Abstract
Recently we demonstrated the existence of a phosphatidylinositol 3-kinase (PI3K)–independent F-actin polymerization during neutrophil pseudopod extension. Here we examine the use of the PI3K-dependent and PI3K-independent pathways of activation by the N-formyl peptide receptor and the chemokine receptors, and the priming of the 2 pathways by granulocyte-macrophage colony-stimulating factor (GM-CSF) and insulin. The inhibition of PI3K activity with wortmannin showed that rate of pseudopod extension stimulated with N-formyl-Met-Leu-Phe (fMLP was mostly dependent on PI3K, while the rate of interleukin-8 (IL-8)–stimulated pseudopod extension was less dependent on PI3K. The incubation of cells with either GM-CSF or insulin increased the rate of pseudopod extension by 50% when the cells were stimulated with IL-8 but not with fMLP. The stimulation with IL-8 phosphorylated the PI3K regulatory subunit. This phosphorylation was enhanced by GM-CSF, which increased PI3K activity and total phosphatidylinositol 3,4,5-trisphosphate (PtdIns(3,4,5)P3) production. The effect of GM-CSF was blocked with wortmannin. In contrast, insulin did not increase p85 phosphorylation and did not enhance PI3K activity or PtdIns(3,4,5)P3 production. The effect of insulin was insensitive to wortmannin; however, it was blocked by an Src homology 2 (SH2)–binding peptide. These data indicate that priming of IL-8 activation with GM-CSF was mediated via the PI3Ks of class IA, while priming with insulin used a PI3K-independent pathway.
Introduction
Directional F-actin polymerization in the lamella region of a migrating human neutrophil is signaled by G-protein–coupled chemotactic receptors (GPCRs). It is known that the ligation of these receptors triggers the release of Gβγ.1 Downstream from the GPCRs, the regulation of F-actin polymerization depends on PI3K activation and PtdIns(3,4,5)P3 production, and involves the activation of protein kinase B (Akt/PKB) and the guanosine triphosphatases (GTPases) Cdc42 and Rac2.2,3 Cdc42 and Rac2 form complexes with the Wiskott-Aldrich syndrome protein (WASp) family proteins and actin-related protein 2/3 (Arp2/3) to promote the formation of free barbed ends,4 which in turn initiate cytoskeletal F-actin polymerization in the lamella region.3 This mechanism of F-actin polymerization provides a useful framework for the understanding of the signaling of actin dynamics in the living cell during motility; however, it is far from complete. The earlier findings that F-actin polymerization is independent of the release of intracellular calcium and the activation of phospholipase C-β suggest that the signaling of migration is distinct from signaling of secretion.5-7 In contrast, there is strong supporting evidence for the dependence of neutrophil migration on the activation of phosphatidylinositol 3-kinase-γ (PI3Kγ) in mice.6,8,9 Similar dependence on PI3Kγ is expected for the migration of the human neutrophil on the basis of the dramatic change of PI3Kγ activity after neutrophil stimulation with chemoattractant10 and the ability of the PI3Kγ product, phosphatidylinositol 3,4,5-trisphosphate (PtdIns(3,4,5)P3), to induce crawling.11 However, mouse neutrophils lacking PI3Kγ activity were still capable of migration,6 which suggests that either the class IA PI3Ks are also involved in the signaling of F-actin polymerization or there is a PI3K-independent F-actin polymerization. Here we focus on the roles of the PI3K-dependent and PI3K-independent pathways of pseudopod extension and their priming by receptor tyrosine kinases (RTKs).
There are 3 classes of phosphoinositol 3-kinases depending on their lipid substrate specificity: class I, II, and III.12 Class I PI3Ks are of particular interest because of their involvement in the receptor-induced cellular responses. This class is composed of 2 subclasses, IA and IB, which consist of heterodimeric proteins with a catalytic and a regulatory subunit. Subclass IA includes the PI3Ks having p110α, p110β, or p110δ as a catalytic subunit and p85α or p85β as a regulatory subunit. Subclass IB is represented by only PI3Kγ, with the catalytic subunit p110γ and the regulatory subunit p101.12 The p110γ catalytic subunit is regulated directly by Gβγ,13 while the role of the regulatory subunit is to increase the overall kinase efficiency.14,15 The PI3Ks from class IA are commonly recruited to the membrane and activated via their p85 subunit.12 There is experimental evidence for the direct regulation of p110β by Gβγ,16-19 which provides a possibility for the existence of PI3Kγ-independent signaling. To distinguish between PI3K-dependent and PI3K-independent signaling, we used the fungal metabolite wortmannin, which selectively binds to the highly conserved residue Lys802 of the p110 subunits20 and inhibits their activity.21 To test for the involvement of PI3K of class IA in the signaling of activation, we assayed for p85 phosphorylation and p85/p110 activity. We also used granulocyte-macrophage colony-stimulating factor (GM-CSF) and insulin to explore the involvement of the PI3K-dependent and PI3K-independent pathways in the priming of GPCR-dependent neutrophil activation. GM-CSF is known to phosphorylate p85 and activate p85/p110.22 Therefore, it is plausible to assume that GM-CSF will increase the possible effect of p85/p110 in the signaling of chemotaxis. Insulin activates PI3K via the production of phosphorylated substrates, such as insulin receptor substrate–1 (IRS-1), IRS-2, IRS-3, IRS-4, adapter protein Shc, and Grb-associated binder 1 (GAB-1),23 that bind to the Src homology 2 (SH2) domains of p85. The binding of these substrates to p85 may or may not activate p110.24
Materials and methods
Cell preparation
Human neutrophils were freshly isolated from whole blood obtained by venipuncture or from deep finger pricks from consenting, healthy adult volunteers (mean age, 28 ± 9.5 years) into vacutainers anticoagulated with K2EDTA (K2 ethylenediaminetetraacetic acid). The neutrophils were isolated by a one-step density gradient centrifugation on Polymorphoprep (Nycomed, Oslo, Norway) at 500g for 35 minutes at 23°C. The neutrophil fraction was washed once with buffer I (1.26 mM CaCl2, 0.81 mM MgSO4, 5.37 mM KCl, 0.44 mM KH2PO4, 4.17 mM NaHCO3, 136.89 mM NaCl, 0.34 mM Na2HPO4, 5.55 mM D-glucose) and resuspended in an autologous plasma/buffer I solution (1:3 vol/vol). The isolated cells were used for the bulk measurements or for the micropipette experiments; the cells were added to the manipulation chamber filled with an autologous plasma/buffer I solution (1:3 vol/vol). Microscopic evaluation of isolated cells indicated that 95% of the cells were neutrophils. The trypan blue dye exclusion test showed that more than 98% of the cells were viable.
The attractants and inhibitors were initially diluted in either ethanol or dimethyl sulfoxide at a maximum final concentration of 0.8% and 0.2%, respectively. The presence of ethanol or dimethyl sulfoxide had no measurable effect on the rates of pseudopod extension (data not shown). The cells were incubated with the inhibitors at room temperature in the following conditions, except where otherwise indicated: wortmannin (10 to 1000 nM, 15 minutes) (Sigma Chemical, St Louis, MO); phosphatidylinositol 4,5-bisphosphate (PIP2)–binding peptide (Ac-K(Dansyl)GGGQRLFQVKGRR) (50 μM, 15 minutes) (the peptide was synthesized in the UNC Microprotein Sequencing and Peptide Synthesis Facility, Chapel Hill, NC; its molecular weight and purity were confirmed by mass spectrometry); GM-CSF (30 pM, 0 to 120 minutes); insulin (320 μU/mL, 20 minutes) (Sigma); and Ac-D-Y(2-malonyl)-V-P-M-L-NH2 trimethyl ester (PI3K-SH2-OMT) (50 μM, 30 minutes) (Biomol Research Laboratories, Plymouth Meeting, PA). The incubation of cells for 30 minutes with PI3K-SH2-OMT was sufficient for reaching an intracellular saturation level (Figures S1-S3 on the Blood website; see the Supplemental Figures link at the top of the online article). Approval for these studies was obtained from the Institutional Review Board at Duke University (Durham, NC), and informed consent was provided according to the Declaration of Helsinki.
Micromanipulation
The experimental temperature-controlled chamber was 2-mm thick. The bottom half of the chamber was covered with a cover glass strip and was open at both sides to allow for micromanipulation. The micropipettes used to manipulate the cells were made from capillary glass tubing (A-M Systems, Everett, WA) pulled with a vertical pipette puller (Model 730; David Kopf Instruments, Tujunga, CA). The tip of the pipettes was cut by means of a microforge. The micropipettes were mounted on the microscope stage and inserted coaxially into the experimental chamber. The micropipettes were connected to a manometer system, which allowed control of the pipette-chamber pressure difference. The temperature of the experimental chamber was controlled by flow of thermostated water and was measured with a thermocouple. All micromanipulation experiments were performed at 37°C.
In the experiments of pseudopod extension, a passive neutrophil with a smooth membrane surface and a uniform optical density was chosen. The cell was held in a supporting pipette with a 5-μm internal diameter. Another pipette with an internal diameter of 1 μm was filled with a solution of N-formyl-Met-Leu-Phe (fMLP) or interleukin 8 (IL-8) (Sigma) and positioned 1 μm from the cell surface. The fMLP or IL-8 solution was blown over the cell, which initiated the extension of a single pseudopod. Pseudopod extension was observed by means of an inverted Nikon (Tokyo, Japan) microscope equipped with a 60 × oil-immersion objective. The microscope images were recorded with a charge-coupled device (CCD) camera (Cohu, San Diego, CA). A real-time counter and the chamber temperature were multiplexed onto the recorded images by means of a multiplexer (Vista Electronics, La Mesa, CA). The recorded images were analyzed with Metamorph Imaging software (Universal Imaging, Downing-town, PA), and the rate of pseudopod extension was calculated from the measured pseudopod lengths and time lapses. Approximately 90% of the neutrophils tested produced pseudopods. The nonresponding fraction of cells was not reported in the averages of the rates of pseudopod extension.
Determination of the total cellular F-actin
Neutrophils were suspended at a final concentration of 1 × 106/mL in buffer I supplemented with 1 mg/mL bovine serum albumin (BSA). The cells were incubated with or without inhibitors for 30 minutes at room temperature as indicated in the Figure legends and subsequently stimulated with fMLP or IL-8 (100 nM final concentration). The reaction was stopped at the appropriate time by fixation with 4% paraformaldehyde. The cells were washed once and resuspended in buffer I. The cells were then permeabilized and stained with a solution containing 20 mg/mL monooleoylphosphatidylcholine (Avanti Polar Lipids, Alabaster, AL) and 100 μM Alexa Fluor 488–conjugated phalloidin (Molecular Probes, Eugene, OR) for 20 minutes at room temperature. The cells were washed 3 times in buffer I, and the phalloidin fluorescence was quantitated by means of flow cytometry (FACStarPLUS; Becton Dickinson, Franklin Lakes, NJ). The samples were run in triplicate, and the average F-actin content of the population was expressed as the mean ± standard deviation (SD) of the fluorescence intensity.
Determination of the total cellular PtdIns(3,4,5)P3
Neutrophils were suspended at a concentration of 1 × 108/mL in buffer II (30 mM HEPES [N-2-hydroxyethylpiperazine-N ′-2-ethanesulfonic acid], 110 mM NaCl, 10 mM KCl, 1 mM MgCl2, 5.55 mM glucose, 1.53 mM CaCl2) supplemented with 1 mg/mL BSA and 1 mCi/mL (37.0 MBq/mL) [32P]orthophosphate (ICN Biomedicals, Irvine, CA). Control experiments were performed in the same buffer without calcium with equivalent results (data not shown). The cells were incubated at 37°C in a shaking water bath for 90 minutes, washed 3 times with buffer II, and resuspended at 2 × 107/mL in buffer II. Aliquots (500 μL of 2 × 107/mL) labeled cells were incubated for 30 minutes at 37°C as indicated in the Figure legends and subsequently stimulated with fMLP or IL-8 (100 nM final concentration). The reaction was stopped by the addition of 1.5 mL chloroform/methanol (1:2 vol/vol) containing the antioxidant butylated hydroxytoluene (BHT) (0.63 mg/mL) and phosphoinositides (Sigma) (10 μg/mL) as cold carrier. Another 3 mL chloroform/2.4 M HCl (1:1 vol/vol) was added to generate aqueous and organic phases. The resulting organic lower phase was removed, and the aqueous upper phase was washed 4 times with 1 mL chloroform. The combined lower phases were then washed once with 2 mL methanol/1 N HCl (1:1 vol/vol). The lower phase was removed and evaporated to dryness under a stream of N2. For thin-layer chromatography, 20 × 20–cm Silica Gel 60 plates (EMD Chemicals, Gibbstown, NJ) impregnated with 1.2% potassium oxalate were activated before spotting for 15 minutes at 110°C. The phospholipids were resuspended in 100 μL chloroform/methanol (2:1 vol/vol) and spotted. The test tubes that had contained the phospholipids were washed with an additional 35 μL chloroform, and the solution was spotted on the same spots. The thin-layer chromatography (TLC) plates were then developed in one dimension in chloroform/acetone/methanol/acetic acid/water (80:30:26:24:14 vol/vol/vol/vol/vol). Radioactive spots were detected with autoradiography with the use of Kodak (Rochester, NY) BioMax x-ray film and compared with 32P-labeled standards (kindly provided by John D. York, Duke University). The total cellular phosphatidylinositol 3,4,5-trisphosphate (PIP3) was quantified by means of a phosphorimager (Molecular Dynamics, Piscataway, NJ) and by scraping and counting in a liquid scintillation counter with comparable results. Samples were run in triplicate, and the total cellular PIP3 was expressed as a percentage of the total amount of phospholipid in each sample. Statistical analysis was performed with the paired 2-sample Student t test.
Antibodies
Rabbit polyclonal antibody for Tyr508 of phosphorylated p85α (anti–pp85α) was from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibody (antiphosphotyrosine [anti-PY]) and p85α polyclonal antibody (anti-p85α) covalently coupled to protein A–agarose were from Upstate Biotechnology (Lake Placid, NY).
Immunoprecipitation
Neutrophils were suspended at a final concentration of 1 × 108/mL in buffer I supplemented with 1 mg/mL BSA. Then, 50 μL cells were preincubated with inhibitor or vehicle for the indicated period of time at 37°C and subsequently stimulated with either fMLP or IL-8 (100 nM final concentration) or an equivalent volume of the appropriate diluent. The stimulation was stopped by the addition of 2 mL ice-cold buffer III (buffer I supplemented with 1 mM MgCl2, 0.1 mM Na3VO4). The samples were immediately centrifuged and washed twice in ice-cold buffer IV (50 mM HEPES, pH 7.4; 1 mM EDTA; 137 mM NaCl; 1 mM CaCl2; 1 mM MgCl2; 0.1 mM Na3VO4). The cells were lysed in 1 mL ice-cold buffer V (50 mM HEPES, pH 7.4; 1 mM EDTA; 137 mM NaCl; 1 mM CaCl2; 1 mM MgCl2; 50 mM NaF; 2 mM Na3VO4; 1% [vol/vol] Nonidet P-40, 10% [vol/vol] glycerol; 2 mM phenylmethylsulfonyl fluoride [PMSF]; 10 mM Na4P2O7; 10 μg/mL aprotonin; 10 μg/mL leupeptin; 1 μM pepstatin) and incubated on ice for 10 minutes with intermittent vortex mixings. The lysates were cleared by centrifugation at 12 000g for 10 minutes at 4°C. Protein concentrations were determined by means of a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL), standardized to BSA.
PI 3-kinase was immunoprecipitated by adding 5 μg of the indicated antibody to the cell lysates for 2 hours at 4°C with constant shaking. The immunoprecipitates were collected following a 2-hour incubation at 4°C with 50 μL of a 1:1 (vol/vol) suspension of Protein A Sepharose (Amersham Biosciences, Piscataway, NJ). The precipitates were washed twice with buffer IV and 3 times with buffer VII (50 mM HEPES, pH 7.4; 40 mM MgCl2; 600 mM NaCl) and resuspended in 50 μL buffer VII.
For antiphosphotyrosine immunoprecipitates, lysates were prepared under reducing conditions. Initially, 500 μL cell suspensions were added to equal amounts of buffer VI (50 mM Tris-HCl [tris(hydroxymethyl)aminomethane)–HCl], pH 8.0; 150 mM NaCl; 2 mM EDTA; 50 mM NaF; 2 mM NaVO4; 20 mM NaP2O4; 10 μg/mL leupeptin; 10 μg/mL aprotinin; 1 μM pepstatin; 1 mM phenylmethylsulfonyl fluoride; 1% sodium dodedyl sulfate [SDS]; and 0.6% β-mercaptoethanol), preheated to 95°C, and incubated for 10 minutes. The lysates were centrifuged at 12 000g for 10 minutes at room temperature. The denaturing agents were then removed by filtering through a Sephadex G-10 column (Amersham Biosicences). The cellular lysates were then incubated with 10 μg agarose-conjugated antiphosphotyrosine antibodies for 5 hours at 4°C with constant shaking on a rotator platform. The beads were collected by centrifugation and washed twice with buffer V and 3 times with buffer I. Then, 45 μL boiling Laemmli buffer (62.5 M Tris-HCl, pH 6.8; 4% SDS; 5% β-mercaptoethanol; 8.5% glycerol; 2.5 mM NaVO4;10 μg/mL aprotinin; 10 μg/mL leupeptin; and 0.025% bromphenol blue) was added to the supernatants and boiled for 10 minutes before electrophoresis.
Immunoblotting
The immunoprecipitated proteins were subjected to 7.5% to 12% sodium dodecyl sulfate–polyacrylamide gradient gel electrophoresis (SDS-PAGE) and transferred to Immobilon polyvinylidene difluoride membranes (Millipore, Bedford, MA). Nonspecific sites were blocked by means of 2% gelatin in Tris-buffered saline–Tween (TBS-Tween) (25 mM Tris-HCl, pH 7.8; 190 mM NaCl; 0.15% Tween 20) for 1 hour at 37°C. After blocking, the membrane was incubated overnight at 4°C with the indicated primary antibody at a final dilution of 1/200 to 1/1000 in fresh blocking solution. The membranes were washed 3 times at room temperature in TBS-Tween for a total duration of 30 minutes and then incubated with secondary antibodies (Abs) labeled with horseradish peroxidase (HRP)–labeled sheep antimouse or donkey antirabbit immunoglobulin G (IgG) (Amersham Biosciences) for 1 hour at 37°C at a final dilution of 1/20 000 in fresh blocking solution. The membranes were washed 3 times with TBS-Tween, and the protein bands were viewed and quantitated by means of a LumiImager with LumiAnalyst software (Roche, Indianapolis, IN). In some cases, the membranes were stripped with Immuno Pure IgG Elution buffer (Pierce), washed, and reprobed with Abs specific for total p85 protein to determine the ratio between phosphorylated and total kinase.
Measurement of in vitro PI3-kinase activity
PI 3-kinase activity was measured by adding 10 μL sonicated PtdIns vesicles (Avanti Polar Lipids) and 10 μCi (0.37 MBq) γ-32P] adenosine triphosphate (γ-32P]ATP) (ICN Biomedicals) at 37°C to the immunoprecipitates. The reaction was carried out for 10 minutes and then quickly stopped by the addition of 100 μL methanol in 1 N HCl (1:1 vol/vol) and then 200 μL chloroform/methanol (1:1 vol/vol). The samples were centrifuged, and the lower organic phase was evaporated to dryness under a stream of N2. The lipids were redissolved in 100 μL chloroform in methanol (2:1 vol/vol), spotted onto activated oxalate-treated Silica Gel 60 plates, and developed with the use of a solvent system of chloroform, methanol, water, and 28% ammonia (50:40:7:3 vol/vol/vol/vol). The TLC plates were dried and exposed to Kodak BioMax x-ray film for 6 hours at –80°C. The 32P-labeled phosphoinositide 3-phosphate band was detected by autoradiography and [32P] incorporation was measured by densitometry and liquid scintillation counting with comparable results.
Results
Dependence of the rate of pseudopod extension on chemoattractant concentration
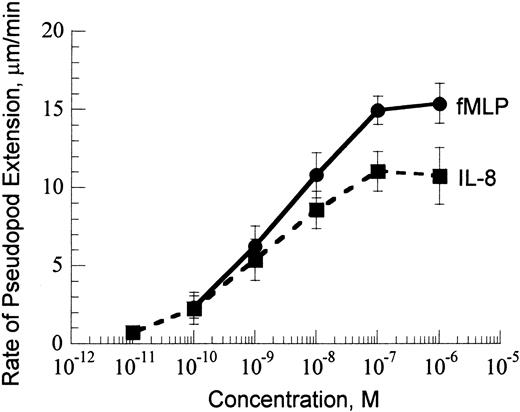
Our assay of stimulating the formation of a single pseudopod from initially passive neutrophils25 studies the dynamics of F-actin polymerization in the activated cell by measuring the rate of pseudopod extension. The rate of pseudopod extension increased with the increase of chemoattractant concentration from 10–11 to 10–7 M (Figure 1). Above 10–7 M, the rate of pseudopod extension was independent of chemoattractant concentration.
Rate of pseudopod extension versus chemoattractant concentration at 37°C. Data are shown for fMLP (solid line) and IL-8 (dashed line).
Rate of pseudopod extension versus chemoattractant concentration at 37°C. Data are shown for fMLP (solid line) and IL-8 (dashed line).
Effect of wortmannin and PtdIns(4,5)P2-binding peptide
The current paradigm of neutrophil motility ascribes a pivotal role of PI3Ks and their lipid products in signaling.26 The p110 catalytic subunit of the PI3Ks produces PtdIns(3,4,5)P3 from phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) and phosphatidylinositol 3,4-bisphosphate (PtdIns(3,4)P2) from phosphatidylinositol 4-phosphate.27 Since PtdIns(3,4,5)P3 alone is capable of stimulating neutrophil polarization11 and the amount of PtdIns(3,4)P2 in the cell is small compared with the other phosphatidylinositol lipids,27 it is assumed that the PI3K-dependent F-actin polymerization is signaled predominantly via PtdIns(3,4,5)P3 production from PtdIns(4,5)P2 as substrate. The critical role of p110γ in this process is demonstrated by the absence of PtdIns(3,4,5)P3 in activated neutrophils from PI3Kγ-null mice.8 We used wortmannin to specifically inhibit the p110 subunit and to study the effect of this inhibition on the rate of pseudopod extension. The incubation of cells with increasing concentrations of wortmannin decreased the rate of fMLP-stimulated pseudopod extension; however, above a 500 nM concentration, wortmannin had no additional effect on the rate of pseudopod extension. The incubation of cells with 1 μM wortmannin decreased the rate of fMLP-stimulated pseudopod extension by 80% (Figure 2A) and the rate of IL-8–stimulated pseudopod extension by 55% (Figure 3A).
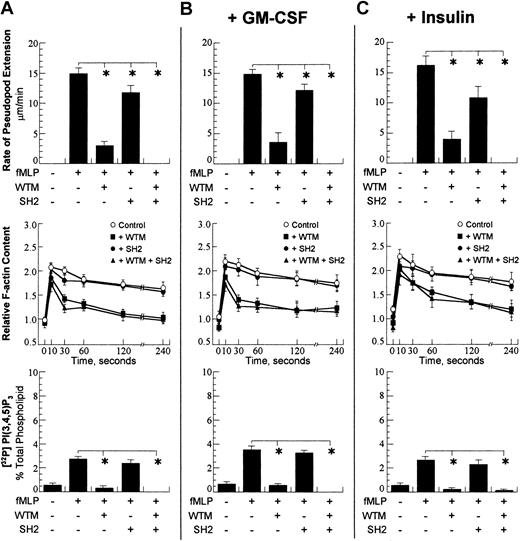
Cellular responses after activation with 100 nM fMLP. A concentration of 10–7 M chemoattractant was chosen. (A) Rate of pseudopod extension (top row), relative cellular F-actin (middle row), and total cellular PtdIns(3,4,5)P3 (bottom row) were performed as detailed in “Materials and methods.” The presence and absence of chemoattractant (fMLP), wortmannin (WTM), and PI3K-SH2-OMT (SH2) is denoted with (+) and (–). The wortmannin concentration was 1 μM, and the PI3K-SH2-OMT concentration was 50 μM. On the graph for the total F-actin content, open circles show the control (fMLP only); closed squares show the data for incubation with wortmannin; closed circles are the data for incubation with PI3K-SH2-OMT; and closed triangles are the data for simultaneous incubation with wortmannin and PI3K-SH2-OMT. (B) Data for the same neutrophil responses as in panel A for cells pretreated for 30 minutes with 30 pM GM-CSF. (C) Data for the same neutrophil responses as in panel A for cells pretreated for 30 minutes with 320 μU/mL insulin. The data points for pseudopod extension are expressed as the mean ± SD of at least 10 cells. For total cellular F-actin content and total PtdIns(3,4,5)P3, the samples were run in triplicate, and the data are expressed as the mean ± SD. *P < .01.
Cellular responses after activation with 100 nM fMLP. A concentration of 10–7 M chemoattractant was chosen. (A) Rate of pseudopod extension (top row), relative cellular F-actin (middle row), and total cellular PtdIns(3,4,5)P3 (bottom row) were performed as detailed in “Materials and methods.” The presence and absence of chemoattractant (fMLP), wortmannin (WTM), and PI3K-SH2-OMT (SH2) is denoted with (+) and (–). The wortmannin concentration was 1 μM, and the PI3K-SH2-OMT concentration was 50 μM. On the graph for the total F-actin content, open circles show the control (fMLP only); closed squares show the data for incubation with wortmannin; closed circles are the data for incubation with PI3K-SH2-OMT; and closed triangles are the data for simultaneous incubation with wortmannin and PI3K-SH2-OMT. (B) Data for the same neutrophil responses as in panel A for cells pretreated for 30 minutes with 30 pM GM-CSF. (C) Data for the same neutrophil responses as in panel A for cells pretreated for 30 minutes with 320 μU/mL insulin. The data points for pseudopod extension are expressed as the mean ± SD of at least 10 cells. For total cellular F-actin content and total PtdIns(3,4,5)P3, the samples were run in triplicate, and the data are expressed as the mean ± SD. *P < .01.
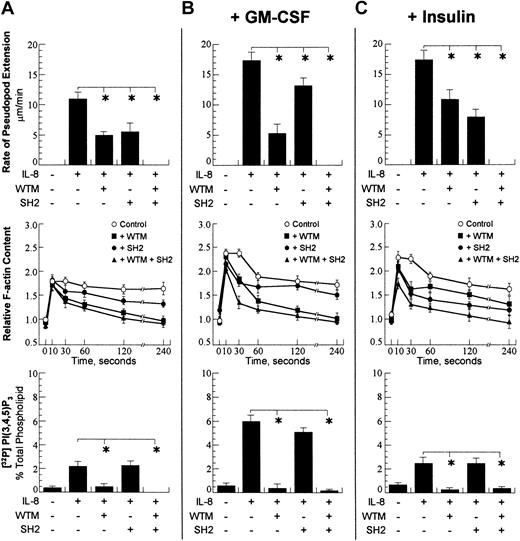
Cellular responses after activation with 100 nMIL-8. A concentration of 10–7 M chemoattractant was chosen. (A) Rate of pseudopod extension (top row), relative cellular F-actin (middle row), and total cellular PtdIns(3,4,5)P3 (bottom row) were performed as detailed in “Materials and methods.” The presence and absence of chemoattractant (fMLP), wortmannin (WTM), and PI3K-SH2-OMT (SH2) is denoted with (+) and (–). The wortmannin concentration was 1 μM, and the PI3K-SH2-OMT concentration was 50 μM. On the graph for the total F-actin content, open circles show the control (fMLP only); closed squares show the data for incubation with wortmannin; closed circles are the data for incubation with PI3K-SH2-OMT; and closed triangles are the data for simultaneous incubation with wortmannin and PI3K-SH2-OMT. (B) Data for the same neutrophil responses as in panel A for cells pretreated for 30 minutes with 30 pM GM-CSF. (C) Data for same neutrophil responses as in panel A for cells pretreated for 30 minutes with 320 μU/mL insulin. The data points for pseudopod extension are expressed as the mean ± SD of at least 10 cells. For total cellular F-actin content and total PtdIns(3,4,5)P3, the samples were run in triplicate, and the data are expressed as the mean ± SD. *P < .01.
Cellular responses after activation with 100 nMIL-8. A concentration of 10–7 M chemoattractant was chosen. (A) Rate of pseudopod extension (top row), relative cellular F-actin (middle row), and total cellular PtdIns(3,4,5)P3 (bottom row) were performed as detailed in “Materials and methods.” The presence and absence of chemoattractant (fMLP), wortmannin (WTM), and PI3K-SH2-OMT (SH2) is denoted with (+) and (–). The wortmannin concentration was 1 μM, and the PI3K-SH2-OMT concentration was 50 μM. On the graph for the total F-actin content, open circles show the control (fMLP only); closed squares show the data for incubation with wortmannin; closed circles are the data for incubation with PI3K-SH2-OMT; and closed triangles are the data for simultaneous incubation with wortmannin and PI3K-SH2-OMT. (B) Data for the same neutrophil responses as in panel A for cells pretreated for 30 minutes with 30 pM GM-CSF. (C) Data for same neutrophil responses as in panel A for cells pretreated for 30 minutes with 320 μU/mL insulin. The data points for pseudopod extension are expressed as the mean ± SD of at least 10 cells. For total cellular F-actin content and total PtdIns(3,4,5)P3, the samples were run in triplicate, and the data are expressed as the mean ± SD. *P < .01.
Since p110 uses PtdIns(4,5)P2 for the production of PtdIns(3,4,5)P3, the sequestering of PtdIns(4,5)P2 was expected to have an adverse affect on pseudopod extension. We used the PtdIns(4,5)P2-binding peptide synthesized after the pleckstrin homology (PH) domain of gelsolin28 to sequester cellular PtdIns(4,5)P2 and in this way reduced the produced PtdIns(3,4,5)P3. Cell incubation with the PtdIns(4,5)P2-binding peptide for 15 minutes was sufficient to transfer the peptide into the cells, and in these conditions the PtdIns(4,5)P2-binding peptide decreased the rate of pseudopod extension similarly to wortmannin (data not shown). The incubation of cells with 25 μM PtdInsP2-binding peptide reduced the rate of fMLP-stimulated pseudopod extension by 75% and the IL-8–stimulated pseudopod extension by 45% (data not shown).
The direct measurement of the total cellular PtdIns(3,4,5)P3 in passive neutrophils and in activated cells provides important information for PI3K activity. The exposure of neutrophils for 60 seconds to 10–7 M of either fMLP or IL-8 increased the amount of PtdIns(3,4,5)P3 4- to 5-fold (Figures 2A and 3A). In the cells exposed to either fMLP or IL-8, the presence of wortmannin reduced the level of PtdIns(3,4,5)P3 to control level (Figures 2A and 3A). This result showed that wortmannin blocked PI3K activity and reduced total cellular PtdIns(3,4,5)P3 production.
Wortmannin significantly reduced the amount of cytoskeletal F-actin at 1 minute after activation; however, it had a smaller effect on the total cellular F-actin.29 Since the total F-actin includes cytoskeletal and noncytoskeletal F-actin, it was concluded by Niggli and Keller29 that wortmannin affected cytoskeletal F-actin polymerization. The comparison of the effect of wortmannin on the rate of pseudopod extension and the total F-actin at 60 seconds after activation showed that wortmannin affected pseudopod extension and total F-actin polymerization similarly. The comparison of the effect of wortmannin on the rate of pseudopod extension, the total F-actin content, and overall PtdIns(3,4,5)P3 production (Figures 2A and 3A) showed that the p110 inhibitor reduced PtdIns(3,4,5)P3 to control level and significantly decreased both the total F-actin content and the rate of pseudopod extension; however, it was incapable of arresting pseudopod extension.
Effect of PI3K-SH2-OMT
Our previous work showed that the wortmannin-independent pseudopod extension was dependent on Src tyrosine kinase.25 This finding suggested that the modulation of the chemotactic responses by the RTKs was mediated via a PI3K-independent pathway. The activation of RTKs is coupled with protein phosphorylation and/or a production of phosphorylated substrates that bind to the SH2 domains present in some of the signaling molecules, such as PI3K. We tested the inhibitor PI3K-SH2-OMT,30,31 which was synthesized after the SH2-binding domain of the PDGF receptor, for its ability to inhibit pseudopod extension.
The measured rate of the fMLP-stimulated pseudopod extension in the presence of 50 μM PI3K-SH2-OMT was decreased by 20% (Figure 2A), whereas the rate of IL-8–stimulated pseudopod extension was decreased by 50% (Figure 3A). A similar decrease in the rate of pseudopod extension was measured for cells incubated with 20 μM Src tyrosine kinase inhibitor, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo(3,4-d)pyrimidine (PP2).25 The incubation of cells with both 1 μM wortmannin and 50 μM PI3K-SH2-OMT abolished pseudopod extension (Figures 2A and 3A), which showed that PI3K-SH2-OMT inhibited a PI3K-independent pathway.
The incubation of cells with PI3K-SH2-OMT had no significant effect on PtdIns(3,4,5)P3 production after activation with either fMLP or IL-8 (Figures 2A and 3A). This confirmed the earlier conclusion that the drug inhibited a PI3K-independent pathway without affecting PI3K activity. PI3K-SH2-OMT had negligible effect on total F-actin polymerization stimulated with fMLP, while it did slightly decrease the total F-actin stimulated with IL-8.
The simultaneous incubation of cells with both 1 μM wortmannin and 50 μM PI3K-SH2-OMT abolished pseudopod extension and reduced the amount of total F-actin 60 seconds after activation to the same level as wortmannin alone (Figures 2A and 3A). The production of PtdIns(3,4,5)P3 after simultaneous incubation with both wortmannin and PI3K-SH2-OMT was slightly below control level, which suggests some synergy between the action of wortmannin and PI3K-SH2-OMT.
Effect of GM-CSF and insulin
We were interested in the involvement of the PI3K-dependent and PI3K-independent pathways in neutrophil priming by RTKs. Since RTKs affect PI3K activity by either phosphorylation of p85 or production of phosphorylated substrates that bind to p85, we measured p85 phosphorylation. The activation of cells with IL-8 was coupled with some p85 phosphorylation, while the activation with fMLP was not (Figure 4A). The IL-8–dependent phosphorylation of p85 suggested that chemokine receptors used PI3K class IA in the signaling of pseudopod extension. We used GM-CSF and insulin, which allowed activation of the tyrosine kinase pathway via distinct mechanisms. GM-CSF activates PI3K via p85 phosphorylation.22 Insulin produces phosphorylated substrates, such as IRS-1, IRS-2, IRS-3, IRS-4, Shc, and GAB-1 that bind to the SH2 domain of p85.23 These substrates may activate PI3K,23 or they may not.24
Phosphorylation of p85 and measurement of p110 activity. A concentration of 10–7 M chemoattractant was chosen. (A) Cells in control conditions. Top subpanel: Neutrophil suspensions were stimulated with or without 100 nM fMLP or 100 nM IL-8 for 60 seconds as indicated. The p85α was immunoprecipitated by means of a polyclonal antibody against p85α, and processed for immunoblotting (IB) as detailed in “Materials and methods” with p85α (anti-p85α), phosphorylated p85 (anti–pp85), or antiphosphotyrosine (anti-PY) antibodies. The data shown are representatives of at least 3 experiments, with identical results, on separate cell preparations. Bottom subpanel: In vitro measurement of PtdIns 3-kinase activity in neutrophils. The cells were activated with 100 nM fMLP (•) or 100 nM IL-8 (○) for the indicated periods of time. Then, p85 was coimmunoprecipitated with p110 from the activated cellular lystates, and p110 kinase activity was measured by [32P] incorporation into PtdIns substrate. The panels above the graph depict the result of a representative TLC plate, and the average densitometric values as a percentage of control ± SD shown are calculated from 3 independent experiments. (B) Same as panel A for cells pretreated for 30 minutes with 30 pM GM-CSF. (C) Same as panel A for cells pretreated for 30 minutes with 320 μU/mL insulin.
Phosphorylation of p85 and measurement of p110 activity. A concentration of 10–7 M chemoattractant was chosen. (A) Cells in control conditions. Top subpanel: Neutrophil suspensions were stimulated with or without 100 nM fMLP or 100 nM IL-8 for 60 seconds as indicated. The p85α was immunoprecipitated by means of a polyclonal antibody against p85α, and processed for immunoblotting (IB) as detailed in “Materials and methods” with p85α (anti-p85α), phosphorylated p85 (anti–pp85), or antiphosphotyrosine (anti-PY) antibodies. The data shown are representatives of at least 3 experiments, with identical results, on separate cell preparations. Bottom subpanel: In vitro measurement of PtdIns 3-kinase activity in neutrophils. The cells were activated with 100 nM fMLP (•) or 100 nM IL-8 (○) for the indicated periods of time. Then, p85 was coimmunoprecipitated with p110 from the activated cellular lystates, and p110 kinase activity was measured by [32P] incorporation into PtdIns substrate. The panels above the graph depict the result of a representative TLC plate, and the average densitometric values as a percentage of control ± SD shown are calculated from 3 independent experiments. (B) Same as panel A for cells pretreated for 30 minutes with 30 pM GM-CSF. (C) Same as panel A for cells pretreated for 30 minutes with 320 μU/mL insulin.
The priming of superoxide production by GM-CSF is widely recognized32 ; however, the role of GM-CSF in chemotaxis is controversial.33 There are reports showing that GM-CSF has no significant effect on chemotaxis34 ; however, other reports show that it either blocks chemotaxis35 or enhances it.36 One possibility for the discrepancies in the reported effects of GM-CSF on chemotaxis is the possible involvement of adhesion receptors, which are easily primed. We started our experiments with characterizing the effect of the time of incubation with GM-CSF on the rate of pseudopod extension and the effect of growth factor concentration (Figures S1-S3).
The measurement of the rate of pseudopod extension stimulated with IL-8 versus the time of incubation with GM-CSF showed that for the first 30 minutes the rate of pseudopod extension increased continuously. After the first 30 minutes, the rate of pseudopod extension remained almost constant for 1 hour and then eventually returned to its initial value. There was also an insignificant increase of the cellular PtdIns(3,4,5)P3 within the first minutes of incubation with GM-CSF, which persisted for the duration of our experiments (Figures S1-S3). The rate of pseudopod extension stimulated with fMLP was independent of GM-CSF, while the rate of pseudopod extension stimulated with IL-8 increased and had a maximum at concentrations from 30 to 50 pM. We used 30 pM GM-CSF, because this is a commonly used concentration in the studies of superoxide anion production. All measurements of pseudopod extension with GM-CSF were performed between the 45th and the 75th minute of incubation with the growth factor.
GM-CSF had a small effect on the total F-actin polymerization and PtdIns(3,4,5)P3 production after activation with fMLP (Figure 2B). All fMLP-induced responses were strongly dependent on wortmannin, while PI3K-SH2-OMT had only a minor effect. Overall, GM-CSF had no effect on the fMLP-induced responses, which suggested that the fMLP-induced responses were independent of GM-CSF. In contrast, the IL-8–stimulated responses were strongly dependent on GM-CSF. The IL-8–stimulated pseudopod extension increased by 50%, and the total F-actin content increased as well as the total cellular PtdIns(3,4,5)P3 (Figure 3B). The effect of GM-CSF on the rate of pseudopod extension and PtdIns(3,4,5)P3 production was completely blocked by wortmannin, while the total F-actin was significantly reduced. The incubation of cells with PI3K-SH2-OMT reduced the rate of pseudopod extension with the same amount as in the absence of GM-CSF, while PtdIns(3,4,5)P3 production and total F-actin content were not significantly affected. These data suggest that the priming of the IL-8–stimulated responses by GM-CSF involves activation of p85/p110. To determine whether p85/p110 was indeed involved, we measured p85 phosphorylation and p110 activity (Figure 4B). After a 30-minute incubation with GM-CSF, a significant phosphorylation of p85 was observed. The activity of p85/p110 was also increased, and this increase was the greatest after IL-8 activation. The comparison of the data for PtdIns(3,4,5)P3 in Figure 2A and 2B shows that GM-CSF has only a minor effect on the fMLP-dependent PtdIns(3,4,5)P3 production.
The insulin receptor provides an alternative pathway for activating PI3K by binding of phosphorylated substrates including IRS-1 to the SH2 domain of p85.37 The activation of the insulin receptor activates PI3K and induces PtdIns(3,4,5)P3 production; however, after 30 minutes the cellular PtdIns(3,4,5)P3 returns to the control level.38 We measured the effect of time of incubation on the fMLP- and IL-8–stimulated rate of pseudopod extension and IL-8–induced PtdIns(3,4,5)P3 production (Figures S1-S3). The IL-8–stimulated pseudopod extension increased by almost 50% after the first 5 minutes of incubation with insulin and remained at this level for 2 hours. The cellular PtdIns(3,4,5)P3 increased 4-fold at 30 seconds after the initial exposure to insulin and returned to control level after 10 minutes (Figures S1-S3 and Rordorf-Nikolic et al37 ). The dependence of the rate of pseudopod extension on insulin concentration showed that 320 μU/mL insulin was sufficient to induce a maximum response. The fMLP-stimulated pseudopod extension was independent of insulin (Figure 2C).
The priming with insulin increased the IL-8–stimulated rate of pseudopod extension and total F-actin content; however, it had no effect on PtdIns(3,4,5)P3 production (Figure 3C). This suggested that insulin affected F-actin polymerization independently of PI3K activation. The measurement of p85 phosphorylation after incubation with insulin showed that there was only a small increase in the level of IL-8–dependent p85 phosphorylation compared with control. The measurement of p85/p110 activity also showed a small increase relative to control (Figure 4). However, the effects of insulin on p85 phosphorylation and p85/p110 activity were much smaller compared with the effects of GM-CSF. Overall, these data show that the effect of insulin on F-actin polymerization is independent of PI3K. Consistent with this conclusion was the finding that wortmannin decreased the rate of IL-8–stimulated pseudopod extension by the same amount as in control (Figure 3A,C), while PI3K-SH2-OMT almost completely blocked the effect of insulin. The simultaneous incubation with wortmannin and PI3K-SH2-OMT abolished pseudopod extension and reduced the total F-actin content similarly to control. These data taken together suggest that insulin primes the PI3K-indepent pathway and PI3K-SH2-OMT inhibits insulin priming.
Discussion
Our results demonstrate that the PI3K-dependent and PI3K-independent signaling of pseudopod extension is used differently by the different chemotactic receptors. The pseudopod extension stimulated by the N-formyl peptide receptor was dependent mostly on PI3K activation, which was evident from the dependence of this pathway on wortmannin. This pathway represented 80% of the total rate of pseudopod extension and a significant amount of the total F-actin. The PI3K-independent F-actin polymerization was blocked with the peptide PI3K-SH2-OMT synthesized after the SH2-binding domain of the PDGF receptor.31 These data are consistent with the currently popular model of chemotaxis where PI3K activation has a central role in signaling.26 In contrast, PI3K was differentially used in different experimental conditions by the chemokine receptors. In control conditions, the PI3K-dependent pathway represented 55% of the rate of pseudopod extension. The priming with GM-CSF increased the PI3K-dependent pseudopod extension up to 60% of the total response, whereas the priming with insulin increased the PI3K-independent pseudopod extension up to 65%. The existence of a PI3K-independent pathway of chemotaxis is consistent with the existence of PI3K-independent Rac activation.39 Our previous data25 showed that the PI3K-independent pathway is inhibited with diphenyleneiodonium (DPI), which prevents reactive oxygen species formation. This suggests that the reactive oxygen species–associated Rac may be involved in the signaling of pseudopod formation.
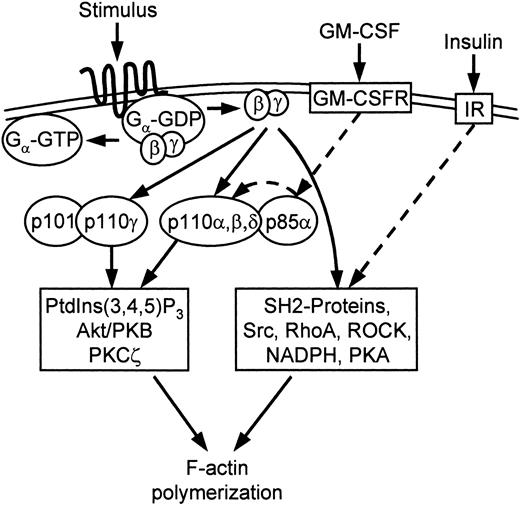
The effect of priming by RTKs provided additional insight into the signaling of chemotaxis. The involvement of the PI3Ks of class IA in the signaling of chemotaxis was evident in the cells primed with GM-CSF. The activation of the GM-CSF receptor is coupled with substantial phosphorylation of a variety of receptor tyrosine kinase–coupled proteins.40 One of the phosphorylated proteins is p85,41 which provided an excellent opportunity to test the hypothesis for the involvement of p85/p110 in the signaling of chemotaxis. The coupling of the significant p85 phosphorylation in the cells incubated with GM-CSF with the increase of p85/p110 activity12,42 and PtdIns(3,4,5)P3 production, and the increase of the rate of pseudopod extension in the same conditions by 50%, led us to the conclusion that p85/p110 was involved in the signaling of pseudopod extension and was used by the GM-CSF receptors for the priming of chemotaxis (Figure 5). This conclusion was confirmed by the ability of wortmannin to inhibit the effects of GM-CSF.
Model for signaling of cytoskeletal F-actin polymerization. Gβγ released after GPCR ligation activates PI3K-dependent and PI3K-independent pathways of F-actin polymerization. The PI3K pathway may involve the class IA PI3Ks, which are primed (dashed arrow) with GM-CSF via p85 phosphorylation. The PI3K-pathway is also dependent on Akt/PKB and PKC-ζ.25 The PI3K-independent pathway is primed with insulin (dashed arrow) via an unknown mechanism, which does not require PI3K activation. The PI3K-independent pathway is dependent on Src tyrosine kinases, RhoA, Rho-associated kinase (ROCK), NADPH oxidase, and PKA.25 The inhibition of this pathway by PI3K-SH2-OMT shows that it is dependent on an unspecified SH2 domain protein (Figures S1-S3).
Model for signaling of cytoskeletal F-actin polymerization. Gβγ released after GPCR ligation activates PI3K-dependent and PI3K-independent pathways of F-actin polymerization. The PI3K pathway may involve the class IA PI3Ks, which are primed (dashed arrow) with GM-CSF via p85 phosphorylation. The PI3K-pathway is also dependent on Akt/PKB and PKC-ζ.25 The PI3K-independent pathway is primed with insulin (dashed arrow) via an unknown mechanism, which does not require PI3K activation. The PI3K-independent pathway is dependent on Src tyrosine kinases, RhoA, Rho-associated kinase (ROCK), NADPH oxidase, and PKA.25 The inhibition of this pathway by PI3K-SH2-OMT shows that it is dependent on an unspecified SH2 domain protein (Figures S1-S3).
The effects of insulin on IL-8–induced neutrophil activation were different from the effects of GM-CSF. While insulin increased the rate of pseudopod extension and total F-actin similarly to GM-CSF, it did not increase PtdIns(3,4,5)P3 production and had only a small effect on p85 phosphorylation and p85/p110 activity. The increase of the rate of pseudopod extension was independent of wortmannin and was blocked by PI3K-SH2-OMT. These data suggested that insulin primed the PI3K-independent pathway of chemotaxis. While the mechanism of this priming is unknown, the blocking of this pathway with the Src tyrosine kinase inhibitor PP2 and the NADPH oxidase inhibitor DPI25 suggests possible involvement of the PI3K-independent Rac activation.39
In conclusion, our data provide the first direct evidence for the involvement of PI3Ks of class IA in the signaling of chemotaxis by the chemokine receptors. This pathway is primed with GM-CSF via p85 phosphorylation and p85/p110 activation. Our data also provide the first evidence for the priming of the PI3K-independent pathway of F-actin polymerization by insulin. It is possible that more than one PI3K-independent pathway is present in the neutrophil, which is suggested by the varying effects of insulin after fMLP and IL-8 activation. However, these pathways may share common signaling molecules because all PI3K-independent responses were blocked by PI3K-SH2-OMT.
Prepublished online as Blood First Edition Paper, May 22, 2003; DOI 10.1182/blood-2002-09-2936.
Supported in part by grant HL57629 from the National Institutes of Health (NIH) (D.V.Z.). D.C. is a recipient of a fellowship from NIH, Research Training Grant GM08555. Blood drawing was supported by grant M01-RR-30 from NIH to the General Clinical Research Centers Program at Duke University.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank John D. York for sharing his laboratory with us for the radioactive measurements.

![Figure 4. Phosphorylation of p85 and measurement of p110 activity. A concentration of 10–7 M chemoattractant was chosen. (A) Cells in control conditions. Top subpanel: Neutrophil suspensions were stimulated with or without 100 nM fMLP or 100 nM IL-8 for 60 seconds as indicated. The p85α was immunoprecipitated by means of a polyclonal antibody against p85α, and processed for immunoblotting (IB) as detailed in “Materials and methods” with p85α (anti-p85α), phosphorylated p85 (anti–pp85), or antiphosphotyrosine (anti-PY) antibodies. The data shown are representatives of at least 3 experiments, with identical results, on separate cell preparations. Bottom subpanel: In vitro measurement of PtdIns 3-kinase activity in neutrophils. The cells were activated with 100 nM fMLP (•) or 100 nM IL-8 (○) for the indicated periods of time. Then, p85 was coimmunoprecipitated with p110 from the activated cellular lystates, and p110 kinase activity was measured by [32P] incorporation into PtdIns substrate. The panels above the graph depict the result of a representative TLC plate, and the average densitometric values as a percentage of control ± SD shown are calculated from 3 independent experiments. (B) Same as panel A for cells pretreated for 30 minutes with 30 pM GM-CSF. (C) Same as panel A for cells pretreated for 30 minutes with 320 μU/mL insulin.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/6/10.1182_blood-2002-09-2936/6/m_h81834950004.jpeg?Expires=1769105258&Signature=B99QKKKy5UPY2w3tw3jkKB2yF8IOG0SpDZzwHH2yGZNRaBQXq33YthVpyIMvXwK6JXQXKSuHBtbL3hn6eeIPBi7hfWugCmhbjpd5dTqRAMp1wO9UWfFoihPVxr-ORSLWoeAnfj-nZuoNycb4Tg7cw-BrPemT3rEWpnGk4OSli7AxaZshvZGJ4jpEfxPFyKimx6ehMcaMbYP6SfnepGcz4xAchPnK-9L9RB6LNjd1mG-wtNWjOnjJda3rO4KcDwG4a62XrToAUdZjMRJrmvprxb0-BWBCEGQz8TUjh2bmh2uo3ArjaG2XSFYPdmCgM7s3dcYl9IccbRcTU1NN71N10g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal