Abstract
Although CD4+CD25+ regulatory T cells are pivotal in the prevention of autoimmunity and appear to mediate transplantation tolerance, little is known concerning their antigen specificity. Here we describe the induction of a human CD4+CD25+ regulatory T-cell line specific for a defined peptide alloantigen (human leukocyte antigen A2 [HLA-A2] 138-170) by priming purified CD4+CD25+ cells ex vivo. The regulatory cells were anergic and retained their ability to suppress antigen-driven responses of CD4+CD25– cells. They inhibited not only interleukin 2 (IL-2) secretion by CD4+CD25– T cells specific for the same peptide but also direct alloresponse of naive CD4+CD25– T cells stimulated by semiallogeneic dendritic cells (DCs) in the presence of the peptide (“linked suppression”). They also suppressed the response of CD4+ T cells specific for viral and bacterial antigens. The suppressive T-cell line showed sustained high CD25 expression. These findings suggest that peripheral CD4+CD25+ regulatory cells are a precommitted cell lineage from which cells with specificity for non–self-peptides can be selected. This may pave the way for inducing and expanding peptide antigen-specific regulatory T cells ex vivo for cell therapy in transplantation, allergy, and autoimmune disease.
Introduction
The mechanisms of immunologic tolerance are conventionally divided into 2 categories, central and peripheral. Central tolerance results from intrathymic deletion of T cells with high affinity for thymically expressed antigen. The mechanisms that contribute to peripheral T-cell tolerance include deletion, the induction of anergy, and active immunoregulation. Characterization of the recently described population of specialized regulatory T cells that coexpress CD4 and CD25 has blurred the boundary between central and peripheral tolerance.1,2 CD4+CD25+ regulatory T cells act in the periphery to prevent autoimmune reactions.3 However, they appear to arise in the thymus and are selected on self–major histocompatibility complex (MHC)–peptide complexes.3
Although this cell population is one of several types of regulatory cells4-10 that have been characterized in recent years, it does appear to play a key role in the prevention of autoimmunity1-3 and to be responsible for maintaining transplantation tolerance.11-13 First described in the mouse,1,2 we and others have described an equivalent cell population in human peripheral blood14-18 and in the thymus.19 Like their murine counterparts,1,2 human CD4+CD25+ T cells are functionally anergic, suppress the responses of CD4+CD25– T cells to a variety of stimuli, and act in a contact-dependent manner.14-19 Although some clues exist concerning the mechanisms whereby these cells effect suppression, including a possible role for cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) and membrane-bound transforming growth factor β (TGF-β), their precise mechanism of action remains to be defined.3
Limited data exist concerning the specificity of CD4+CD25+ T cells in the regulation of autoimmune responses. In autoimmune thyroiditis and diabetes models, 2 lines of evidence have implicated that CD4+ regulatory T cells have specificity for tissue-specific autoantigens.20,21 In transplantation tolerance, data from several experimental models point to the conclusion that the CD4+ regulatory T cells that maintain transplantation tolerance have indirect allospecificity for donor antigens.12,22 Although ex vivo expansion of murine CD4+CD25+ cells specific for allogeneic antigens (direct allospecificity) has been described, CD4+CD25+ regulatory T cells with specificity for a defined peptide antigen are still unknown.23
Given that the indirect pathway of the alloimmune response appears to be a key driver of chronic transplant rejection, the induction of regulatory T cells with the capacity to control this response is a crucial goal in the pursuit of transplantation tolerance.24 In this study we have explored the possibility that regulatory T cells with antigen specificity against an allopeptide (indirect allospecificity) can be induced and selected from human peripheral blood CD4+CD25+ cells. The selected regulatory T cells were characterized for their phenotype and function. These data highlight the possibility that “customized” CD4+CD25+ regulatory T cells with antigen specificity for peptides can be induced and expanded ex vivo for therapeutic purposes in transplantation, allergy, and autoimmune disease.
Materials and methods
Cell line
The murine cytotoxic T-lymphocyte line (CTLL)–2 proliferates in a dose-dependent manner with murine interleukin 2 (IL-2) and IL-4 but proliferates only to human IL-2 or IL-15. Cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 20 μg/mL gentamicine (Sigma, Poole, United Kingdom) in the presence of 10 U/mL recombinant human IL-2 (Roche, Basel, Switzerland).
Peptides
The peptide corresponding to residue 138-170 of human leukocyte antigen A2 [HLA-A2], tetanus toxin (TT:947-967), and influenza hemagglutinin (HA:307-319) peptides were synthesized using a peptide synthesizer and then purified by high-pressure chromatography in the Immunology Unit of the Imperial Cancer Research Fund (University College London Medical School, London, United Kingdom). The purity of these peptides was more than 90%. The sequence of the A2 peptide was as follows: MAAQTTKHKWEAAHVAEQLRAYLEGTCVEWLRR.
HLA typing
Lymphocytes were typed for HLA class I and class II antigens by the tissue typing service at Hammersmith Hospital. The HLA phenotypes of the blood donors used in this study are HLA-A1, B45, DR7, DR10; HLA-A29, A32, B14, B44, DR7; HLA-A3, A24, B18, B38, DR7, DR12; and HLA-A24, B37, B61, DR10, DR16.
Purification of CD4+CD25– and CD4+CD25+ T-cell populations
Peripheral blood mononuclear cells (PBMCs) from a healthy blood donor were separated by density gradient centrifugation over Lymphoprep (Nycomed, Birmingham, United Kingdom). For purification of CD4+ T cells, PBMCs were first incubated in RPMI 1640 medium supplemented with 2% FCS at 37°C twice for 45 minutes to remove adherent cells. The nonadherent cells were then collected and washed. Non-CD4+ cells were depleted by incubation with a cocktail of antibodies: anti-CD8 (OKT8), anti-CD14 (clone BA8; Diaclone, Bolton, United Kingdom), anti-CD16 (clone B-E16; Diaclone), anti-CD19 (clone BC3; Diaclone), anti-CD33 (clone WM53; Diaclone), and anti-CD56 (clone BA19; Diaclone) followed by magnetic bead (Dynal, Wirral, United Kingdom) separation. CD4+CD25– cells were obtained by incubation with anti-CD25 magnetic bead (Dynal), and CD4+CD25+ cells were obtained by using Detachbeads (Dynal). The purity of CD4+CD25– and CD4+CD25+ populations was determined by flow cytometry and was more than 90%.
Dendritic cells
Dendritic cells were prepared from adherent cells derived from frozen PBMCs in RPMI 1640 medium supplemented with 5% human serum (Sigma, United Kingdom) and 20 μg/mL gentamicine. Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF; 1000 U/mL) and IL-4 (1000 U/mL; R&D Systems, Oxon, United Kingdom) were added on the initial day of culture. Cytokines were replenished every other day (days 2 and 4) by removing 0.3 mL of the medium and adding back 0.5 mL fresh medium containing cytokines. On day 5 or 6, DCs were harvested and used for priming and restimulation of T-cell cultures.
Generation of antigen-specific T-cell lines (TCLs)
Freshly isolated CD4+CD25+ (1 × 106) and CD4+CD25– T cells (10 × 106) were used as responder cells. Immature DCs were pulsed with the HLA-A2 (138-170) peptide (10 μg/mL) for 4 hours and then washed and irradiated (60 Gy). Responder T cells were then stimulated with the DCs in a 24-well plate (3 wells for CD4+CD25– T cells and 1 well for CD4+CD25+ T cells) in RPMI 1640 medium (1.5 mL per well) that was supplemented with 10% human serum (Sigma), 2 mM L-glutamine, and 20 μg/mL gentamicine. Two days later, 10 U/mL rIL-2 (Roche) was added to the CD4+CD25– T-cell culture or 10 U/mL rIL-2 (Roche) plus 10 ng/mL rIL-7 (R&D Systems) was added to the CD4+CD25+ T-cell culture. Ten days after priming, T cells were collected and restimulated weekly with irradiated immature DCs pulsed with the peptide (10 μg/mL) in the presence of 10 U/mL rIL-2 (for CD4+CD25– T cells) or 10 U/mL rIL-2 plus 10 ng/mL IL-7 (for CD4+CD25+ T cells and for the initial 4 weeks). The generation of tetanus toxin (TT:947-967) and influenza hemagglutinin (HA:307-319) peptide-specific CD4+ T-cell lines was similar to that of the A2 peptide–specific CD4+CD25– T-cell line, except freshly purified CD4+ T cells were used as responder cells.
T-cell proliferation assays
Antigen-specific CD4+CD25– T cells (20 000 cells per well) were cultured with irradiated (60 Gy) DCs (2000 cells per well) in 96-well round-bottom microplate. Allopeptide HLA-A2 (138-170) (2.5 μg/well) was added directly at the initiation of the culture. The final volume per well was 200 μL. The cultures were labeled with [3H]thymide ([3H]TdR) (0.5 μCi/well [0.0185 MBq/well]) after 48 hours of incubation and harvested 18 hours later. [3H]TdR incorporation was then measured in a Betaplate liquid scintillation counter (Wallac, Milton Keynes, United Kingdom). Mean counts per minute (cpm) of triplicate cultures and standard deviation of the mean were calculated.
Suppression assays
CD4+CD25– or CD4+ T cells (20 000 cells per well) were stimulated by irradiated (60Gy) DCs or DCs prepulsed with 10 μg/mL peptide in the presence of different numbers of CD4+CD25+ T cells. When CD3/CD28 Dynal beads were used as stimulators, the concentration of the beads was 0.1 μL/well. For antibody blocking assays neutralizing anti–TGF-β1 monoclonal antibodies (mAbs; clone 9016.2; R&D Systems), anti–IL-10 mAbs (clone 9D7; Becton Dickinson, Oxford, United Kingdom), anti–IL-4 mAbs (clone 3007.11; R&D Systems), and anti–CTLA-4 mAbs (clone BNI3; Immunotech, Marseille, France) were added at the initiation of the culture at a concentration of 10 μg/mL. Supernatants were removed after 20 or 48 hours or 5 days (for naive CD4+ T cells as responders) of stimulation. IL-2 production in the supernatants was measured by CTLL-2 cell proliferation.
Transwell experiments
Transwells of pore size 0.4 μm were used (Costar, High Wycombe, United Kingdom). Equal numbers of CD4+CD25– and CD4+CD25+ T cells (500 000 cells) were stimulated together by 2 μL CD3/CD28 Dynal beads, or CD4+CD25– T cells and CD4+CD25+ T cells were stimulated separately in the bottom and top chamber by CD3/CD28 beads. Supernatants were harvested 20 hours later and IL-2 contents were measured by CTLL-2 cell proliferation.
CTLL-2 cell proliferation assays
Fifty microliters of supernatants was added to CTLL-2 cells (10 000 cells per well) in 150 μL of medium. The cultures were labeled with [3H]TdR (0.5 μCi/well [0.0185 MBq/well]) after 24 hours of incubation and harvested 18 hours later. [3H]TdR incorporation was then measured in a Betaplate liquid scintillation counter. Mean cpm of triplicate cultures and standard deviation of the mean were calculated. In each experiment different concentrations of standard recombinant IL-2 were used as positive controls.
Intracellular cytokine staining
Phycoerythrin (PE)–anti–IL-10 (JES3; Pharmingen, San Diego, CA), fluorescein isothiocyanate (FITC)–conjugated anti–γ-interferon (IFN) (B27; Caltag, Burlingame, CA), and tri-color (TC)–conjugated anti-CD4 (S3.5; Caltag) were used in flow cytometry. Mouse immunoglobulin G1 (IgG1) labeled with FITC, PE, and TC, all from Caltag, were used as isotype controls. Briefly, CD4+CD25– and CD4+CD25+ T cells were stimulated by phorbol myristate acetate (PMA; 5 ng/mL; Sigma) and Ionomycin (500 ng/mL; Sigma) for 6 hours. Brefeldin A (10 μg/mL; Sigma) was added for the last 5 hours. Cells were fixed with 4% paraformaldehyde (Sigma) for 10 minutes at 4°C. They were then washed once with cold phosphate-buffered saline (PBS), twice with PBS containing 0.25% saponin (Sigma), and finally with PBS containing 0.25% saponin and 5% FCS. Antibodies were added and cells were incubated for 45 minutes at room temperature and then washed with PBS containing 0.25% saponin and 5% FCS. Cells were analyzed on a FACScalibur (Becton Dickinson) using Cell Quest software (Becton Dickinson).
Results
Induction of human CD4+CD25+ cell line specific for a peptide antigen of HLA-A2 (138-170)
CD25+ and CD25– populations were selected using magnetic beads from human peripheral blood CD4+ T cells. Cell purities of 90% or more were routinely achieved. A representative flow cytometric profile of the purified cells is shown in Figure 1A. The T-cell populations were derived from an HLA-A2–negative DR7, 10 heterozygous individual. In order to establish A2 peptide–specific T-cell lines the T-cell populations were incubated with autologous, peripheral blood–derived immature DCs pulsed with an HLA-A2 peptide comprising residues 138-170.25 The T-cell cultures were maintained by weekly restimulation with peptide-pulsed DCs. We chose to use immature dendritic cells to stimulate the CD4+CD25+ T-cell lines on the basis that these cells are likely to be responsible for maintaining regulatory cells in vivo as they circulate through primary lymphoid tissue. Starting from 1 × 106 enriched CD4+CD25+ cells, sufficient cell numbers were achieved after 4 rounds of restimulation for detailed analysis of their specificity and function. However, suppressive activity was confirmed in limited assays before each restimulation step (data not shown). The responses of the CD4+CD25– and CD4+CD25+ T-cell lines to the HLA-A2 peptide were tested. As shown in Figure 1B, the CD4+CD25– T cells proliferated in a peptide-dependent manner. In contrast, the CD4+CD25+ T cells were anergic in that they failed to proliferate in response to DCs plus peptide unless exogenous IL-2 was added. Furthermore, they did not proliferate in the presence of peptide derived from residues 103-120 of HLA-A2 antigen (data not shown).
Induction of HLA-A2 (138-170) allopeptide-specific CD4+CD25– and CD4+CD25+ T-cell lines. (A) CD25 expression on purified CD4+, CD4+CD25–, and CD4+CD25+ cells by flow cytometric analysis. (B) CD4+CD25– and CD4+CD25+ T-cell lines were stimulated by autologous DCs in the absence or presence of the A2 (138-170) allopeptide. After 48 hours of culture, [3H]thymidine was added and the cells were harvested 16 hours later. The concentration of rIL-2 was 5 U/mL. Each bar shows the mean cpm ± standard deviation (SD). (C) The CD4+CD25– and CD4+CD25+ T-cell lines were stimulated by autologous DCs pulsed with the A2 peptide in the presence of exogenous IL-2 (10 U/mL; 10 ng/ml IL-7 was added in the culture of CD4+CD25+ T cells) for one week and then cell numbers were counted. (D-E) CD25 expression on gated CD4+ cells was analyzed after CD4+CD25– (gray open curve) and CD4+CD25+ (filled curve) T cells were stimulated by the A2 (138-170) peptide–pulsed autologous DCs in the presence of IL-2 for one week (D) and then reactivated for 16 hours by CD3/CD28 Dynalbeads (E). (F) To measure intracellular cytokines IFN-γ and IL-10, the CD4+CD25– and CD4+CD25+ T cells were stimulated by ionomycin and PMA for 6 hours; Brefeldin A was added for the last 5 hours. Cells were then stained and analyzed by flow cytometry. The numbers of cytokine-producing cells are shown in percentages. Results are representative of at least 2 independent experiments.
Induction of HLA-A2 (138-170) allopeptide-specific CD4+CD25– and CD4+CD25+ T-cell lines. (A) CD25 expression on purified CD4+, CD4+CD25–, and CD4+CD25+ cells by flow cytometric analysis. (B) CD4+CD25– and CD4+CD25+ T-cell lines were stimulated by autologous DCs in the absence or presence of the A2 (138-170) allopeptide. After 48 hours of culture, [3H]thymidine was added and the cells were harvested 16 hours later. The concentration of rIL-2 was 5 U/mL. Each bar shows the mean cpm ± standard deviation (SD). (C) The CD4+CD25– and CD4+CD25+ T-cell lines were stimulated by autologous DCs pulsed with the A2 peptide in the presence of exogenous IL-2 (10 U/mL; 10 ng/ml IL-7 was added in the culture of CD4+CD25+ T cells) for one week and then cell numbers were counted. (D-E) CD25 expression on gated CD4+ cells was analyzed after CD4+CD25– (gray open curve) and CD4+CD25+ (filled curve) T cells were stimulated by the A2 (138-170) peptide–pulsed autologous DCs in the presence of IL-2 for one week (D) and then reactivated for 16 hours by CD3/CD28 Dynalbeads (E). (F) To measure intracellular cytokines IFN-γ and IL-10, the CD4+CD25– and CD4+CD25+ T cells were stimulated by ionomycin and PMA for 6 hours; Brefeldin A was added for the last 5 hours. Cells were then stained and analyzed by flow cytometry. The numbers of cytokine-producing cells are shown in percentages. Results are representative of at least 2 independent experiments.
The HLA-DR restriction of the T-cell line derived from CD4+CD25– T cells was examined using DCs that shared either DR7 or DR10 with the responder. Proliferation of the T cells derived from CD25– cells was detected when the A2 peptide was presented by either DR allele, although the predominant response appeared to be DR7 restricted (data not shown). Importantly, the allopeptide-specific CD4+CD25+ T cells could be expanded by weekly restimulation with the peptide-pulsed immature DCs in the presence of exogenous IL-2. However, the degree of expansion achieved was at least 5-fold lower than that seen with the T-cell line derived from CD25– cells (Figure 1C).
Flow cytometric analysis of the surface phenotypes of the 2 T-cell lines revealed a striking difference in their CD25 expression (Figure 1D-E). After being cultured in IL-2 medium weekly, all cells derived from the CD4+CD25+ population expressed high CD25, while only 70% of T cells derived from CD4+CD25– cells showed expression of CD25. The mean fluorescence intensity (MFI) of CD25 expression on CD4+CD25+ cells was 3.9 times higher than that on CD4+CD25– cells (Figure 1D). After being reactivated by CD3/CD28 Dynal beads CD25, expression by CD4+CD25– T cells was transiently enhanced, and CD4+CD25+ T cells retained their high CD25 expression even in the absence of IL-2 (Figure 1E). The CD4+CD25+ cell line did not produce any IL-2 after activation (Figure 2). Furthermore, the MFI of CD25 expression on CD4+CD25+ cells was still 2.5 times higher than that on CD4+CD25– cells (Figure 1E).
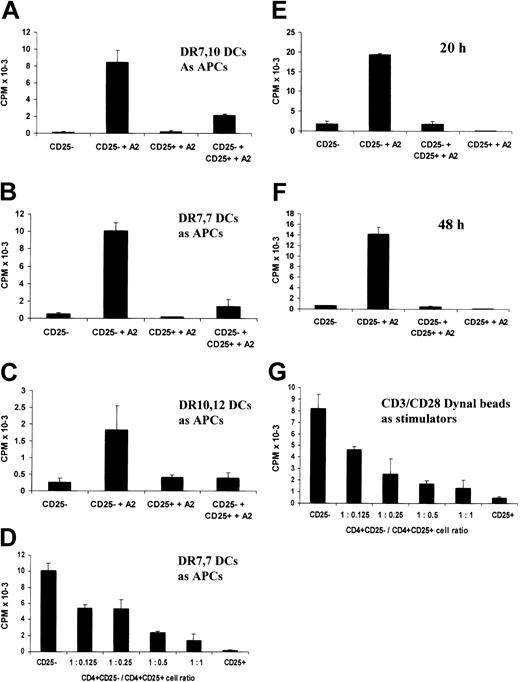
The in vitro–cultured CD4+CD25+ T-cell line retains suppressive properties. (A-F) CD4+CD25– T cells were stimulated by DCs pulsed with the A2 (138-170) peptide in the presence of equal numbers (A-C, E-F) or different numbers (D) of CD4+CD25+ T cells. After 20 hours or 48 hours (F) of culture, supernatants were harvested and their IL-2 contents were measured by CTLL-2 cell proliferation. (A) Heterozygous autologous HLA-DR 7, DR10 DCs were used as APCs. For panels B and D-F, HLA-DR7 homozygous DCs were used as APCs, and for panel C, HLA-DR10, DR16 DCs were used as APCs. (G) CD4+CD25– T cells were stimulated by CD3/CD28 Dynal beads in the presence of different numbers of the CD4+CD25+ T cells for 20 hours. IL-2 secretion in the culture was measured by CTLL-2 cell proliferation. Each bar shows the mean cpm ± SD. Results are representative of at least 2 independent experiments.
The in vitro–cultured CD4+CD25+ T-cell line retains suppressive properties. (A-F) CD4+CD25– T cells were stimulated by DCs pulsed with the A2 (138-170) peptide in the presence of equal numbers (A-C, E-F) or different numbers (D) of CD4+CD25+ T cells. After 20 hours or 48 hours (F) of culture, supernatants were harvested and their IL-2 contents were measured by CTLL-2 cell proliferation. (A) Heterozygous autologous HLA-DR 7, DR10 DCs were used as APCs. For panels B and D-F, HLA-DR7 homozygous DCs were used as APCs, and for panel C, HLA-DR10, DR16 DCs were used as APCs. (G) CD4+CD25– T cells were stimulated by CD3/CD28 Dynal beads in the presence of different numbers of the CD4+CD25+ T cells for 20 hours. IL-2 secretion in the culture was measured by CTLL-2 cell proliferation. Each bar shows the mean cpm ± SD. Results are representative of at least 2 independent experiments.
Analysis of intracellular cytokine profiles by flow cytometry showed that the CD4+CD25– T-cell line produced largely the Th1 cytokine IFN-γ and to a lesser extent IL-10. By contrast, the CD4+CD25+ cells produced very low levels of these cytokines (Figure 1F). IL-2 production by the CD4+CD25+ cells was never detected (Figures 2, 3, 4, 5).
Suppression is cell-contact dependent but cytokine independent. (A) CD4+CD25– T cells were stimulated by CD3/CD28 Dynal beads in the presence or absence of equal numbers of CD4+CD25+ T cells in the same chamber, or CD4+CD25– and CD4+CD25+ T cells were stimulated separately in the bottom and top chambers of transwells by CD3/CD28 Dynal beads. After 20 hours of stimulation, IL-2 production in the culture was measured by CTLL-2 cell proliferation. (B) CD4+CD25– T cells were stimulated by HLA-DR7 homozygous DCs pulsed with the A2 (138-170) peptide in the presence of CD4+CD25+ T cells at a 1:1 ratio. Antibodies (10 μg/mL) were added at the initiation of culture. After 20 hours of stimulation, IL-2 production in the culture was measured by CTLL-2 cell proliferation. Each bar shows the mean cpm ± SD. Results are representative of 2 independent experiments.
Suppression is cell-contact dependent but cytokine independent. (A) CD4+CD25– T cells were stimulated by CD3/CD28 Dynal beads in the presence or absence of equal numbers of CD4+CD25+ T cells in the same chamber, or CD4+CD25– and CD4+CD25+ T cells were stimulated separately in the bottom and top chambers of transwells by CD3/CD28 Dynal beads. After 20 hours of stimulation, IL-2 production in the culture was measured by CTLL-2 cell proliferation. (B) CD4+CD25– T cells were stimulated by HLA-DR7 homozygous DCs pulsed with the A2 (138-170) peptide in the presence of CD4+CD25+ T cells at a 1:1 ratio. Antibodies (10 μg/mL) were added at the initiation of culture. After 20 hours of stimulation, IL-2 production in the culture was measured by CTLL-2 cell proliferation. Each bar shows the mean cpm ± SD. Results are representative of 2 independent experiments.
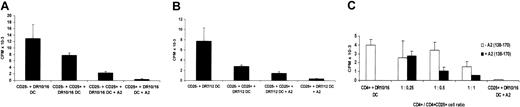
The CD4+CD25+ T cells effect “linked” or “unlinked” suppression. (A-B) Freshly isolated CD4+CD25– T cells were stimulated by semiallogeneic HLA-DR10, DR16 DCs or HLA-DR7, DR12 DCs in the presence of equal numbers of CD4+CD25+ T cells with or without the A2 (137-180) allopeptide. Supernatants were harvested after 5 days of culture and IL-2 secretion by CD4+CD25– T cells was measured by CTLL-2 cell proliferation. (C) Freshly isolated CD4+ T cells were used as responders instead of CD4+CD25– T cells as in panels A-B, and different numbers of CD4+CD25+ T cells were added at the initiation of the culture with (▪) or without (□) the A2 peptide. Each bar shows the mean cpm ± SD. Results are representative of at least 3 independent experiments.
The CD4+CD25+ T cells effect “linked” or “unlinked” suppression. (A-B) Freshly isolated CD4+CD25– T cells were stimulated by semiallogeneic HLA-DR10, DR16 DCs or HLA-DR7, DR12 DCs in the presence of equal numbers of CD4+CD25+ T cells with or without the A2 (137-180) allopeptide. Supernatants were harvested after 5 days of culture and IL-2 secretion by CD4+CD25– T cells was measured by CTLL-2 cell proliferation. (C) Freshly isolated CD4+ T cells were used as responders instead of CD4+CD25– T cells as in panels A-B, and different numbers of CD4+CD25+ T cells were added at the initiation of the culture with (▪) or without (□) the A2 peptide. Each bar shows the mean cpm ± SD. Results are representative of at least 3 independent experiments.
The CD4+CD25+ T cells suppress pathogen-driven responses of CD4+ T cells. (A-B) Tetanus toxin (TT:947-967) or haemagglutinin (HA:307-319) peptide-specific CD4+ T-cell lines were stimulated by DCs pulsed with the tetanus and the A2 (138-170) peptide or the HA and the A2 peptide in the presence of different numbers of CD4+CD25+ T cells. After 20 hours of stimulation, IL-2 production in the culture was measured by CTLL-2 cell proliferation. (C) Tetanus-specific CD4+ T-cell line was stimulated by the tetanus-loaded DCs in the presence of different numbers of CD4+CD25+ T cells and the A2 peptide–loaded DCs. After 20 hours of stimulation, IL-2 production in the culture was measured by CTLL-2 cell proliferation. Each bar shows the mean cpm ± SD. Results are representative of at least 2 independent experiments.
The CD4+CD25+ T cells suppress pathogen-driven responses of CD4+ T cells. (A-B) Tetanus toxin (TT:947-967) or haemagglutinin (HA:307-319) peptide-specific CD4+ T-cell lines were stimulated by DCs pulsed with the tetanus and the A2 (138-170) peptide or the HA and the A2 peptide in the presence of different numbers of CD4+CD25+ T cells. After 20 hours of stimulation, IL-2 production in the culture was measured by CTLL-2 cell proliferation. (C) Tetanus-specific CD4+ T-cell line was stimulated by the tetanus-loaded DCs in the presence of different numbers of CD4+CD25+ T cells and the A2 peptide–loaded DCs. After 20 hours of stimulation, IL-2 production in the culture was measured by CTLL-2 cell proliferation. Each bar shows the mean cpm ± SD. Results are representative of at least 2 independent experiments.
Taken together, these data indicate that an antigen-specific CD4+CD25+ T-cell line against a peptide can be established from human peripheral blood CD4+CD25+ cells. The cells show sustained high CD25 expression, and their cytokine production profiles were similar to those of CD4+CD25+ regulatory cells when studied ex vivo.14,16,18
In vitro–expanded, antigen-specific CD4+CD25+ cells retain suppressive properties
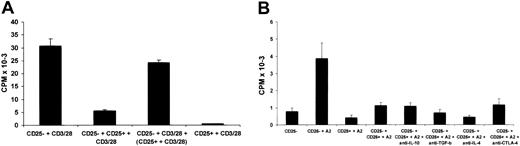
Freshly isolated CD4+CD25+ T cells suppress the proliferation of CD4+CD25– cells by inhibiting IL-2 secretion in response to a variety of activating stimuli.2,14,16,18 A series of experiments were performed to determine whether the allopeptide-specific CD4+CD25+ T-cell line retained suppressive capacity. The T cells derived from the CD4+CD25– starting population were cocultured with autologous DCs pulsed with the A2 peptide in the presence or absence of the CD4+CD25+ T-cell line at a ratio of 1:1. IL-2 secretion was measured by CTLL-2 proliferation by removing culture supernatants after 20 hours of stimulation. As shown in Figure 2A, the CD4+CD25+ T cells markedly inhibited IL-2 production (Figure 2A). Similar data were acquired when HLA-DR7 homozygous DCs or HLA-DR10, DR16 DCs were used as antigen-presenting cells (APCs; Figure 2B-C). Moreover, the CD4+CD25+ T cells inhibited IL-2 production in a dose-dependent fashion. Fifty percent inhibition was seen at a ratio of suppressor cells to responder cells of 1:8; the percentage inhibition increased to 85% at a ratio of 1:1 (Figure 2D), and in some experiments IL-2 production by the CD4+CD25– T cells was virtually abolished at a 1:1 ratio (Figure 2E-F). Furthermore, the inhibition of IL-2 secretion by the CD4+CD25– T cells was observed not only after 20 hours of coculture but also after 48 hours of coculture (Figure 2E-F).
To determine whether the suppression effected by the CD4+CD25+ T-cell line was APC dependent, the CD4+CD25+ or CD4+CD25– T-cell line was stimulated by CD3/CD28 Dynal beads in the absence of any accessory cells. Again, IL-2 production was inhibited in a dose-dependent manner indicating that APCs were not required for, or the target of, suppression (Figure 2G). Furthermore, we did not observe any CD4+CD25– cell death induced by CD4+CD25+ T cells, which may have accounted for the suppression (data not shown).
Suppression is cell-contact dependent and cytokine independent
To investigate the role of soluble factors in the suppression mediated by the CD4+CD25+ T-cell line, a transwell system was used. When the 2 T-cell lines were cocultured with CD3/CD28 Dynal beads, marked inhibition of IL-2 secretion was observed. In contrast, when the 2 T-cell lines were separated by a semipermeable membrane, minimal suppression was observed (Figure 3A). This result suggests that soluble factors are not responsible for the suppression and further indicated that these phenomena cannot be accounted for by IL-2 consumption by the CD4+CD25+ T cells.
The possible involvement of IL-10, TGF-β, and IL-4 was further tested by the addition of neutralizing antibodies against each of these cytokines in suppression assays. None of these antibodies reversed the suppression effected by the CD4+CD25+ cells (Figure 3B). In addition, an anti–CTLA-4 antibody had no effect (Figure 3B).
The CD4+CD25+ cells effect “linked” or “unlinked” suppression
The potential in vivo utility of in vitro–generated CD4+CD25+ regulatory T cells would be greatly enhanced if these cells effected linked suppression.26-28 To test this possibility, the ability of the CD4+CD25+ T-cell line with indirect allospecifity for HLA-A2 to suppress the direct alloresponse of naive CD4+CD25– T cells was tested. Substantial inhibition of the alloresponse against heterozygous semiallogeneic DCs was observed particularly when the HLA-A2 peptide was added to the cultures (Figure 4). The degree of inhibition seen in the absence of A2 peptide may reflect some cross-reactivity of the CD4+CD25+ cell line on the HLA class II alloantigens expressed by the DR10, 16 and DR7, 12 DCs. The amplification of suppression by the addition of the A2 peptide illustrates the need for the CD4+CD25+ T cells to be activated through T-cell–receptor (TCR) ligation to acquire their suppressive function, further demonstrating their antigen specificity to the A2 peptide. This was illustrated again by the dose-response titration (Figure 4C). As described above (Figure 2), the CD4+CD25+ T-cell line caused cell dose–dependent suppression. This was most marked in the presence of the A2 peptide. We further observed that the alloresponse against semiallogeneic DR10, 16 DCs could also be inhibited when A2 peptide–pulsed DR7, 10 or DR7, 7 DCs were added (data not shown), indicating that the CD4+CD25+ cells can also effect nonlinked suppression.
The CD4+CD25+ cell line suppresses pathogen-specific CD4+ T-cell responses
It has been reported that CD4+CD25+ T cells isolated from one TCR-transgenic mouse strain are able to suppress antigen-specific responses of CD4+CD25– T cells from mice transgenic for a different TCR.29 This suggests that once activated, CD4+CD25+ regulatory T cells could contribute to the contraction phase of effector CD4+ T cells that expand in response to a pathogen. We next tested the capacity of CD4+CD25+ cells specific for the HLA-A2 peptide to suppress the responses of CD4+ T-cell lines specific for tetanus toxin (TT:947-967) and for influenza hemagglutinin (HA:307-319) peptides generated from the same individual. As shown in Figure 5A, more than 80% inhibition of IL-2 secretion by tetanus-specific CD4+ T cells was seen at a ratio of suppressor cells to responder cells of 1:8 when the A2 and tetanus peptides were presented on the same DCs. A similar degree of suppression was seen in the CD4+ T-cell response against the HA peptide in the presence of DCs pulsed with both the A2 and HA peptides (Figure 5B). Inhibition of the tetanus-specific response by the CD4+CD25+ cells could also be achieved when separate populations of tetanus-loaded DCs and DCs pulsed with the A2 peptide were present (Figure 5C). Minimal suppression was observed when the A2 peptide–loaded DCs were not added (data not shown). These data indicate that antigen-specific human CD4+CD25+ cells can effect bystander suppression of CD4+ T-cell responses against bacterial or viral antigens.
Discussion
The data presented here demonstrate that T-cell lines can be established and expanded from the human CD4+CD25+ regulatory population ex vivo, that cell lines with defined peptide specificity can be selected, and that the in vitro–expanded cells retain their suppressive properties and high CD25 expression.
The antigen specificity of CD4+CD25+ regulatory cells remains to be defined. Several lines of evidence suggest that these cells have specificity for tissue-specific self-antigens.20,21 This was illustrated by the observation in an adoptive transfer model that CD4+ (probably CD4+CD45RC–) regulatory T cells from peripheral lymph nodes and spleen of athyroid rats were not capable of preventing the development of autoimmune thyroiditis but were still able to inhibit the development of diabetes.20 CD4+CD25+ T cells from the pancreatic lymph nodes of prediabetic mice could also transfer protection against diabetes if given in sufficient numbers to other prediabetic animals. No protection was conferred by the transfer of the equivalent population from anatomically distant lymph nodes.21 Despite this probable specificity for antigens derived from self-proteins it appears that the CD4+CD25+ regulatory population has the capacity for allorecognition. Some of the most compelling evidence for this comes from adoptive transfer studies in models of transplantation tolerance. In such models the tolerance can be transferred by CD4+ T cells to a naive host and the transferred tolerance appears to be specific for the alloantigens carried by the tolerized graft.22 The relevance of CD4+CD25+ cells to this phenomenon has recently been demonstrated by Hara et al12 who showed that tolerance could be transferred selectively by CD4+CD25+ T cells and that this was alloantigen specific. In this model, the induction of CD4+CD25+ regulatory cells with apparent specificity for allogeneic antigens was achieved by anti-CD4 antibody therapy and allogeneic lymphocyte transfusion. It is not known whether these regulatory cells were derived from peripheral naive CD4+ T cells or from a pre-existing CD4+CD25+ population. Our data suggest that cells capable of cross-reacting with foreign peptide antigens can be selected from naturally occurring CD4+CD25+ regulatory T cells.
We showed that the CD4+CD25+ T cells specific for HLA-A2 peptide suppressed not only the response of CD4+CD25– cells with the same antigen specificity but also effected bystander inhibition of the response of CD4+ T cells to viral or bacterial antigens. This suggests that antigen-specific CD4+CD25+ regulatory cells may actively participate in the contraction phase of effector CD4+ T cells after viral or bacterial infection, implying their new potential role in the homeostasis of memory CD4+ T cells. Although our in vitro studies indicate that this regulation does not require that CD4+CD25+ and effector CD4+ T cells are activated through the same APC, given that cell contact is indispensable for the suppression, it is most likely that the necessary proximity is only achieved in vivo when the antigens for which the suppressor and effector cells are specific are presented by the same APC.28 The HLA-A2 peptide–specific CD4+CD25+ T cells showed sustained high CD25 expression compared with CD4+CD25– cells specific for the same peptide. This is in line with the data from Levings et al30 showing that human CD4+CD25+ T-cell clones expressed high levels of CD25.
Interestingly, it appears that the regulatory cells that maintain transplantation tolerance have indirect, rather than direct, allospecificity. These experimental findings are mirrored by recent observations in clinical transplant recipients. While we have failed to detect regulation of direct pathway antidonor alloresponses in stable transplant patients, depletion of CD4+CD25+ T cells reveals significant indirect pathway antidonor alloresponses in a fraction of stable transplant patients.31,32 Given that the indirect antidonor alloresponse appears to be an important immunologic driver of more chronic forms of transplant rejection, it can be argued that regulation of this response is the key to the establishment of long-term transplantation tolerance.24 The data described in this study raise the possibility that allopeptide-specific CD4+CD25+ regulatory T cells can be selected and expanded ex vivo as a potential therapeutic tool in manipulating both direct and indirect alloresponses in vivo. They also imply that peptide antigen–specific CD4+CD25+ regulatory T cells may have potential use as cell therapy in autoimmune disease and allergy.
Prepublished online as Blood First Edition Paper, May 29, 2003; DOI 10.1182/blood-2003-04-1164.
Supported by the Medical Research Council (MRC) (R.I.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. Induction of HLA-A2 (138-170) allopeptide-specific CD4+CD25– and CD4+CD25+ T-cell lines. (A) CD25 expression on purified CD4+, CD4+CD25–, and CD4+CD25+ cells by flow cytometric analysis. (B) CD4+CD25– and CD4+CD25+ T-cell lines were stimulated by autologous DCs in the absence or presence of the A2 (138-170) allopeptide. After 48 hours of culture, [3H]thymidine was added and the cells were harvested 16 hours later. The concentration of rIL-2 was 5 U/mL. Each bar shows the mean cpm ± standard deviation (SD). (C) The CD4+CD25– and CD4+CD25+ T-cell lines were stimulated by autologous DCs pulsed with the A2 peptide in the presence of exogenous IL-2 (10 U/mL; 10 ng/ml IL-7 was added in the culture of CD4+CD25+ T cells) for one week and then cell numbers were counted. (D-E) CD25 expression on gated CD4+ cells was analyzed after CD4+CD25– (gray open curve) and CD4+CD25+ (filled curve) T cells were stimulated by the A2 (138-170) peptide–pulsed autologous DCs in the presence of IL-2 for one week (D) and then reactivated for 16 hours by CD3/CD28 Dynalbeads (E). (F) To measure intracellular cytokines IFN-γ and IL-10, the CD4+CD25– and CD4+CD25+ T cells were stimulated by ionomycin and PMA for 6 hours; Brefeldin A was added for the last 5 hours. Cells were then stained and analyzed by flow cytometry. The numbers of cytokine-producing cells are shown in percentages. Results are representative of at least 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/6/10.1182_blood-2003-04-1164/6/m_h81834922001.jpeg?Expires=1769122631&Signature=OJPjH33ZJ-Sa-nGbuJhLoTkePaPjBZxPrN1M3hToE1U~9SuOvhp747DrPY-ft-yZKBJCQvSAaYJ4QEDOf0OIqAbFTjna6T4k4qd-gSjEe6d8z6f4Pjik8dQAbUBwXzsNiqJTennJom9cA1k0eEQQfAPalccBEJ0Q1i1pqymigyopM-W6mo7x6mjl5MZgaoL6BL-u-t3uod5Au0jaGZiq5~sk~P5SnQ3nXsWJh5amn6sCJaotyas-wLUPl4FhcE3rz8Ju6FZYm2iGyogQOslCC~uyW9JFdlYQ5~OFC~mvBEhNvm~nVnujDkk5kilUeo0IritsHIW6zK5MX9aDEOAIQg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal