Abstract
The rapid exocytosis of von Willebrand factor (VWF) in response to vascular injury can be attributed to the fact that VWF is stored in the Weibel-Palade bodies (WPBs) of endothelial cells. We describe a system for examining the ability of VWF to drive both the formation of a storage compartment and the function of that compartment with respect to regulated secretion. Transient transfection of HEK293 cells with wild-type human VWF cDNA leads to the formation of numerous elongated organelles that resemble WPBs. These “pseudo-WPBs” exhibit the internal structure, as well as the ability to recruit membrane proteins including P-selectin, of bona fide WPBs. Finally, VWF was efficiently secreted upon stimulation by phorbol ester. We used this system to examine 3 VWF mutations leading to von Willebrand disease that affect VWF multimerization and constitutive secretion. Surprisingly we find that all 3 mutants can, to some extent, make pseudo-WPBs that recruit appropriate membrane proteins and that are responsive to secretagogues. The most striking defects are a delay in formation and a reduction in the length and number of pseudo-WPBs in proportion to the clinical severity of the mutation. Studies of pseudo-WPB formation in this system thus yield insights into the structure-function relationships underpinning the ability of VWF to form functional WPBs. (Blood. 2003;102:2452-2458)
Introduction
The glycoprotein von Willebrand factor (VWF) circulates in plasma as multimers ranging in size from 5 × 105 to 20 × 106 Da and is implicated in primary and secondary hemostasis.1 Its role in secondary hemostasis is to act as a carrier for clotting factor VIII, protecting it from proteolytic inactivation in the circulation. In the primary hemostatic response, VWF is secreted by endothelial cells and recruits platelets to the site of vascular injury. The VWF precursor spans 2813 amino acids and is composed of a signal peptide (22 amino acids), an N-terminal propeptide comprising the D1 and D2 domains (741 amino acids), and the mature VWF polypeptide (2050 amino acids) consisting of D′-D3-A1-A2-A3-D4-B1-B2-B3-C1-C2-CK domains.2,3 Within the cell, VWF exists both as an uncleaved proprotein called proVWF (propeptide plus mature VWF) and as a cleaved mature VWF. The C-terminal part of VWF is implicated in dimerization of the protein, which is followed by further extensive multimerization dependent on formation of disulfide bonds in both the propeptide and the N-terminal region of mature VWF.
The cell biology of VWF has been the focus of several studies.4,5 VWF is translocated in the endoplasmic reticulum (ER), where it undergoes several modifications, including N-linked oligosaccharide addition and disulfide-bond formation leading to C-terminal dimerization. It then passes through the Golgi to the trans-Golgi network (TGN), where N-terminal multimerization occurs. VWF is secreted by both constitutive and regulated pathways. Constitutive secretion is thought to occur directly from the TGN to the plasma membrane, whereas regulated exocytosis operates on VWF stored in specific organelles called Weibel-Palade bodies (WPBs), which are found only in endothelial cells. It has been demonstrated that the content of WPBs consists mainly of VWF. This stored VWF forms a readily releasable pool that can be secreted upon stimulation by a secretagogue during the primary hemostatic response. Two integral membrane proteins are known to be recruited to WPBs: the leukocyte adhesion receptor P-selectin6,7 and the tetraspanin CD63.8 Heterologous expression of VWF can drive the formation of WPB-like organelles in several, but not all, cell types (reviewed in Hannah et al4 ). These pseudogranules are capable of regulated exocytosis and of recruiting appropriate membrane proteins in a specific and signal-dependent manner. Because it is responsible for driving the formation of WPBs including the recruitment of P-selectin, functional VWF not only is central to hemostasis but is also critical to the ability of the endothelium to mount an inflammatory response.9
Mutations in the VWF gene are responsible for von Willebrand disease (VWD), the most common inherited bleeding disorder in humans. Three types of VWD are recognized.10 Types 1 and 3 are characterized by a quantitative deficiency in plasma VWF, partial or complete, respectively, and type 2 by a qualitative deficiency affecting aspects of VWF function such as binding of VWF to factor VIII and to platelets. Mutations can also affect the ability of VWF to multimerize. For example, Cys788Arg and Cys1225Gly can be described as both type 1 and type 2 mutations since they fail to bind factor VIII and also show reduced secretion of VWF.11 The mutation Arg273Trp can result in type 1 or type 3 VWD, with no current explanation for the different phenotypes observed in homozygous individuals in families affected by this mutation.12 Following expression in COS-7 cells of recombinant VWF (rVWF) proteins having each of these 3 mutations, Allen and coworkers11,12 showed that the Cys1225Gly mutation causes a mild reduction in multimerization and constitutive secretion; the Cys788Arg mutation has significant defects in both; the Arg273Trp mutation forms only low molecular weight multimers and its constitutive secretion is severely affected.
In this study, we present the effects of these 3 mutations on the biogenesis and function of WPBs. We have introduced plasmids encoding either wild-type (WT) or mutant recombinant proteins in a heterologous system to monitor the morphology and the formation of what we have called pseudo-WPBs, the recruitment of the WPB membrane proteins P-selectin and CD63 to these organelles, and the regulated secretion of VWF from the pseudo-WPBs. We show that, while they exhibit significant and reproducible differences in their ability to do so, the WT and mutant rVWFs are all able to induce the formation of elongated organelles containing VWF, which can recruit membrane proteins and be secreted upon stimulation.
Materials and methods
Cell culture and transfection
HEK293 cells were provided by BD Biosciences (Oxon, United Kingdom) and were grown in alpha-minimum essential medium (alpha-MEM) (Life Technologies, Paisley, United Kingdom), 10% fetal calf serum, 50 μg/mL gentamicin (Life Technologies). Human umbilical vein endothelial cells (HUVECs) were obtained from Cascade Biologicals (Portland, OR). The cells were grown in M199 (Gibco BRL, Grand Island, NY) supplemented with 20% fetal calf serum (Hyclone, Logan, UT), 10 U/mL heparin (Sigma, St Louis, MO), 50 μg/mL gentamicin, and 30 μg/mL endothelial cell growth supplement (Sigma). Cells were transiently transfected by Nucleofection (Amaxa, Cologne, Germany) according to the manufacturer's instructions, leading to a transfection efficiency of 40% for WT and mutant rVWF with a variation of 10% between experiments.
Expression vectors
The plasmid allowing enhanced green fluorescent protein (EGFP) expression of EGFP-CD63 has been described.13 Signal sequence horseradish peroxidase (ssHRP)-P-selectin was used to detect P-selectin as previously described.14 Recombinant pSV7D expression plasmids containing full-length cDNAs encoding wild-type human VWF and the Cys1225Gly, Cys788Arg, and Arg273Trp rVWF variants have been described previously. For the studies described here, the full-length cDNAs were removed as EcoRI fragments of the pSV7D expression plasmids and cloned into the EcoRI site of the pCI-neo mammalian expression vector (Promega, Southampton, United Kingdom).
Antibodies and confocal immunofluorescence microscopy
Sheep polyclonal antihuman VWF was purchased from Serotec (Oxford, United Kingdom). HRP-coupled anti-VWF and rabbit polyclonal anti-HRP antibodies were purchased from DAKO (Cambridgeshire, United Kingdom). Rabbit polyclonal antihuman calreticulin was purchased from Cambridge BioScience (Cambridge, United Kingdom). Secondary antibodies coupled to fluorophores were purchased from Jackson (Luton, United Kingdom). For immunofluorescence studies, antibody staining and confocal microscopy were carried out as previously described.15 In all the figures, data were collected 48 hours after nucleofection and were piled-up z-series, unless otherwise stated. To measure the length of WPBs (Figure 4A), Volocity software (Improvision, Coventry, United Kingdom) was used. Briefly, we randomly selected about 30 cells from 4 independent experiments containing at least 20 pseudo-WPBs and having VWF present in pseudo-WPBs only (no ER staining); pictures were obtained by confocal microscopy and opened with Volocity (Improvision, Coventry, United Kingdom) where the length of each isolated spot of VWF staining was measured with the “Measurements” tool. To count the number of WPBs (Figure 4B), we looked at 80 cells from 4 different experiments.
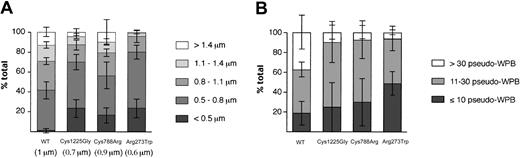
Formation of shorter and fewer pseudo-WPBs following expression of mutant rVWF. HEK293 cells were transiently transfected with WT rVWF or rVWF variants and immunolabeled for VWF 2 days later. (A) The Volocity program was used to determine the length of at least 450 pseudo-WPBs for WT and all 3 mutants following transfection. The graph represents the percentage of pseudo-WPBs in each category (fewer than 0.5 μm, then increasing in 0.3-μm steps to more than 1.4 μm). Minimum and maximum lengths are 0.4 to 3.6 μm for WT rVWF; 0.4 to 2.8 μm for rVWF-Cys1225Gly; 0.4 to 3.4 μm for rVWF-Cys788Arg; and 0.4 to 2.0 μm for rVWF-Arg273Trp. The average length is indicated in parentheses. Each bar represents the mean ± standard deviation of all WPBs from about 30 cells in 4 independent experiments. (B) We counted the number of pseudo-WPBs in 20 randomly chosen cells. The graph represents the percentage of cells in each category (fewer than 10; from 11 to 30; and more than 30 pseudo-WPBs per cell). Each bar represents the mean ± standard deviation of 4 independent experiments.
Formation of shorter and fewer pseudo-WPBs following expression of mutant rVWF. HEK293 cells were transiently transfected with WT rVWF or rVWF variants and immunolabeled for VWF 2 days later. (A) The Volocity program was used to determine the length of at least 450 pseudo-WPBs for WT and all 3 mutants following transfection. The graph represents the percentage of pseudo-WPBs in each category (fewer than 0.5 μm, then increasing in 0.3-μm steps to more than 1.4 μm). Minimum and maximum lengths are 0.4 to 3.6 μm for WT rVWF; 0.4 to 2.8 μm for rVWF-Cys1225Gly; 0.4 to 3.4 μm for rVWF-Cys788Arg; and 0.4 to 2.0 μm for rVWF-Arg273Trp. The average length is indicated in parentheses. Each bar represents the mean ± standard deviation of all WPBs from about 30 cells in 4 independent experiments. (B) We counted the number of pseudo-WPBs in 20 randomly chosen cells. The graph represents the percentage of cells in each category (fewer than 10; from 11 to 30; and more than 30 pseudo-WPBs per cell). Each bar represents the mean ± standard deviation of 4 independent experiments.
Electron microscopy
HEK293 cells were nucleofected with the WT rVWF and mutant rVWF plasmids and plated onto 6-well plates (for embedding pellets) or onto 10-mm diameter coverslips (for embedding en face). At 2 or 3 days after nucleofection, cells were fixed with 2% paraformaldehyde/1.5% glutaraldehyde in 0.1 M sodium cacodylate buffer for 20 minutes at room temperature. The cells were then postfixed in 1% osmium tetroxide/1.5% potassium ferricyanide and treated with tannic acid as described previously.16 The samples were then embedded in epon by conventional procedures. For samples embedded en face, coverslips were removed by plunging the samples into liquid nitrogen, leaving the cells embedded on the epon. Then, 60-nm sections were cut by means of a Leica Ultracut UCT microtome (Leica, Vienna, Austria). Sections were stained with lead citrate and viewed with a transmission electron microscope (EM420; Philips, Eindhoven, the Netherlands).
VWF multimer analysis
HEK293 cells were incubated for 3 days in normal growth medium, which was then collected. Then, 100 nM phenylmethylsulfonyl fluoride (PMSF) and protease inhibitors (Roche, Basel, Switzerland) were added. To quantify the amount of intracellular VWF, cells were lysed for 60 minutes at 4°C in lysis medium (100 mM Tris, 1 mM EDTA [ethylenediaminetetraacetic acid], 150 mM NaCl, 0.5% deoxycholic acid, 0.02% sodium azide, 0.5% Triton X-100) in presence of protease inhibitors. All the samples were centrifuged, and prior to electrophoresis, the supernatant was concentrated about 10 times by means of Centricon centrifugal filter devices (Millipore, Billerica, Spain) according to the manufacturer's instructions.
VWF multimers were separated by electrophoresis in 1.5% (wt/vol) agarose gels containing 0.1% (wt/vol) sodium dodecyl sulfate (SDS). Following electrophoresis, VWF was detected in the gel by means of rabbit antihuman VWF (Dako, Glostrup, Denmark) and visualized by means of alkaline phosphatase-conjugated swine antirabbit immunoglobulins (Dako, Denmark) and the AP Conjugate Substrate kit (Biorad, Hertfordshire, United Kingdom).17
Constitutive and regulated VWF secretion and VWF ELISA
To quantify constitutive and regulated secretion, HEK293 cells were transfected to express VWF and left for 48 hours to recover. They were rinsed and then incubated in normal growth medium (when appropriate, signal originating from bovine VWF has been subtracted from the data presented) for 4 hours to quantify constitutive secretion or for various times at 37°C in release medium (alpha-MEM medium, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] pH 7.3, 0.2% bovine serum albumin) in the absence or presence of phorbol 12-myristate 13-acetate (PMA) (Sigma) at a final concentration of 100 ng/mL (160 nM). All media samples were then collected and centrifuged for 5 minutes at 10 000g, and the supernatant was then analyzed further. To quantify the amount of VWF inside the cells following stimulated release, cells were lysed for 30 minutes at 4°C in lysis medium (alpha-MEM medium, 10 mM HEPES pH 7.3, 0.2% bovine serum albumin, 0.1% Triton X-100), in the presence of protease inhibitors. The lysis medium was then collected and centrifuged for 5 minutes at 10 000g. VWF in all samples was measured by solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) as described.15 To analyze the kinetics of secretion, we stimulated the cells for 15, 30, 45, and 60 minutes with PMA. On the basis of previous work by Ewenstein et al,18 regulated secretion for WT and mutant rVWF was analyzed after a 30-minute PMA stimulation.
Results
VWF expression in HEK293
To establish a system for examining the ability of VWF and its mutants to drive the formation of functional pseudo-WPBs, several established cell lines were screened. To avoid any possible complication arising from the presence of endothelial-specific machinery affecting the formation of WPBs and also to examine mutations having recessive effects, only nonendothelial cell lines were tested. Readily transfectable lines were assessed for the following characteristics: (1) formation of VWF+ elongated organelles, (2) VWF multimer formation, (3) recruitment, by VWF+ organelles, of membrane proteins normally found on WPBs, and (4) secretion of stored VWF upon secretagogue stimulation. Several cell lines, including AtT-20, T24, and HEK293, have previously been used to express VWF,4 but it was not clear whether any of these lines could meet all the criteria for a suitable system listed above. For example, the T24 cell line makes elongated organelles that can be released upon stimulation after transfection with a VWF expression plasmid, but the recruitment of membrane proteins has not been tested19 (note that in the cited report, the ECV304 cells are T24 cells20 ). Nucleofection was used to transiently express WT rVWF in several cell lines. When WT rVWF was expressed in T24 cells, formation of storage organelles was induced, but only in very few cells, and most of the organelles were not elongated (data not shown). We found that the VWF+ organelles formed after WT rVWF expression in AtT-20 cells are often more round than elongated (data not shown). This phenomenon can also be seen in published data describing VWF expression in AtT-20 cells.21 While the significance of the shape of any VWF-containing organelle is not clear, we thought it was important to work with a storage granule that resembles as closely as possible the bona fide WPBs. Finally, the HEK293 cell line has been reported to express VWF at a high level,21 and to form VWF+ elongated organelles with internal striations,4 but had not been fully examined for membrane protein recruitment or for regulated exocytosis of VWF. The suitability of HEK293 was examined further.
rVWF expression and pseudo-WPB formation
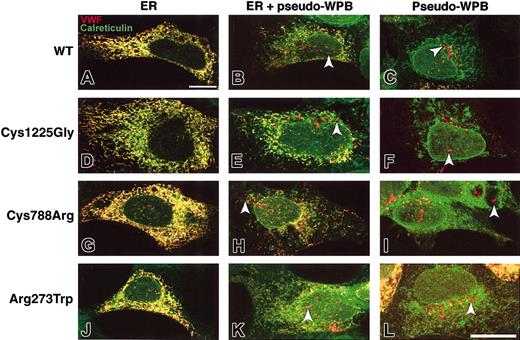
VWF subcellular localization was observed after HEK293 cells had been nucleofected so as to express WT and mutant rVWF. Cells were plated after nucleofection, left for 2 days, and then processed for confocal indirect immunofluorescence microscopy. Costaining with antibodies against VWF and the endoplasmic reticulum marker calreticulin shows that WT rVWF and the rVWF variants have a similar subcellular localization (Figure 1). Three categories of cells were identified; in the first one, VWF was visible only in a meshlike structure, identified as ER by the distribution of calreticulin. In the second group, VWF colocalized with calreticulin but was also visible in rod-shaped organelles identified as pseudo-WPBs. The last category comprised cells where VWF was visible only in pseudo-WPBs. In particular, rVWF-Arg273Trp and rVWF-Cys788Arg expression can induce the formation of some elongated organelles (Figure 1H,I,K,L).
Subcellular distribution of WT and mutant rVWF. HEK293 cells were transiently transfected with either WT rVWF (A-C); rVWF-Cys1225Gly (D-F); rVWF-Cys788Arg (G-I); or rVWF-Arg273Trp (J-L), and were coimmunolabeled for VWF and the endoplasmic reticulum marker calreticulin. In panels A, D, G, and J, VWF exhibits ER staining only (in yellow); in panels B, E, H, and K, VWF exhibits ER (in yellow) and pseudo-WPB staining (in red; arrowheads); in panels C, F, I, and L, VWF exhibits pseudo-WPB staining only (in red; arrowheads). Bar is 20 μm.
Subcellular distribution of WT and mutant rVWF. HEK293 cells were transiently transfected with either WT rVWF (A-C); rVWF-Cys1225Gly (D-F); rVWF-Cys788Arg (G-I); or rVWF-Arg273Trp (J-L), and were coimmunolabeled for VWF and the endoplasmic reticulum marker calreticulin. In panels A, D, G, and J, VWF exhibits ER staining only (in yellow); in panels B, E, H, and K, VWF exhibits ER (in yellow) and pseudo-WPB staining (in red; arrowheads); in panels C, F, I, and L, VWF exhibits pseudo-WPB staining only (in red; arrowheads). Bar is 20 μm.
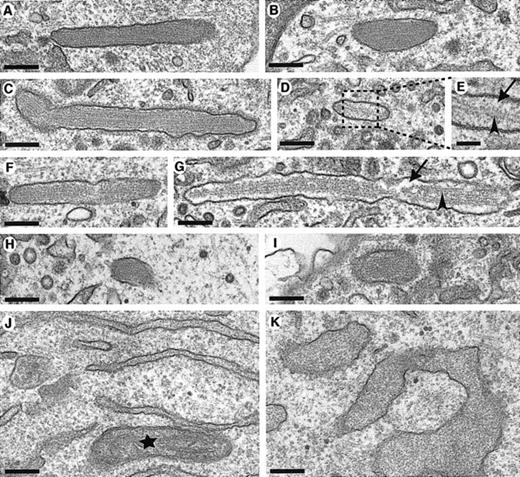
To further characterize the pseudo-WPBs, their ultrastructure was examined by transmission electron microscopy (Figure 2). As we have previously reported,4 WT rVWF induced the formation of pseudo-WPBs indistinguishable from the WPBs found in endothelial cells. In particular, the membrane-bound organelle was filled with internal striations (Figure 2A-B). In HEK293 cells expressing rVWF-Cys1225Gly and rVWF-Cys788Arg, elongated pseudo-WPBs were also visible (Figure 2C-G). However, in these organelles, an empty space between the striations and the membrane was sometimes observed, and very few striations were seen in some of the pseudo-WPBs (Figure 2D,E,G). A likely interpretation is that these were pseudo-WPBs in which the VWF condensation process was not complete. Some striated pseudo-WPBs were detected after rVWF-Arg273Trp expression (Figure 2H-I), although they were rare and much shorter. In agreement with the report of prolonged interaction between this mutant protein and ER chaperones,22 a grossly swollen ER filled with electron-dense material was observed in these cells (Figure 2K), which suggests that the accumulated mutant rVWF fails to exit the ER owing to defects in folding.
Pseudo-WPB formation after WT and mutant rVWF expression. HEK293 cells were transiently transfected with either WT rVWF in (A,B,J); rVWF-Cys1225Gly (C-E); rVWF-Cys788Arg (F-G), or rVWF-Arg273Trp (H,I,K), and were observed by transmission electron microscopy 2 days (WT, Cys788Arg, and Cys1225Gly) or 3 days (Arg273Trp) later. Sections were made en face, as shown in panels A-I, or in a pellet in panels J-K. Longitudinal sections of membrane-bound pseudo-WPBs with internal striations are shown in panels A-I. Note that in some of the pseudo-WPBs, there are few striations, as shown in panel G (arrowhead), or only one striation, shown in panels D-E (arrowhead), and that there is empty space in these organelles, shown in panels E and G (arrows). The inset in panel E shows as enlargement of the region boxed with a dashed line in panel D. After WT rVWF expression, the ER is normal as shown in panel J, but rVWF-Arg273Trp expression induces the deformation of the ER, which becomes swollen. The star in panel J indicates a mitochondrion. Scale bar is 200 nm (50 nm in panel E).
Pseudo-WPB formation after WT and mutant rVWF expression. HEK293 cells were transiently transfected with either WT rVWF in (A,B,J); rVWF-Cys1225Gly (C-E); rVWF-Cys788Arg (F-G), or rVWF-Arg273Trp (H,I,K), and were observed by transmission electron microscopy 2 days (WT, Cys788Arg, and Cys1225Gly) or 3 days (Arg273Trp) later. Sections were made en face, as shown in panels A-I, or in a pellet in panels J-K. Longitudinal sections of membrane-bound pseudo-WPBs with internal striations are shown in panels A-I. Note that in some of the pseudo-WPBs, there are few striations, as shown in panel G (arrowhead), or only one striation, shown in panels D-E (arrowhead), and that there is empty space in these organelles, shown in panels E and G (arrows). The inset in panel E shows as enlargement of the region boxed with a dashed line in panel D. After WT rVWF expression, the ER is normal as shown in panel J, but rVWF-Arg273Trp expression induces the deformation of the ER, which becomes swollen. The star in panel J indicates a mitochondrion. Scale bar is 200 nm (50 nm in panel E).
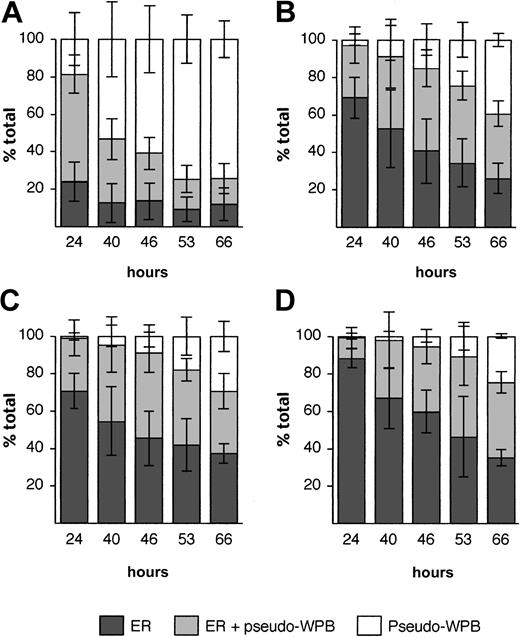
To quantify any defects in pseudo-WPB formation following expression of WT and mutant rVWF, several morphological criteria were used. The observation that at a given time after transfection, the proportion of cells containing only pseudo-WPBs was higher in cells expressing WT rVWF than in cells expressing mutant rVWF, led us to follow the evolution of the ER versus pseudo-WPB localization of rVWF over several days. The proportion of cells with ER staining was found to fall from 80% to less than 30% over 3 days for WT rVWF (Figure 3). A decrease in ER staining was also seen for all the mutant rVWF, although the reduction was less dramatic, leaving 60% to 70% of the cells with ER staining after 66 hours (Figure 3). This indicated that WT rVWF exited the ER faster than the mutant rVWF proteins. Determination of the lengths of pseudo-WPBs (“Materials and methods”) showed that pseudo-WPBs made from mutant rVWF were shorter, especially for the Arg273Trp mutation, than those made of WT rVWF (Figure 4A). Finally, 48 hours after transfection, WT rVWF expression led to the formation of more than 30 pseudo-WPBs per cell in 40% of the cells, compared with fewer than 10% of cells expressing mutant rVWF (Figure 4B). These significant differences between the WT and mutant rVWF probably arise from the effects of the mutations within VWF.
Retention of mutant rVWF in the ER and delay in pseudo-WPB formation. HEK293 cells were transiently transfected with WT rVWF (A) or mutated rVWF (Cys1225Gly, panel B; Cys788Arg, panel C; Arg 273Trp, panel D) and immunolabeled for VWF and calreticulin at different times after transfection. At each time point, 50 cells were observed, and the percentage of cells was plotted according to the VWF subcellular localization (Figure 1). Each bar represents the mean ± standard deviation of 4 independent experiments.
Retention of mutant rVWF in the ER and delay in pseudo-WPB formation. HEK293 cells were transiently transfected with WT rVWF (A) or mutated rVWF (Cys1225Gly, panel B; Cys788Arg, panel C; Arg 273Trp, panel D) and immunolabeled for VWF and calreticulin at different times after transfection. At each time point, 50 cells were observed, and the percentage of cells was plotted according to the VWF subcellular localization (Figure 1). Each bar represents the mean ± standard deviation of 4 independent experiments.
Multimer analysis
To analyze multimerization of rVWF, cell lysate and media samples were separated on 1.5% agarose gels under nonreducing conditions, and the VWF multimers were detected immunologically (Figure 5). As with various other heterologous expression systems,23,24 rVWF is present primarily as dimers in HEK293 cell lysates (Figure 5A). However, a full range of multimers is visible in the growth medium. As already shown,11,12 rVWF-Cys1225Gly lacks only very high molecular weight forms, whereas rVWF-Cys788Arg and rVWF-Arg273Trp are more severely affected, showing only dimers and low molecular weight multimers.
Multimer analysis following rVWF expression. HEK293 cells were transiently transfected with WT and mutant rVWFs. Samples of cell lysate (A) and media (B) were prepared as described in “Materials and methods” and were electrophoresed in 1.5% agarose/0.1% SDS gels under nonreducing conditions. Multimers were visualized by means of a polyclonal rabbit antihuman VWF serum and alkaline phosphatase-conjugated swine antirabbit immunoglobulins.
Multimer analysis following rVWF expression. HEK293 cells were transiently transfected with WT and mutant rVWFs. Samples of cell lysate (A) and media (B) were prepared as described in “Materials and methods” and were electrophoresed in 1.5% agarose/0.1% SDS gels under nonreducing conditions. Multimers were visualized by means of a polyclonal rabbit antihuman VWF serum and alkaline phosphatase-conjugated swine antirabbit immunoglobulins.
Membrane protein recruitment
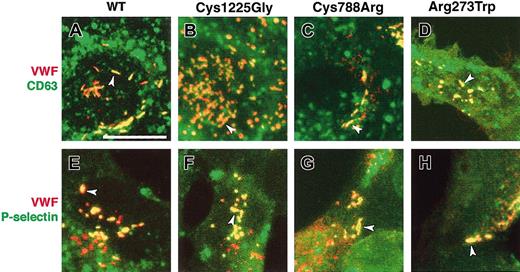
In addition to storing VWF, another important function of WPBs is to support the rapid surface appearance of P-selectin and other membrane proteins. To examine the ability of WT rVWF to drive the formation of WPBs that can acquire appropriate membrane proteins, WT rVWF was coexpressed with 2 WPB membrane proteins. Coexpression of WT rVWF and 2 chimeric proteins, EGFP-CD63 and HRP-P-selectin, revealed that both were recruited to the membrane of pseudo-WPBs (Figure 6A,E). In accordance with previous studies in other cell types, these 2 proteins are also associated with other intracellular compartments, primarily those of the endocytic pathway, and are therefore also visible at other cellular locations.15,25 To test the effects of the mutations affecting VWF, the same markers were coexpressed with the 3 mutant rVWF proteins; in each case, the results were indistinguishable from those obtained after WT rVWF expression (Figure 6). WPBs are often categorized as lysosome-related organelles (LROs), one characteristic of which is to share proteins with late endosomes or lysosomes.26 Further, LROs are often derived by modification of compartments of the endocytic pathway. The membrane acquisition by rVWF in HEK293 cells was therefore further characterized by examining the localization of a series of representative markers for colocalization with rVWF: early endosomal antigen 1 (EEA1) for early endosomes, transferrin receptor for recycling endosomes, cathepsin D and lysosome-associated membrane protein 1 (Lamp1) for lysosomes, and TGN46 for the trans-Golgi network. None of these proteins colocalized with WT or mutant rVWF (data not shown).
Membrane protein recruitment by WT and mutant rVWF. HEK293 cells were transiently cotransfected with either WT rVWF (A,E); rVWF-Cys1225Gly (B,F); rVWF-Cys788Arg (C,G); or rVWF-Arg273Trp (D,H). The cells were also transiently cotransfected with EGFP-CD63 (A-D) or HRP-P-selectin (E-H), and were immunolabeled for VWF (in red, panels A-H) and HRP (in green, panels E-H) 2 days later. The GFP in panels A-D (in green) was visible without staining. In each panel, the majority of pseudo-WPBs are yellow (arrowheads), indicating a colocalization of VWF and the marker. CD63 and P-selectin are also visible in other vesicles (as described in “Membrane protein recruitment”), where there is no colocalization with VWF. Scale bar measures 20 μm.
Membrane protein recruitment by WT and mutant rVWF. HEK293 cells were transiently cotransfected with either WT rVWF (A,E); rVWF-Cys1225Gly (B,F); rVWF-Cys788Arg (C,G); or rVWF-Arg273Trp (D,H). The cells were also transiently cotransfected with EGFP-CD63 (A-D) or HRP-P-selectin (E-H), and were immunolabeled for VWF (in red, panels A-H) and HRP (in green, panels E-H) 2 days later. The GFP in panels A-D (in green) was visible without staining. In each panel, the majority of pseudo-WPBs are yellow (arrowheads), indicating a colocalization of VWF and the marker. CD63 and P-selectin are also visible in other vesicles (as described in “Membrane protein recruitment”), where there is no colocalization with VWF. Scale bar measures 20 μm.
Secretion of VWF
Regulated exocytosis not only is crucial for VWF function but also provides a measure of the extent to which WPBs are fully functional, which in turn depends on the ability of VWF to drive the acquisition of appropriate membrane proteins. We have therefore analyzed the ability of WT rVWF and the 3 variants to form secretagogue-responsive compartments in HEK293 cells. The constitutive secretion of all 3 variants has previously been shown in COS-7 cells to be affected by the mutations. We confirmed this result in HEK293 cells: the quantity of constitutively secreted VWF compared with the total expressed VWF (secreted plus intracellular) is reduced by 1.5- to 2-fold when WT and mutant rVWF are compared (WT, 10.9% ± 1.6%; Cys1225Gly, 5.4% ± 0.1%; Cys788Arg, 8.4% ± 3.2%; Arg273Trp, 5.6% ± 0.1%).
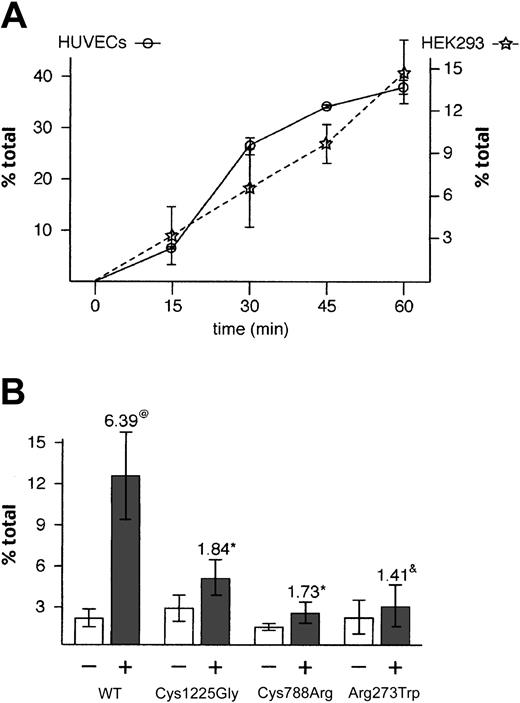
Although HEK293 cells are not thought to have regulated secretory organelles, their ability to make pseudo-WPBs that are able to recruit membrane proteins, suggested that these pseudo-WPBs could be secreted upon stimulation. Indeed, exocytosis of rVWF was observed upon stimulation by the calcium ionophore A23187 (2.3-fold increase; data not shown) and by the phorbol ester PMA, which induced a 6-fold increase over backgound in WT rVWF secretion over a 30-minute period (Figure 7B). To further characterize this PMA response, and in particular to determine how it differs from the effects of this secretagogue on endothelial cells, we analyzed the regulated secretion of VWF from HUVECs and HEK293 cells in parallel (Figure 7A). After 1 hour, about 40% of total VWF has been secreted by HUVECs; the shallow sigmoidal curve suggests that this represents the total releasable VWF. This is in agreement with the results of Ewenstein and coworkers.18 The kinetics of release from HEK293 are more linear, but pseudo-WPB secretion is clearly stimulated by PMA, although less efficiently than in HUVECs. We note that after 15 minutes, both systems have released 20% of the total amount of VWF secreted after 1 hour. Because of its ability to efficiently trigger the regulated secretion of WT rVWF, we used PMA to look at secretion of mutant rVWF, choosing a time point (30 minutes) at which secretion is still rapidly rising as being most likely to reveal differences between WT and mutant rVWF.
Stimulated secretion of WT and mutant rVWF. HEK293 cells were transiently transfected with WT rVWF or rVWF variants, and the quantity of VWF was measured by ELISA 2 days later with (+) or without (-) PMA stimulation. (A) Each point represents the amount of VWF secreted upon stimulation minus constitutive secretion at the indicated time. Error bars represent standard deviation (n = 3). (B) Each bar represents secreted VWF as a fraction of total VWF (secreted plus intracellular) ± standard deviation (n = 6). The numbers above the stimulated release bars (+) indicate the fold increase compared with the nonstimulated bars. Symbols (@ and*) indicate that these differences are statistically significant after Student t test (@P < 1.10-5;*P < .02). &This difference is not statistically significant (P < .2).
Stimulated secretion of WT and mutant rVWF. HEK293 cells were transiently transfected with WT rVWF or rVWF variants, and the quantity of VWF was measured by ELISA 2 days later with (+) or without (-) PMA stimulation. (A) Each point represents the amount of VWF secreted upon stimulation minus constitutive secretion at the indicated time. Error bars represent standard deviation (n = 3). (B) Each bar represents secreted VWF as a fraction of total VWF (secreted plus intracellular) ± standard deviation (n = 6). The numbers above the stimulated release bars (+) indicate the fold increase compared with the nonstimulated bars. Symbols (@ and*) indicate that these differences are statistically significant after Student t test (@P < 1.10-5;*P < .02). &This difference is not statistically significant (P < .2).
We find that although the response to PMA is reduced, the secretagogue does induce a response from both rVWF-Cys1225Gly- and rVWF-Cys788Arg-expressing cells; the lower increase is likely to reflect the fact that these cells had less rVWF stored in pseudo-WPBs compared with WT rVWF (Figures 3 and 4). A 1.4-fold increase in secretion of rVWF-Arg273Trp was also detected. This result was not statistically significant, but we believe that it is consistent with the secretion of the very low number of small pseudo-WPBs found in these cells. Importantly, this increased secretion upon stimulation in WT and mutant rVWF-expressing cells indicated that the basic molecular machinery necessary for targeting and fusion to the plasma membrane was being recruited at the membrane of pseudo-WPBs.
Discussion
We have characterized a heterologous system in which VWF expression recapitulates both the storage and the regulated secretion that occur naturally in endothelial cells. Some of the results presented here have already been found in other heterologous systems, but it was important to confirm that the HEK293 cell does not behave differently toward, for example, COS-7 and AtT-20 cells with respect to the basic biochemical processing of VWF. WT rVWF expression in HEK293 cells leads to its constitutive secretion and its storage in elongated organelles morphologically indistinguishable from bona fide WPBs, which we called pseudo-WPBs. We find that those proteins known to be present in the membrane of WPBs in endothelial cells are also recruited to the membrane of pseudo-WPBs. Finally, we have shown regulated secretion of VWF in pseudo-WPBs after stimulation by a secretagogue.
We used this system to examine 3 human variants of VWF. Expression of these mutants in COS-7 cells demonstrated that their constitutive secretion and multimerization were mildly to severely affected.11,12 We confirmed these results after expression of mutant rVWF in HEK293 cells. Constitutive secretion is affected in HEK293, although in our experiments the 3 mutations induce a similar reduction in secretion. We also found that multimerization of rVWF-Cys1225Gly and rVWF-Cys788Arg are, respectively, mildly and severely affected. However, our results suggest that rVWF-Arg273Trp does form some low molecular weight multimers, and this mutation has a similar effect on multimerization to rVWF-Cys788Arg in this system.
We report now that all 3 mutants can induce the formation of pseudo-WPBs, but that there are significant differences between WT- and mutant rVWF-containing organelles. The first difference is a delay in the formation and a reduction in the number of pseudo-WPBs. This is accompanied by retention of recombinant mutant VWF within the ER, which becomes grossly swollen. This delay in pseudo-WPB formation and the associated reduction in pseudo-WPB numbers correlate directly with the clinical severity of the mutation. We suspect that the consequence of a delay in WPB formation is that recovery from stimulation in vivo will be delayed. Unfortunately, appropriate clinical data confirming this clear prediction have not been possible to obtain. Further studies with different variants will allow this hypothesis to be tested. In addition, the pseudo-WPBs made by the mutants are shorter than for WT rVWF. The functional consequences of a reduction in the quantal size of VWF release is as yet unknown.
It has been shown by several groups that VWF multimerization and storage are not linked.21,27,28 We confirmed this result by expressing human VWF variants forming only very low molecular weight multimers in a system that allows us to go further and quantify any link between multimerization and both number and length of WPBs. The multimeric status of total intracellular rVWF is largely dimeric, as reported in 3T3, COS, and AtT-20 cells.24 However, since WPBs contain the highest molecular weight multimers visible in the growth medium,29 we deduce that pseudo-WPBs contain medium molecular weight multimers following rVWF-Cys1225Gly expression and only very low molecular weight multimers after rVWF-Cys788Arg and rVWF-Arg273Trp expression. Nonetheless, these last 2 mutations still induce pseudo-WPB formation.
It has been proposed that the elongation of WPBs and their internal striations are due to noncovalent interactions between VWF molecules, regardless of their multimerization status.28 Our data are consistent with this hypothesis. Expression of rVWF-Cys788Arg and rVWF-Arg273Trp leads to a similar reduction in multimer formation but induces very different morphologic phenotypes, leading respectively to a severe and a mild phenotype in terms of pseudo-WPB formation. This result is in contradiction with a direct link between multimerization status and pseudo-WPB length but could be explained by a differential impact of these mutations on the formation of noncovalent interactions. It is thought that the VWF propeptide acts as chaperone for the mature VWF. The Arg273Trp mutation, which affects the propeptide, could induce its misfolding, leading to a strong effect on its chaperone activity. This in turn could prevent the formation of noncovalent interactions between VWF molecules and therefore the elongation of pseudo-WPBs. In contrast, Cys788Arg affects a cysteine implicated in an intrachain disulfide bond in mature VWF30 and could induce misfolding, thereby preventing multimer formation but not noncovalent interactions. Alternatively, the difference between Cys788Arg and Arg273Trp could be due to the retention of most of the VWF inside the ER in the latter compared with the Cys788Arg mutation, leading in turn to too few molecules to effectively trigger elongation.
It is already known that Cys1142 and one or more of Cys1222, Cys1225, and Cys1227 are involved in intersubunit disulfide-bond formation.31 A mutation in a cysteine involved in an intersubunit disulfide bond is likely to affect VWF multimerization. In the case of rVWF-Cys1225Gly, only high molecular weight forms were absent after expression in HEK293 cells. We therefore hypothesize that Cys1225 is not one of the cysteines involved in intersubunit disulfide bonding, but that it forms an intrachain bond, most likely affecting the folding of rVWF-Cys1225Gly, albeit not to a great extent, since multimerization is almost normal, leading to ER retention of only a small proportion of rVWF. Consistent with this, it has been shown that rVWF-Cys1149Arg and rVWF-Cys1060Arg, which are involved in intrachain bonds, do not prevent formation of low and intermediate molecular weight VWF multimers.17,32
To be able to fully assess the functioning of VWF, we believe that analysis of its ability to form WPBs, using expression of VWF in a heterologous system, should be part of a comprehensive study. The system should be such that expression of WT rVWF leads to formation of elongated organelles with internal striations that recruit appropriate membrane proteins and that respond to secretagogue. We describe a highly effective system for such analyses. Using simple transient expression in HEK293 cells by nucleofection, we find a high level of VWF expression and the formation of storage organelles, which we have characterized as indistinguishable from bona fide WPBs by a variety of criteria. These criteria include an analysis for the first time of the ability of VWF variants to drive the recruitment of CD63 and P-selectin. This system could, in the future, replace VWF expression in COS or AtT-20 cells, the 2 other cells lines widely used thus far, and should yield useful insights into the structure-function relationships of VWF by allowing monitoring of each step from dimerization to WPB secretion.
Prepublished online as Blood First Edition Paper, June 5, 2003; DOI 10.1182/blood-2003-02-0599.
Supported by a Marie Curie Fellowship of the European Community (G.M.) and an MRC grant (D.F.C.); S.L.M. was supported by a grant awarded under the European Union Fifth Framework program.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal