Bisphosphonates are analogues of endogenous pyrophosphate in which a carbon atom replaces the central atom of oxygen. This carbon substitution makes these compounds resistant to hydrolysis and allows 2 additional chains of variable structure. One of these side chains usually contains a hydroxyl moiety that allows high affinity for calcium crystals and bone mineral. The differences at the other side chain produce marked differences in the antiresorptive potency of different bisphosphonates. In fact, the newer bisphosphonates, such as ibandronate and zoledronic acid, show 10 000- to 100 000-fold more potency than the older agents such as etidronate.1
Exposure to bisphosphonates may not only reduce bone loss but may also have an antimyeloma effect. Recent animal studies have shown that the aminobisphosphonates also have potent antiangiogenic activity that may contribute to these drugs' antibone resorptive effect as well as provide additional mechanisms by which these drugs may have antimyeloma effects as well.1
A new potential antitumor mechanism for these compounds was recently reported for aminobisphosphonates.2 These drugs were shown to induce expansion of Vγ9Vδ2 T cells in peripheral blood mononuclear cell cultures and enhance cytotoxicity of malignant plasma cells in bone marrow cultures by these Vγ9Vδ2 T lymphocytes. Thus, there is increasing evidence that bisphosphonates, especially the nitrogen-containing compounds, can lead to direct and indirect effects that result not only in less bone loss but less tumor burden as well. In support of this, Wilhelm et al3 now report the results of a pilot study of low-dose interleukin-2 (IL-2) in combination with pamidronate in patients with low-grade non-Hodgkin lymphoma or multiple myeloma. They selected 9 patients by positive in vitro proliferation of Vγ9Vδ2 T cells in response to pamidronate and IL-2, and show that 5 of the patients had significant in vivo activation/proliferation of Vγ9Vδ2 T cells. Three patients also achieved objective responses, indicating that Vγ9Vδ2 T cells might contribute to this antilymphoma effect.
To evaluate the antitumor activity of Vγ9Vδ2 T cells in vivo, we initiated a pilot study of low-dose zoledronic acid in 9 cancer patients with bone metastases (3 females affected by breast cancer; 6 males affected by prostate cancer; median age, 66 years [range, 54-83 years]). The objective of this study was to evaluate the in vivo effect of zoledronic acid on recently identified subsets of Vγ9Vδ2 cells that display different functional activities: CD45RA+CD27+ naive and CD45RA–CD27+ memory Vγ9Vδ2 cells strongly proliferate but lack immediate effector functions, while CD45RA–CD27– Vγ9Vδ2 cells proliferate poorly but produce interferon γ (IFNγ) and exert cytotoxicity.4 Both of these functions are strictly involved in their antitumor activity. Patients were treated with 4 mg zoledronic acid via 15-minute intravenous infusion every 3 weeks. Peripheral blood mononuclear cells (PBMCs) were collected before treatment (time 0), and 1 month (time 1) and 3 months (time 2) after the first administration. PBMCs were also taken from 15 age- and sex-matched healthy control subjects.
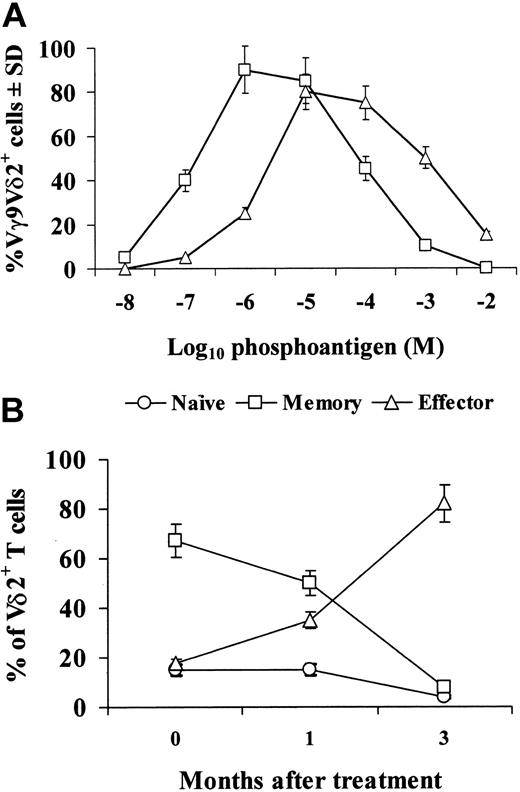
Preliminary experiments (Figure 1A) showed that zoledronic acid was as able as the phosphoantigen isopentenylpyrophosphate (IPP) to induce expansion of Vγ9Vδ2+ T cells upon culture in vitro in the presence of IL-2. The relative percentages of naive, memory, and effector subsets of Vγ9Vδ2 cells were comparable in control and cancer patients before treatment with zoledronic acid (data not shown). However, treatment with zoledronic acid caused a decrease in the percentages of naive and memory Vγ9Vδ2 cells, which was evident 1 month after the initial treatment and dramatic after 3 months (Figure 1B): in fact, at this stage, naive and memory cell subsets were less than 10% of the whole Vγ9Vδ2 population, while in control subjects these 2 subsets usually account for about 70% of Vγ9Vδ2 cells. The decrease of naive and Vγ9Vδ2 cells was paralleled by an increase in the percentage of the effector subset, which accounted for about 80% of the whole Vγ9Vδ2 population after 3 months from the initial treatment with zoledronic acid. As the percentage and absolute numbers of Vγ9Vδ2 cells in PBMCs were not modified during treatment, we interpret these results to indicate that zoledronic acid in vivo expands a subset of effector Vγ9Vδ2 cells, while decreasing the naive and memory subsets.
Zoledronic acid activates in vitro Vγ9Vδ2 cells and induces subset distribution in cancer patients in vivo. (A) PBMCs were incubated in vitro with IPP (▵) or zoledronic acid (□) at the indicated final concentrations and 10 U/mL IL-2. Then, 7 days later, cells were collected and the percentage of Vγ9Vδ2+ cells within the CD3+ population was assessed. (B) Vγ9Vδ2 T-cell subsets were analyzed by 3-color fluorescence-activated cell-sorter (FACS) analysis before or after in vivo treatment with zoledronic acid. The following antibodies were used in different combinations: anti-Vδ2 fluorescein isothiocyanate (FITC, IMMU389; Coulter, Miami, FL), anti-CD27 phycoerythrin (PE) (M-T271; BD Pharmingen, San Diego, CA), and anti-CD45RA PE–cytochrome 5 (Cy5, 2H4; Coulter). Data were acquired on a FACSCalibur instrument (BD Biosciences, San Diego, CA) and analyzed using CellQuest software (BD Immunocytometry Systems, San Jose, CA). Error bars indicate standard deviation.
Zoledronic acid activates in vitro Vγ9Vδ2 cells and induces subset distribution in cancer patients in vivo. (A) PBMCs were incubated in vitro with IPP (▵) or zoledronic acid (□) at the indicated final concentrations and 10 U/mL IL-2. Then, 7 days later, cells were collected and the percentage of Vγ9Vδ2+ cells within the CD3+ population was assessed. (B) Vγ9Vδ2 T-cell subsets were analyzed by 3-color fluorescence-activated cell-sorter (FACS) analysis before or after in vivo treatment with zoledronic acid. The following antibodies were used in different combinations: anti-Vδ2 fluorescein isothiocyanate (FITC, IMMU389; Coulter, Miami, FL), anti-CD27 phycoerythrin (PE) (M-T271; BD Pharmingen, San Diego, CA), and anti-CD45RA PE–cytochrome 5 (Cy5, 2H4; Coulter). Data were acquired on a FACSCalibur instrument (BD Biosciences, San Diego, CA) and analyzed using CellQuest software (BD Immunocytometry Systems, San Jose, CA). Error bars indicate standard deviation.
Different subsets of Vγ9Vδ2 cells have been reported to exert different functions.4 Therefore, we assessed whether modification of Vγ9Vδ2 cell subset distribution was accompanied by alterations in the functions they usually perform. As shown in Figure 2A, proliferative activity of Vγ9Vδ2 cells upon in vitro stimulation with IPP, which is a property of naive and memory cells, strongly decreased upon in vivo treatment with zoledronic acid. Conversely, production of IFNγ by Vγ9Vδ2 cells upon in vitro stimulation with IPP, which is a property of effector cells, consistently increased upon in vivo treatment with zoledronic acid.
Proliferation and IFNγ production by Vγ9Vδ2 cells from cancer patients before and after treatment with zoledronic acid. PBMCs were cultured at 37°C, in the presence of 5% CO2, at 106/mL in 96-well flat-bottomed plates (0.2 mL/well), with isopentenylpyrophosphate (IPP; Sigma Chemical, St Louis, MO; 100 μM/mL final concentration) and 20 U/mL final concentration human recombinant IL-2 (▪) or with IL-2 alone as a control (□). Proliferation (A) was measured 7 days later by adding 1 μCi/well (0.037 MBq) [3H]thymidine (Amersham, Arlington Heights, IL) during the last 6 hours of culture. Cells were then harvested and [3H]thymidine incorporation was measured with a liquid scintillation β-counter. Results are expressed as mean counts per minute (cpm) of triplicate wells ± standard deviation (SD). IFNγ levels in the 48-hour culture supernatants (B) were assessed by 2 monoclonal antibodies (mAbs) sandwich enzyme-linked immunosorbent assay (ELISA) assay following the manufacturer's recommendations (R&D Systems, Minneapolis, MN).
Proliferation and IFNγ production by Vγ9Vδ2 cells from cancer patients before and after treatment with zoledronic acid. PBMCs were cultured at 37°C, in the presence of 5% CO2, at 106/mL in 96-well flat-bottomed plates (0.2 mL/well), with isopentenylpyrophosphate (IPP; Sigma Chemical, St Louis, MO; 100 μM/mL final concentration) and 20 U/mL final concentration human recombinant IL-2 (▪) or with IL-2 alone as a control (□). Proliferation (A) was measured 7 days later by adding 1 μCi/well (0.037 MBq) [3H]thymidine (Amersham, Arlington Heights, IL) during the last 6 hours of culture. Cells were then harvested and [3H]thymidine incorporation was measured with a liquid scintillation β-counter. Results are expressed as mean counts per minute (cpm) of triplicate wells ± standard deviation (SD). IFNγ levels in the 48-hour culture supernatants (B) were assessed by 2 monoclonal antibodies (mAbs) sandwich enzyme-linked immunosorbent assay (ELISA) assay following the manufacturer's recommendations (R&D Systems, Minneapolis, MN).
Although the data here reported strengthen the results of Wilhelm et al3 (ie, the high IFNγ concentrations in serum and the slow response profiles of most of lymphoma patients), a major difference to the study by Wilhelm et al is that they used a combination of pamidronate and low-dose IL-2 in vivo. In principle, IL-2 application in vivo might not have influenced their results, as indicated by the findings that aminobisphosphonate-induced Vγ9Vδ2 T-cell activation and IFNγ production is strictly dependent on the presence of monocytes,5 and that Vγ9Vδ2 T cells can be quickly and antigen-nonspecifically activated, even in the absence of IL-2, by monocyte-derived IL-12 and tumor necrosis factor α (TNFα).6
All together, our results indicate that in vivo treatment with zoledronic acid induces Vγ9Vδ2 cells to mature toward an IFNγ-producing effector phenotype, which may induce more effective antitumor responses.

![Figure 2. Proliferation and IFNγ production by Vγ9Vδ2 cells from cancer patients before and after treatment with zoledronic acid. PBMCs were cultured at 37°C, in the presence of 5% CO2, at 106/mL in 96-well flat-bottomed plates (0.2 mL/well), with isopentenylpyrophosphate (IPP; Sigma Chemical, St Louis, MO; 100 μM/mL final concentration) and 20 U/mL final concentration human recombinant IL-2 (▪) or with IL-2 alone as a control (□). Proliferation (A) was measured 7 days later by adding 1 μCi/well (0.037 MBq) [3H]thymidine (Amersham, Arlington Heights, IL) during the last 6 hours of culture. Cells were then harvested and [3H]thymidine incorporation was measured with a liquid scintillation β-counter. Results are expressed as mean counts per minute (cpm) of triplicate wells ± standard deviation (SD). IFNγ levels in the 48-hour culture supernatants (B) were assessed by 2 monoclonal antibodies (mAbs) sandwich enzyme-linked immunosorbent assay (ELISA) assay following the manufacturer's recommendations (R&D Systems, Minneapolis, MN).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/6/10.1182_blood-2003-05-1655/6/m_h81835050002.jpeg?Expires=1769205653&Signature=rTgHAnrjcK5Ai35TdGfquuqgSH92kU0awYKDs3GzkIhZqb1J8BM6mVdnlsHFoGhecMY-7AQ2B2~nHZ9uX1xGGRVTgRJ9ZenEjrP~4r4xE~gWD2GVvm6soAor~SviG~EJwHAapr1GNSLxHk9mXoSIFFoRnHq93nCk5K~9AddfX1T0fhzbutxzdpd67kmWaMHCoMaK2wtwfsEU3yXWjjdXzLLSCNqZXq2yL~F5S9u2lIhlvTn1OcVC~KTy3bE-eidw4ha4~rkUVeZ1EWV8HBi15hNi5BjWSllKCjBH0vscBNItMXWD2WtKFLjSvJcW1cSS5Ht18NGfqFO5s8AcY-fRYA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal