node.2,3 Antigen presentation by DCs activates helper T cells to express CD40 ligand (CD40L), which in turn activates CD40+ DCs.4 Similarly, we have provided some evidence that lymphocyte activation gene-3 (LAG-3; CD223),5-7 an activation antigen expressed on activated T cells in human tissues,8,9 could activate and mature APCs through its specific binding to major histocompatibility complex (MHC) class II (class II) molecules expressed on immature DCs.10,11
Class II molecules are expressed at high levels on the surface of APCs and play an important role as signal transducing receptors.12-14 Multimerization of class II molecules following engagement with antibodies has been shown to mediate proliferation and/or differentiation as well as apoptosis.15-17 It has been proposed that class II-induced apoptosis can be prevented by CD40 counter-signaling.18 However, all these studies on class II signaling have been performed using specific monoclonal antibody (mAb), and the data may not be relevant when one considers the engagement of class II molecules by a natural ligand. Indeed, we have recently reported that a soluble LAG-3 protein, in contrast to class II mAb,17 does not induce apoptosis of immature human DCs but induces the activation/maturation of these cells,9-11 and like sCD40L, the secretion of the macrophage-derived chemokine (MDC)/CCL22 and thymus and activation-regulated chemokine (TARC)/CCL17 known to direct the migration of maturing DCs to lymph nodes.19 This soluble LAG-3Ig fusion protein also directly stimulates human monocytes, inducing tumor necrosis factor α (TNF-α) production and enhancing interleukin 12 (IL-12) production when these cells were stimulated with infra-optimal concentrations of sCD40L.10
In the myelomonocytic THP-1 cells, the ability of class II to activate protein tyrosine kinases (PTKs) is dependent on its association with membrane rafts.20 Class II recruitment to membrane rafts may result in its localization to specific microdomains in the plasma membrane that are enriched in Src family PTKs.20 These rafts presumably contain one or more intermediate transducer proteins that mediate class II signal transduction. In human T cells, both LAG-3 and class II molecules have been shown to be present in raft microdomains before engagement of the T-cell receptor (TCR) by specific mAb or peptide/MHC complexes.21 In B cells, class II molecules were found to be constitutively present in rafts, and this concentration of class II molecules facilitates antigen presentation.22 In DCs, the functional effect of LAG-3 may be dependent on the presence of class II molecules in rafts, as disrupting the raft structure inhibits the binding of LAG-3 to these cells.11 Thus, the transactivation of raft-associated protein-tyrosine kinases leading to the initiation of intracellular signaling cascades is probably facilitated by the aggregation of rafts. However, the mechanism whereby the ligation of class II molecules initiates phosphotyrosine induction is currently unclear, as the cytoplasmic tails of the class II molecules do not associate with any detectable tyrosine kinases.
Despite its pivotal role in DC function, little is known about the transduction machinery involved in DC maturation. Lipopolysaccharide (LPS) has been shown to activate multiple signaling pathways in immature human DCs with the phosphorylation of Akt, a downstream target of phosphatidyl inositol 3-kinase (PI3 kinase), p42/44 extracellular signal-regulated protein kinase (p42/44ERK), and p38 mitogen-activated protein kinase (p38MAPK).23 With the use of specific inhibitors, researchers have shown these different pathways to play an important role in regulating various aspects of LPS-induced DC maturation.23 Here, we present evidence that engagement of class II signaling by its natural ligand LAG-3 induces rapid protein phosphorylation of PLCγ2 and p72syk as well as activation of PI3 kinase/Akt, p42/44ERK, and p38MAPK. The latter kinases were shown to be implicated in the LAG-3–induced maturation process of DCs. Substantial differences were found when class II molecules were ligated with LAG-3 versus specific antibody.
Materials and methods
Reagents
Recombinant soluble human LAG-3Ig molecules were generated by fusing the extracellular domain of hLAG-3 to a human immunoglobulin G1 (IgG1) Fc portion.24 The resulting recombinant protein was produced in Chinese hamster ovary cells and purified as described24 (Dr M. Subramanyam and M. Tepper, Ares Advanced Technology, Randolph, MA). Preparations contained less than 1 endotoxin unit (EU)/mg as determined by the limulus amoebocyte lysate assay (Biowhittaker, Walkersville, MD).
The 17B4 mAb specific for the LAG-3.1 extracellular domain epitope (the extra-loop of immunoglobulin-like domain 1) has been previously described.7
The specific inhibitors SB203580 (p38MAPK), PD98059 (ERK), LY294002 (PI3 kinase), herbimycin A (tyrosine kinases), and piceatannol (p72syk), all from Calbiochem (Nottingham, United Kingdom), were dissolved in dimethyl sulfoxide (DMSO) and used over a range of concentrations on both LPS- and LAG-3Ig–induced DC maturation to control for the toxicity of the drugs. A 0.1% (vol/vol) concentration of DMSO was used as a negative control where indicated.
Purification of human monocytes and generation of monocyte-derived DCs
Human peripheral blood mononuclear cells (PBMCs) were isolated from venous blood of voluntary healthy donors by Ficoll-Paque density gradient centrifugation (Pharmacia, Uppsala, Sweden). Monocytes were enriched by aggregation in the cold at a concentration of 50 × 106 cells/mL in complete culture medium (1640 RPMI medium supplemented with 10% fetal calf serum [GIBCO, Paisley, Scotland], 2 mM glutamine, and 1 mM pyruvate) for 40 minutes under rotation. The aggregates were separated by sedimentation through 1 mL fetal calf serum and depleted of T cells by rosetting on 2-aminoethylisothiouronium bromide- (AET; Sigma, St Louis, MO) treated sheep red blood cells (SRBCs; BioMerieux, Marcy l'Etoile, France). For this treatment, 2.5 mL SRBCs were incubated with 30 mL 5% AET (wt/vol) for 15 minutes at 37°C, thoroughly washed, and resuspended in 17.5 mL complete culture medium. Enriched monocytes were then resuspended at 3 × 106 cells/mL with 10% of the SRBC suspension and centrifuged on Ficoll-Paque for 25 minutes at 500 rpm and for 20 minutes at 2000 rpm to separate the monocyte fraction from SRBCs and bound T cells. The resulting preparations were consistently more than 90% CD14+ as determined by fluorescence-activated cell sorter (FACS; Elite; Coulter, Miami, FL).
To prepare human immature DCs, the purified monocytes were incubated in 6-well culture plates (5 × 106 cells/3 mL per well) in serum-free RPMI 1640 for 1 hour in a humidified incubator at 37°C and 5% CO2. Nonadherent cells were removed, and adherent cells were cultured in 3 mL/well complete culture medium supplemented with 100 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Novartis, Rueil-Malmaison, France) and 50 ng/mL IL-4 (R&D, Minneapolis, MN). On day 2 and day 4, two-thirds culture medium was replaced by fresh medium containing GM-CSF and IL-4, and the nonadherent cells were harvested on day 6.
For the induction of maturation, immature DCs were resuspended at 1 × 106 cells/mL in complete culture medium with cytokines containing either no stimulus, human IgG1 (10 μg/mL; Chemikon, Temecula, CA), or hLAG-3Ig (10 μg/mL). After 24 hours of culture, cells were harvested and analyzed. When inhibitors were used, cells were incubated for 2 hours at 37°C with the respective inhibitor prior to the addition of the maturation stimulus.
For immunoprecipitation experiments, monocyte-derived DCs purified from PBMCs after a 7-day culture with 50 ng/mL IL-13 and 50 ng/mL GM-CSF were kindly provided by Dr P. Abastado (IDM, Paris, France).
Confocal microscopy
Immature DCs (4 × 105) were incubated for 1 hour at 37°C on coverslips coated with poly-L-Lysine (Sigma Aldrich, Poole, United Kingdom). After 30 minutes of saturation at 37°C with phosphate-buffered saline (PBS)–dry milk 3%, cells were incubated 15 minutes at 4°C for temperature equilibration. All of the following incubations were done at 4°CinPBS–dry milk 3% and washed with cold PBS. Cells were first incubated 30 minutes with LAG-3Ig (30 μg/mL), washed, and incubated 30 minutes in the dark with Alexa Fluor 488 goat antihuman IgG (8 μg/mL; Molecular Probes, Leiden, The Netherlands). Cells were then incubated 30 minutes in the dark with a pan anti–MHC II (I3; 10 μg/mL; Coulter) or CDw78 mAb (FN1; 10 μg/mL; Pharmingen, San Diego, CA), washed, and incubated 30 minutes in the dark with Alexa Fluor 594 goat antimouse IgG (4 μg/mL; Molecular Probes). Cells were then fixed with 1% paraformaldehyde for 30 minutes at 4°C and mounted using Fluoromount G (Southern Biotechnology Associates, Birmingham, AL). For the methyl-β-cyclodextrin (MCD) treatment, cells were incubated 20 minutes at 37°C in RPMI containing 10 mM MCD and washed once with PBS prior adherence on coverslips. Confocal analysis was conducted using a confocal laser scanning microscope (LSM 510 equipped with an air-cooled argon ion laser 488 nm and a helium neon laser 543 nm; Zeiss, Le Pecq, France) configured with an Axiovert 100 M microscope using a Plan Apochromat × 63/1.40 oil objective.
Cytofluorometric analysis
To assess the purity and phenotype of cell preparations, mAbs specific for CD1a, CD3, CD11c, CD14, CD16, CD19, CD54, CD83, MHC I (W6/32), MHC II (I3, IgG2a) (all Coulter), CD32, CD40, CD64, CD80, CD86 (all Pharmingen), and isotype-matched negative controls (Coulter) were used. Cells were incubated with the respective antibody at 10 μg/mL for 30 minutes at 4°C in PBS 1% bovine serum albumin (BSA) and then stained for 30 minutes at 4°C with fluorescein isothiocyanate (FITC)–labeled goat antimouse (GAM) F(ab)′2 (Coulter). Stained cells were analyzed by FACS using an Epics Elite cytometer (Coulter).
Assessment of antigen uptake
For equilibration, 0.5 × 106 DCs were incubated at 1 × 106 cells/mL in complete culture medium for 15 minutes at 4°C or 37°C. FITC-labeled BSA (Sigma) was added at a final concentration of 50 μg/mL, and the cells were incubated for another 30 minutes to allow capture of the antigen. After thorough washing of the cells with cold medium, fluorescence was measured by FACS analysis. Fluorescence in this assay is indicative of BSA uptake.
Cytokine measurement
Culture supernatants were collected at 24 hours and stored at –80°C. Commercially available enzyme-linked immunosorbent assay (ELISA) kits were used according to the manufacturer's instructions to detect IL-12 p40 and TNF-α in DC culture (R&D).
Western blot
After equilibration at 37°C, cells were stimulated with human IgG1, human LAG-3Ig, or a pan class II–specific antibody (I3; IgG2a) for 3 minutes at 37°C following 2 minutes cross-linking with a secondary antibody. After stimulation, cells were rapidly pelleted and lysed at 4°C for 30 minutes at 1 × 107/100 μL in cell lysis buffer containing proteases (20 mM Tris (tris(hydroxymethyl)aminomethane)–HCl pH 7.5, 140 mM NaCl, 1 mM EDTA (ethylenediaminetetraacetic acid), 1% NP-40, 1 mM sodium orthovanadate, 1 μg/mL aprotinin, 1 mM phenylmethylsulfonyl fluoride [PMSF]). Cell debris were removed by 10 minutes of centrifugation at 10 000g and 4°C. An equal volume of 2 × sample loading buffer was added to the supernatants, and the proteins were denatured for 5 minutes at 95°C with 5% β-mercaptoethanol. Total cell lysates per well (75 μg) were separated on a 12% discontinuous sodium dodecyl sulfate (SDS) polyacrylamide gel and transferred to nitrocellulose membranes.
For detection with antibodies specific for the tyrosine-phosphorylated proteins (anti-pTyr 4G10; Upstate Biotechnology, Lake Placid, NY), p72syk (Santa Cruz Biotechnology, Santa Cruz, CA), or PLCγ1 (Upstate Biotechnology), membranes were saturated with 3% gelatin in 200 mM Tris-HCl, pH 7.6, 1.37 M NaCl (Tris-buffered saline [TBS]) for 1 hour at 37°C. The membranes were then incubated in primary and secondary antibody dilutions in 1% gelatin-TBS for 1.5 hours each with slow agitation. For the anti-PLCγ2 antibody (Santa Cruz Biotechnology), 5% dry milk in TBS was used as blocking and antibody dilution buffer. After rigorous washing in TBS 0.1% Tween 20, the signal was detected by enhanced chemiluminescence (ECL; Amersham, Buckinghamshire, United Kingdom). Antibodies directed against phospho-p38 and total p38, phospho-ERK and total ERK, and phospho–c-Akt and total c-Akt (all from New England Biolabs, Hitchin, United Kingdom) were used according to the manufacturer's instructions.
Immunoprecipitation
Cell lysates were precleared for 2 hours at 4°C with 10 μg/mL isotype plus species-matched nonspecific antibody and 20 μL protein A–coupled Sepharose beads (Pharmacia). Precleared lysates were immunoprecipitated by incubation with specific antibody at 10 μg/mL for 2 hours, followed by overnight incubation with protein A–coupled Sepharose beads under gentle agitation. For immunoprecipitation of p72syk, agarose-conjugated antibody (Santa Cruz) was used. Immunoprecipitates were washed 3 times with cell lysis buffer containing antiproteases and dissolved in SDS sample buffer.
Statistics
Data were analyzed by the nonparametric Mann-Whitney U rank test, and differences with P < .05 were considered statistically significant.
Results
LAG-3 and a class II–specific mAb differ in their association to class II molecules expressed on monocyte-derived human DCs
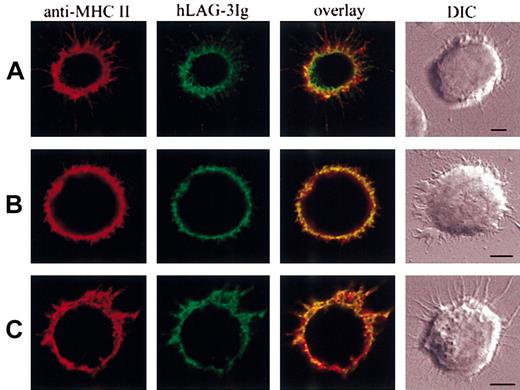
We used immunofluorescence microscopy to examine the relative location of the subset of class II molecules recognized by LAG-3Ig on immature DCs. Cytometric analysis has previously revealed that LAG-3Ig bound to only a fraction of class II molecules on DCs (but not on B cells) and that this binding was dramatically reduced by methyl-β-cyclodextrin (MCD), a compound that disrupts protein association with lipid rafts by extracting cholesterol from the plasma membrane.11 Clear-cut differences in LAG-3Ig and class II–specific mAb labeling were observed by confocal microscopy that were consistent with the idea that only class II molecules in areas of raft aggregation are labeled by LAG-3Ig. Figure 1A shows examples of these structures on one cell that was chosen, as it already had dendritic projections: areas of raft aggregation labeled preferentially by LAG-3Ig are visualized on both the cell membrane and the dendrites. We could not visualize raft structures with the GM1-binding cholera toxin B (CTB) subunit because very little CTB-FITC labeling of immature DCs was detected by cytometric analysis compared with B or T cells. When immature DCs were treated with 10 mM MCD prior to the addition of LAG-3, there was some reduction of the size and the number of areas of raft aggregation, with a more pronounced coclustering between LAG-3Ig and the pan class II–specific I3 mAb (overlay in Figure 1B). Of interest, there was also quite a significant colocalization of the subset of class II molecules recognized by LAG-3Ig with the CDw78 determinant25 expressed by clusters of dimerized or oligomerized class II molecules located in tetraspan microdomains26 (Figure 1C). Overall, these experiments performed on live cells (paraformaldehyde [PFA] was added after labeling) suggest the unique binding properties of LAG-3 on a subset of class II molecules is due to its preferential binding to both class II molecules present in cholesterol-rich lipid rafts and class II molecules located in CDw78 tetraspan microdomains.
Different binding patterns of LAG-3 and class II-specific mAb on human immature DC. (A,C) Immature DCs were incubated on poly-l-lysine–coated surfaces saturated with PBS–dry milk 3% at 37°C. After 15 minutes of temperature equilibration at 4°C, cells were labeled with LAG-3Ig in green and a class II-specific mAb in red (I3 in panel A, CDw78 in panel C). Bound LAG-3Ig and I3 or CDw78 were revealed using Alexa 488– and Alexa 594–conjugated secondary antibodies, respectively. (B) Cells were pretreated with 10 mM MCD prior to adherence and labeling. Scale bars, 5 μm. DIC indicates differential interferential contrast. The pictures are representative of the results obtained in at least 3 different experiments.
Different binding patterns of LAG-3 and class II-specific mAb on human immature DC. (A,C) Immature DCs were incubated on poly-l-lysine–coated surfaces saturated with PBS–dry milk 3% at 37°C. After 15 minutes of temperature equilibration at 4°C, cells were labeled with LAG-3Ig in green and a class II-specific mAb in red (I3 in panel A, CDw78 in panel C). Bound LAG-3Ig and I3 or CDw78 were revealed using Alexa 488– and Alexa 594–conjugated secondary antibodies, respectively. (B) Cells were pretreated with 10 mM MCD prior to adherence and labeling. Scale bars, 5 μm. DIC indicates differential interferential contrast. The pictures are representative of the results obtained in at least 3 different experiments.
Cross-linking of class II molecules on human dendritic cells induces protein tyrosine phosphorylation
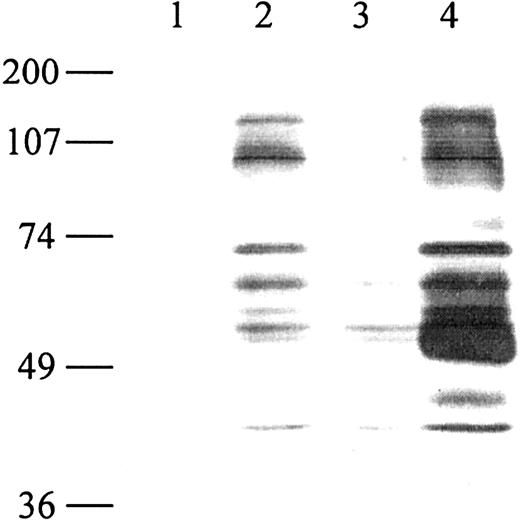
We have previously shown that the incubation of human immature monocyte-derived DCs with the soluble class II ligand LAG-3Ig induces characteristics of cell maturation such as the up-regulation of cell surface molecules, down-regulation of antigen capture, cytokine secretion, and strong T-cell allostimulatory capacities.11 LAG-3Ig, but not an anti–class II Ab (I3 mAb), induces phenotypic maturation (induction of CD83 expression) of immature DCs, whereas simultaneous engagement of class II molecules with LAG-3 and I3 mAb did not change the DC phenotype compared with the use of LAG-3 alone.11 Also, DCs already induced to mature for 24 hours with LPS or CD40L were not affected by the addition of hLAG-3Ig for another 24 hours (data not shown). To gain insight into the molecular mechanism involved in the induction of DC maturation by LAG-3, we have investigated the signaling pathways activated after LAG-3–induced engagement of class II molecules. One of the earliest signaling events after receptor ligation is the tyrosine phosphorylation of intracellular protein kinases. We, therefore, analyzed total cell lysates for the induction of tyrosine phosphorylation after signaling via class II molecules. Class II molecules were ligated to either a human LAG-3Ig or an anti–MHC II antibody, and cellular lysates were analyzed by immunoblotting with anti-pTyr (4G10 mAb). Phosphorylation was observed only in conditions in which the signal was amplified by adding a secondary goat antibody. Cross-linking of human IgG1 induced weak phosphorylation (Figure 2). This induction may be due to Fc receptor–mediated signaling induced by IgG1/GAH immune complexes. Note that Fc receptor signaling has not been found to have any major influence on the maturation effect of LAG-3Ig (used alone without any secondary antibody) on DCs.11 Cross-linking of surface class II molecules with the I3 antibody resulted in the tyrosine phosphorylation of several cellular protein substrates, including proteins with apparent molecular weights of 70, 100, and 130 kd. The signal revealed at 50 kd after antibody cross-linking of class II molecules probably corresponds to the detection of the heavy chain of the mouse anti–class II antibody used for stimulation by the secondary GAM coupled to horseradish peroxidase (HRP). A very similar pattern including the 70-, 100-, and 130-kd proteins was obtained by engagement of class II molecules with LAG-3 plus a secondary goat antibody (Figure 2).
Cross-linking of class II molecules on human dendritic cells induces tyrosine phosphorylation. Monocyte-derived immature DCs (5 × 106) were stimulated at 37°C with medium (lane 1), human LAG-3Ig (lane 2), human IgG1 (lane 3), or I3 (pan class II–specific mAb, lane 4) at 10 μg/mL for 3 minutes followed by cross-linking with a secondary goat antibody directed against human (lanes 2-3) or mouse (lane 4) immunoglobulins at 20 μg/mL for 2 minutes. The cells were lysed, and 75 μg of total cell lysate was loaded per well. Tyrosine phosphorylation of proteins was detected by Western blotting with an antibody specific for phosphotyrosine (anti-pTyr 4G10). The positions of the molecular weight markers are indicated. Lane 1 shows the unstimulated control. Results are representative of 6 experiments performed on different DC samples.
Cross-linking of class II molecules on human dendritic cells induces tyrosine phosphorylation. Monocyte-derived immature DCs (5 × 106) were stimulated at 37°C with medium (lane 1), human LAG-3Ig (lane 2), human IgG1 (lane 3), or I3 (pan class II–specific mAb, lane 4) at 10 μg/mL for 3 minutes followed by cross-linking with a secondary goat antibody directed against human (lanes 2-3) or mouse (lane 4) immunoglobulins at 20 μg/mL for 2 minutes. The cells were lysed, and 75 μg of total cell lysate was loaded per well. Tyrosine phosphorylation of proteins was detected by Western blotting with an antibody specific for phosphotyrosine (anti-pTyr 4G10). The positions of the molecular weight markers are indicated. Lane 1 shows the unstimulated control. Results are representative of 6 experiments performed on different DC samples.
Cross-linking of class II molecules induces phosphorylation of PLCγ2 (but not PLCγ1) and p72syk
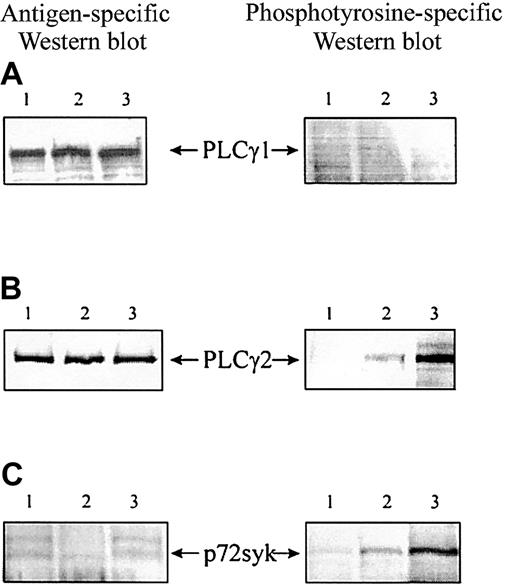
As one of the major phosphorylated substrates is a 130-kd species, we considered the possibility that the identity of this protein may be a phospholipase-Cγ (PLCγ), known to be induced after HLA-DR cross-linking in T cells.27 We, therefore, immunoprecipitated PLCγ1 and 2 using specific antibodies and analyzed their phosphorylation by Western blotting. To obtain the large number of monocyte-derived DCs needed for immunoprecipitation experiments, the cells were cultured slightly differently (described in “Materials and methods”) but showed the same profile of tyrosine phosphorylation after class II cross-linking by LAG-3 or I3 mAb (data not shown). Both lipases, PLCγ1 and 2, are expressed in human monocyte-derived DCs (Figure 3A-B). However, only the PLCγ2 isoform was activated by phosphorylation after class II cross-linking (Figure 3B). Furthermore, immunoprecipitation of p72syk revealed induction of phosphorylation after class II ligation (Figure 3C). In both cases, the degree of phosphorylation was stronger when class II molecules were cross-linked with antibody compared with the natural ligand LAG-3. This finding may be due to higher affinity binding of the antibody or to a different binding site, inducing different conformational changes of the MHC molecules. Following densitometric analysis performed on 3 experiments (different DC samples), LAG-3–induced phosphorylation of both PLCγ2 and p72syk was found to be significant (P < .05). Together, these results indicate that PLCγ2 and the protein kinase p72syk are involved in LAG-3–induced class II signaling.
Cross-linking of MHC class II molecules on human dendritic cells induces phosphorylation of the PLCγ2 and p72syk but not PLCγ1. (A) PLCγ1. (B) PLCγ2. (C) p72syk. After stimulation as described in the legend to Figure 2, DC lysates were precleared for 2 hours and immunoprecipitated by incubation with specific antibody at 10 μg/mL. Tyrosine phosphorylation was analyzed by Western blotting of heat-denatured immunoprecipitates with an antibody specific for phosphotyrosine (4G10). Each membrane was rehybridized with antigen-specific antibody to ensure equal loading. IgG1 (lane 1), human LAG-3Ig (lane 2), I3 (pan class II–specific mAb, lane 3). Results are representative of 3 experiments performed on different DC samples.
Cross-linking of MHC class II molecules on human dendritic cells induces phosphorylation of the PLCγ2 and p72syk but not PLCγ1. (A) PLCγ1. (B) PLCγ2. (C) p72syk. After stimulation as described in the legend to Figure 2, DC lysates were precleared for 2 hours and immunoprecipitated by incubation with specific antibody at 10 μg/mL. Tyrosine phosphorylation was analyzed by Western blotting of heat-denatured immunoprecipitates with an antibody specific for phosphotyrosine (4G10). Each membrane was rehybridized with antigen-specific antibody to ensure equal loading. IgG1 (lane 1), human LAG-3Ig (lane 2), I3 (pan class II–specific mAb, lane 3). Results are representative of 3 experiments performed on different DC samples.
Inhibition of LAG-3–induced phenotypic maturation of human DCs by the p72syk inhibitor piceatannol
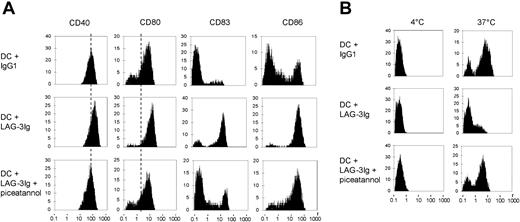
We next examined whether the activation of the protein kinase p72syk is necessary for LAG-3–induced maturation of DCs in the absence of secondary cross-linking. Immature DCs were treated with 10 μg/mL IgG1 or LAG-3Ig, or preincubated for 2 hours with 100 μM piceatannol prior to LAG-3–induced maturation (Figure 4A). As expected,11 LAG-3 but not I3 mAb (not shown) induced the up-regulation of the cell surface receptors CD40, CD80, and CD86 and the expression of the maturation marker CD83. Preincubation with piceatannol inhibited this maturation effect of LAG-3, whereas viability of the cells was not affected (not shown). Therefore, the activation of the kinase p72syk by LAG-3 may play a central role in the transduction of a maturation signal in DCs.
The p72syk inhibitor piceatannol inhibits LAG-3–induced up-regulation of DC surface molecules and LAG-3–induced down-regulation of antigen uptake by DCs. Peripheral blood monocytes were differentiated into immature DCs with GM-CSF and IL-4 for 7 days. Cells were then treated in the presence of GM-CSF and IL-4 with 10 μg/mL IgG1 or LAG-3Ig or preincubated for 2 hours with the p72syk inhibitor piceatannol prior to treatment with LAG-3Ig. After 24 hours of incubation, surface expression of the indicated markers was analyzed by flow cytometry (A). Cells were incubated with BSA-FITC for 30 minutes at 4°C or 37°C, and the BSA uptake was determined by FACS analysis (B). Results are representative of 3 independent experiments.
The p72syk inhibitor piceatannol inhibits LAG-3–induced up-regulation of DC surface molecules and LAG-3–induced down-regulation of antigen uptake by DCs. Peripheral blood monocytes were differentiated into immature DCs with GM-CSF and IL-4 for 7 days. Cells were then treated in the presence of GM-CSF and IL-4 with 10 μg/mL IgG1 or LAG-3Ig or preincubated for 2 hours with the p72syk inhibitor piceatannol prior to treatment with LAG-3Ig. After 24 hours of incubation, surface expression of the indicated markers was analyzed by flow cytometry (A). Cells were incubated with BSA-FITC for 30 minutes at 4°C or 37°C, and the BSA uptake was determined by FACS analysis (B). Results are representative of 3 independent experiments.
The p72syk inhibitor piceatannol inhibits LAG-3–induced down-regulation of antigen uptake by DCs
Up-regulation of the expression of surface markers during the maturation of DCs is generally accompanied by functional changes such as the loss of the capacity to capture antigen. To determine the involvement of p72syk in these changes, we investigated the effects of the inhibitor piceatannol on LAG-3–induced down-regulation of antigen capture. Control IgG1-treated DCs efficiently captured the test antigen BSA-FITC after 30 minutes of incubation at 37°C, whereas LAG-3–matured DCs lost this ability (Figure 4B), and addition of I3 mAb alone or combined to LAG-3 had no effect compared with medium or LAG-3, respectively (data not shown). Preincubation of immature DCs with piceatannol strongly inhibited LAG-3–induced down-regulation of BSA uptake. In conclusion, the activation of p72syk is not only involved in the phenotypic maturation of DCs but also necessary for the acquisition of the functional changes associated with the maturation of DCs.
Activation of PI3 kinase, p38MAPK, and p42/44ERK by LAG-3
The maturation of DCs involves different signaling pathways as shown by immature DCs stimulated with LPS, CD40, or TNF-α.23,28,29 Depending on the maturation stimulus, a different set of kinases is activated. To examine their activation after MHC class II signaling induced by LAG-3, immature DCs were stimulated with either human IgG1 as a control, LAG-3Ig, or the class II–specific mAb I3 and cross-linked with secondary goat antibody for the indicated periods of time. Western blot analysis was performed to detect total and phosphorylated forms of c-Akt (protein kinase B), an effector of the PI3 kinase pathway, p38MAPK, or p42/44ERK. Reblotting of the same membrane by an antibody directed against total antigen was performed to ensure equal loading of each well. LAG-3 induced strong phosphorylation of the signaling protein Akt, as shown in Figure 5A at 2- and 10-minute time points. The I3 mAb (anti–MHC II) also induced the phosphorylation of Akt, albeit to a lesser extent. We detected weak activation of p38MAP kinase after class II signaling (Figure 5B). In addition, p42/44ERK was rapidly but transiently activated after signaling via LAG-3, whereas the I3 mAb induced a weaker phosphorylation (Figure 5C). Altogether, direct involvement of the signaling molecules PI3 kinase and kinases of the MAP family were identified by Western blotting, and differences were observed between the activation status of these transduction molecules following signals induced by LAG-3 or anti–MHC II antibody.
Effects of MHC class II cross-linking on the activation of PI3 kinase, p38MAPK, and p42/44ERK. Peripheral blood monocytes differentiated into immature DCs for 7 days with GM-CSF and IL-4 were stimulated with human IgG1, LAG-3Ig, or a class II–specific mAb (I3) as described in the legend to Figure 2. Cell lysates were analyzed by probing Western blots with phosphorylation-specific antibodies against the PI3 kinase substrate Akt (A), the kinase p38 MAPK (B), and p42/44 ERK (C). Blots were reprobed with antibodies specific for total antigen to verify equal loading of samples. Results are representative of 3 experiments performed on different DC samples.
Effects of MHC class II cross-linking on the activation of PI3 kinase, p38MAPK, and p42/44ERK. Peripheral blood monocytes differentiated into immature DCs for 7 days with GM-CSF and IL-4 were stimulated with human IgG1, LAG-3Ig, or a class II–specific mAb (I3) as described in the legend to Figure 2. Cell lysates were analyzed by probing Western blots with phosphorylation-specific antibodies against the PI3 kinase substrate Akt (A), the kinase p38 MAPK (B), and p42/44 ERK (C). Blots were reprobed with antibodies specific for total antigen to verify equal loading of samples. Results are representative of 3 experiments performed on different DC samples.
PI3 kinase, p38MAPK, and p42/44ERK are differentially involved in DC maturation
To confirm the involvement of these kinases in the signal transduction pathway leading to DC maturation, we examined the effect of specific kinase inhibitors on LAG-3–induced DC maturation. The inhibitor PD98059 suppresses the activation of p42/44ERK by interfering with the upstream MAPK kinase 1 (MKK1/MEK).30 SB203580 and LY294002 are specific inhibitors of the p38MAPK and the PI3 kinase, respectively.31,32 Immature DCs were incubated for 24 hours with 10 μg/mL LAG-3Ig with or without prior treatment with the different inhibitors. As the inhibitors were dissolved in DMSO, immature DCs treated with DMSO alone were used as a control. The expression of cell surface markers was determined by FACS, and the inhibition of LAG-3–induced up-regulation was calculated according to the mean cellular fluorescence. The PI3 kinase inhibitor LY294002 shows the strongest effect at 25 μM (Table 1) and 12.5 μM (data not shown), with complete inhibition of CD80 and CD83 up-regulation and to a lesser extent CD40 and CD86. Despite the low level of activation of the p38MAPK after LAG-3 signaling detected by Western blotting, the kinase seems to play a role in DC maturation, as revealed by the inhibition with SB203580 at 40 μM (Table 1) and 5 μM (not shown). Interestingly, the different cell surface molecules seem to be differentially regulated. Thus, the p38MAPK inhibitor SB203580 strongly inhibits CD80 and CD83 expression but shows no consistent effect on CD86. PD98059, the inhibitor of the ERK pathway, only leads to partial inhibition of CD83 at 50 μM (Table 1) and 12.5 μM (not shown). Blocking tyrosine kinase activation with herbimycin A has an inhibitory effect on the up-regulation of CD80 and CD83 but only very weakly inhibits CD86. This result confirms the involvement of tyrosine phosphorylation in DC maturation induced by LAG-3. These effects were not due to nonspecific toxicity, as no reduction in viability was observed compared with maturation with LAG-3 alone or in the presence of the same concentration of DMSO or to maturation with LPS and the same concentrations of inhibitors (not shown).
Percentage of inhibition of LAG-3–induced up-regulation of CD40, CD80, CD83, and CD86 expression and IL-12 p40 and TNF-α secretion
. | SB203580 (p38MAPKi) 40 μM . | PD98059 (p42/44ERKi) 50 μM . | LY294002 (PI3Ki) 25 μM . | Herbimycin A (TKi) 5 μM . | Piceatannol (p72SYKi) 100 μM . |
|---|---|---|---|---|---|
| CD40, % | ND | ND | 62 | ND | 116 |
| CD80, % | 65 | 26 | 101 | 84 | 90 |
| CD83, % | 77 | 50 | 100 | 66 | 65 |
| CD86, % | 22 | 28 | 65 | 46 | 60 |
| IL-12 p40, % | 86 | 76 | 86 | 56 | 83 |
| TNF-α, % | 52 | 85 | 88 | 76 | 82 |
. | SB203580 (p38MAPKi) 40 μM . | PD98059 (p42/44ERKi) 50 μM . | LY294002 (PI3Ki) 25 μM . | Herbimycin A (TKi) 5 μM . | Piceatannol (p72SYKi) 100 μM . |
|---|---|---|---|---|---|
| CD40, % | ND | ND | 62 | ND | 116 |
| CD80, % | 65 | 26 | 101 | 84 | 90 |
| CD83, % | 77 | 50 | 100 | 66 | 65 |
| CD86, % | 22 | 28 | 65 | 46 | 60 |
| IL-12 p40, % | 86 | 76 | 86 | 56 | 83 |
| TNF-α, % | 52 | 85 | 88 | 76 | 82 |
Peripheral blood monocytes were incubated with GM-CSF and IL-4 for 7 days. Immature DCs were then either treated with DMSO alone (negative control, not shown) or preincubated with the indicated inhibitors for 2 hours and then exposed to 10 μg/mL LAG-3Ig for 24 hours. Data are shown as mean of percentages of at least 3 independent experiments. Surface expression of the indicated markers was assessed by FACS analysis. Mean cellular fluorescence values were used to calculate the percentage of inhibition of LAG-3—induced up-regulation of CD40, CD80, CD83, and CD86. Cell culture supernatants were collected, and cytokine concentration was measured by ELISA. Concentration values were used to calculate the percentage of inhibition of LAG-3—induced up-regulation. Following LAG-3Ig addition, concentrations rose from 7 ± 2 and 0.4 ± 0.1 ng/mL to 61 ± 10 and 3.9 ± 0.1 ng/mL for IL-12 p40 and TNF-α, respectively. ND indicates not determined.
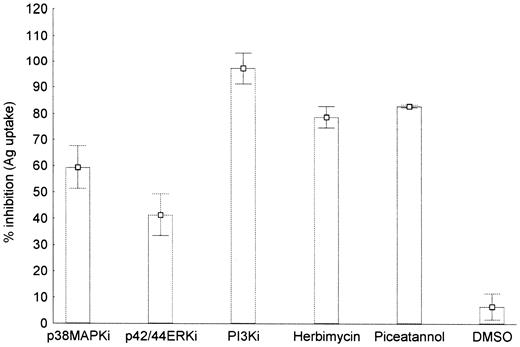
Similar results were obtained when examining the functional effects of DC maturation. LY294002 as well as herbimycin A strongly inhibited the down-regulation of antigen capture by LAG-3, whereas PD98059 and SB203580 had a weaker but still significant (P < .05) effect (Figure 6). These results suggest that PI3 kinase, and to a lesser extent p38MAPK and p42/44ERK, are all involved in the up-regulation of cell surface molecules as well as the functional changes associated with DC maturation.
Effect of SB203580 (p38MAPKi), PD98059 (P42/44ERKi), LY294002 (PI3Ki), and herbimycin A inhibitors on LAG-3–induced down-regulation of antigen uptake by human monocyte-derived dendritic cells. Immature DCs were treated for 24 hours with 10 μg/mL IgG1 or LAG-3Ig after preincubation with each inhibitor for 2 hours as indicated. Cells were then incubated with BSA-FITC for 30 minutes at 4°C or 37°C, and the BSA uptake was determined by FACS analysis. The percentage of FITC-positive cells was used to calculate the percentage of inhibition of LAG-3–induced down-regulation of BSA uptake by the indicated inhibitors. As the inhibitors were dissolved in DMSO, cells treated with the same concentration of DMSO alone (0.1%) were used as a control. Data are given as mean ± SEM of at least 3 independent experiments.
Effect of SB203580 (p38MAPKi), PD98059 (P42/44ERKi), LY294002 (PI3Ki), and herbimycin A inhibitors on LAG-3–induced down-regulation of antigen uptake by human monocyte-derived dendritic cells. Immature DCs were treated for 24 hours with 10 μg/mL IgG1 or LAG-3Ig after preincubation with each inhibitor for 2 hours as indicated. Cells were then incubated with BSA-FITC for 30 minutes at 4°C or 37°C, and the BSA uptake was determined by FACS analysis. The percentage of FITC-positive cells was used to calculate the percentage of inhibition of LAG-3–induced down-regulation of BSA uptake by the indicated inhibitors. As the inhibitors were dissolved in DMSO, cells treated with the same concentration of DMSO alone (0.1%) were used as a control. Data are given as mean ± SEM of at least 3 independent experiments.
LAG-3–induced cytokine secretion is regulated by different signaling pathways
Mature DCs are a major source of immunoregulatory cytokines like TNF-α and IL-12. As previously reported11 and shown in the legend of Table 1, LAG-3 induces strong secretion of these cytokines. Thus, we tested the influence of different inhibitors on LAG-3–induced cytokine secretion. In line with the results on cell surface marker and antigen capture, addition of LY294002 or piceatannol to the culture strongly inhibited the production of both cytokines. Significant inhibition was also observed with SB203580 and herbimycin A, albeit to a different extent depending on the cytokine (Table 1). Interestingly, PD98059 efficiently blocked cytokine production despite having little effect on phenotypic maturation. Therefore, different aspects of DC functions appear to be differentially regulated.
Discussion
When recognition of the class II–peptide complex by a specific TCR occurs, intracellular signals are transduced in the T cell through the TCR and in the APC through class II molecules. In addition, after activation CD8+ MHC class I–restricted cells express a natural class II binder, LAG-3, which could also affect APC activation/differentiation. Although class II signaling has important pleiotropic effects on APC function, the question of how signaling through class II molecules is achieved in these cells remains poorly documented. This study provides evidence that class II signaling induced by LAG-3 in human immature DCs leads to rapid protein phosphorylation of PLCγ2 and p72syk as well as activation of PI3 kinase, p42/44ERK, and p38MAPK.
We observed a stronger protein phosphorylation pattern with the class II–specific mAb I3 compared with that of LAG-3. In contrast, DC maturation was not observed with I3 or with 3 other class II mAbs.11 To reconcile these apparently discrepant observations, we changed the intensity of the signal given to the DCs, considering that signal intensity needs to be well balanced to induce cellular activation and not inhibition or apoptosis. Indeed, ligation with a high-affinity antibody such as I3 may induce a stronger signal than the natural ligand that binds with lower avidity. Scatchard analysis gave a dissociation constant (Kd) of 60 nM and 5 nM for LAG-3Ig and I3 (also termed 9.49), respectively, at 37°C on Daudi B cells.33 However, even at low concentrations (0.1 or 1 μg/mL) I3 does not induce DC maturation, whereas LAG-3Ig keeps its maturation effect after strong cross-linking with secondary antibody (data not shown). LAG-3 binding to DCs is dependent on class II molecules being located in membrane rafts, as it is inhibited by methyl-β-cyclodextrin, which depletes cell membrane cholesterol.11 This leads to the facilitation of raft-associated protein-tyrosine kinase transactivation.20 Therefore, we believe that these functional differences between LAG-3 and I3 are related to differences in their association in raft microdomains. The importance of class II molecules in cholesterol-rich domains of DCs has been underlined recently for the induction of the “cross-priming” (or “cross-presentation”) phenomenon because their presence in rafts is required for induction of CD8+ T-cell responses to exogenous antigen (Ag) by inducing DCs to process this Ag for class I presentation.34 The direct visualization of areas of class II–enriched raft aggregation on immature human DCs using LAG-3, the natural class II ligand, but not a class II–specific mAb (Figure 1), is another compelling evidence for the constitutive presence of a fraction of class II molecules in lipid rafts in DC. The coclustering of the class II LAG-3 binding site partly on cholesterolrich rafts and partly on CDw78 microdomains underscores the importance of membrane compartmentalization of class II molecules in LAG-3–induced DC final maturation and licensing.11 Whether the formation of large-sized arrays of surface class II molecules encompassing both types of microdomains, such as the ones observed when a 1- to 4-μm synapse is formed between an APC and a T cell, can be actively induced by the presence of LAG-3 on activated T cells is currently under investigation.
Antibody-mediated ligation of class II ligands on B lymphocytes resulted in phosphorylation of p72syk.35 In human monocyte-derived DCs, p72syk is phosphorylated following engagement of CD40 by specific mAbs, a process that requires the compartmentalization of CD40 in membrane rafts.29 In the latter cells, we show that p72syk is also phosphorylated following class II signaling induced by both LAG-3 and class II–specific mAbs. The kinase p72syk seems to play an active role in LAG-3–induced DC maturation as shown by the strong inhibitory effect of its inhibitor piceatannol. In addition, we identify a second protein, PLCγ2, which was also phosphorylated following class II signaling. Note that PLCγ1 was not found to be phosphorylated in similar conditions, in contrast to previous results obtained with human T cells.27
Consistent with the findings of others using LPS, CD40 ligation, or contact sensitizers as a maturation stimulus,23,28,36 we showed here that PI3 kinase/Akt, p42/44ERK, and p38MAPK pathways were all activated when immature monocyte-derived DCs were triggered with LAG-3. Interestingly, a class II–specific mAb does not induce the same activation pattern as LAG-3. Although the antibody binding sites were in far greater number than that of LAG-311 and the antibody induces stronger tyrosine phosphorylation, Akt phosphorylation was weaker and slower than that induced by LAG-3. This finding is of particular interest given that specific inhibition experiments identified the Akt/PI3 kinase pathways as playing a key role in DC maturation induced by LAG-3.
The precise reasons for the observed differences in signal transduction induced by LAG-3 compared with a class II–specific mAb remain unknown. However, one could speculate that the almost exclusive binding of LAG-3 to class II molecules associated with rafts rich in signal transduction proteins may play a role.11 Indeed, the selective clustering of molecules already linked to the signal transduction machinery may allow a more rapid and more oriented activation of specific pathways. Even small changes in the balance or the kinetics of inhibitory versus activation signals can lead to dramatic functional consequences and could in this case be the reason for the induction of DC maturation by the natural class II ligand LAG-3 and not by nonspecific aggregation of class II molecules by an antibody. Thus, it may be possible in the future to finely tune the immune response by selective activation of these pathways using other class II ligands with binding characteristics different from that of the dimeric LAG-3Ig fusion protein. Together with our previous in vivo experiments with immunized mice,37,38 this study's dissection of the LAG-3–induced class II signaling pathways represents a further step toward the use of this protein as an adjuvant for subunit vaccines.37,38
Prepublished online as Blood First Edition Paper, May 29, 2003; DOI 10.1182/blood-2003-01-0273.
Supported by a grant from Association pour la recherche sur le cancer and from the European Community (new cancer vaccines program, QLK3-CT-1999-00064). S.A. is a recipient of a fellowship from the Gottlieb-Daimler und Karl-Benz Stiftung.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank V. Nicolas (Imagerie cellulaire–IFR 75-ISIT) for her expert assistance with confocal laser scanning microscopy analysis.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal