Abstract
The platelet fibrinogen receptor, integrin αIIbβ3, is a noncovalent heterodimer of glycoproteins IIb and IIIa. This work was aimed at elucidating the role played by the carboxy-terminal extracellular, trans-membrane, and cytoplasmic regions of the glycoprotein β3 in the formation of functional complexes with α subunits. Progressive carboxy-terminal deletions of β3 revealed that surface exposure of αIIbβ3 or αvβ3 could not occur in the absence of the transmembrane domain of β3. In contrast, internal deletions 616 to 690 of the carboxy-terminal regions of the β3 ectodomain led to surface exposure of constitu tive active receptors in CHO cells, as indicated by the enhanced rate of cell adhesion to immobilized ligands and spontaneous binding to soluble fibrinogen or activation-dependent antibody PAC-1. The functional analysis of cysteine mutations within the 616 to 690 region of β3 or chimeric β3-β7 subunits revealed that disruption of the C663-C687 disulfide bridge endows constitutive activity to the αIIbβ3 receptor. It is concluded that the carboxy-terminal tail of the β3 ectodomain, so-called β tail domain (βTD), is not essential for cell surface expression of β3 receptors. However, a basal, nonactivated, low ligand-affinity state of the β3 integrins demands a normal conformation of this domain. (Blood. 2003;102:2491-2497)
Introduction
The glycoprotein (GP) IIb-IIIa complex, integrin αIIbβ3, is a calcium-dependent, noncovalent heterodimer formed by GPIIb and GPIIIa. This complex is found in the plasma membrane of megakaryocytes, platelets, and some tumor tissues1-3 and functions as a receptor for fibrinogen and other adhesive proteins like the von Willebrand factor, fibronectin, or vitronectin.4 The β3 subunit may also complex the GP αv to form the vitronectin receptor (integrin αvβ3) that shares with αIIbβ3 the binding of fibrinogen although with different affinity.5 The platelet αIIbβ3 complex is essential to maintain a normal hemostasis. Unlike other platelet receptors that are constitutively active, the αIIbβ3 is maintained in a low-affinity state for its ligands. Disruption of the vascular endothelium and exposure of platelets to the action of agonists and adhesive proteins from the subendothelial matrix induces a cellular activation. The activated cells interact with adhesive proteins from the extracellular matrix,6,7 and the αIIbβ3 receptors are able to bind fibrinogen with high affinity (inside out signaling), resulting in platelet aggregation.8 Conversely, ligand-bound αIIbβ3 propagates signals to the interior of the cell (outside-in signaling) leading to enhanced interaction with the cytoskeleton, clustering of receptors (increased ligand avidity), and formation of focal contacts rich in signaling complexes.9,10 The agonist-induced increase in ligand affinity of αIIbβ3 is thought to be the result of conformational changes of the heterodimer11-13 initiated by the interaction of the cytoplasmic tails of α and β3 subunits with cytosolic proteins. Despite the pathophysiologic importance of the platelet αIIbβ3 receptor, the knowledge of the mechanisms controlling its state of activation is rather limited.
Unlike previous reports,14 recent work from our laboratory15 showed that a truncated form of β3 lacking the transmembrane and cytosolic domains failed to associate with αIIb. The present work was aimed at further investigating the role played by the carboxy-terminal domain of β3 in the surface expression and function of β3 heterodimers. The results obtained in this study indicate that surface expression of αIIbβ3 could not occur in the absence of the transmembrane domain of β3. The present study has also revealed that either deletion of the carboxy-terminal region of the β3 ectodomain or disruption of the 663-687 disulfide bridge confers constitutive activity to the β3 integrins, suggesting that this region is involved in maintaining the αIIbβ3 receptor in a resting, nonactivated state.
Materials and methods
Materials
Restriction enzymes were obtained from Boehringer (Mannheim, Germany). The pcDNA3 expression vector was from Invitrogen (San Diego, CA). Oligonucleotides were synthesized by Invitrogen (Paisley, Scotland). Most other reagents were purchased from Sigma Chemical (St Louis, MO), Merck (Darmstadt, Germany), or Calbiochem-Novabiochem (San Diego, CA). Monoclonal antibodies (mAbs) anti-αIIb (2bc1) and anti-β3 (H1a) were obtained in our laboratory; the anti-αvβ3 MAB1976 was purchased from Chemicon (Temecula, CA), and fluorescein isothiocyanate (FITC)-PAC-1 was obtained from Becton Dickinson (San Jose, CA). WOW-1 F(ab′)2 and PAC-1 F(ab′)2 were obtained from Dr S. Shattil (The Scripps Research Institute, La Jolla, CA).
Construction of expression vectors with normal or mutant β3 cDNAs
Wild β3-cDNA cloned in EcoRI and HindIII sites of pBluescript II-KS (Stratagene, La Jolla, CA) was digested with XhoI and partially with BamHI, and subcloned in pcDNA3. pcDNA3-β3Δ616 construct was prepared as described previously.15 To generate truncated β3 at 638, 657, 675, and 693 amino acids, stop codons were introduced by polymerase chain reaction (PCR) using pcDNA3-β3 as template, and oligonucleotide β3-sense (1670-1689): 5′-ACTGCAACTGTACCACGCGT-3′ and the antisense primers β3-AS-638-stop-XhoI: 5′-TATCTCGAGCTAGTCACGGCAG-3′, β3-AS-657-stop-XhoI: 5′-TATCTCGAGTTAGGTACAATTCAC-3′, β3-AS-675-stop-XhoI: 5′-TAACTCGAGTCAACTAGAATCTTC-3′, and β3-AS-693-stop-XhoI: 5′-TATCTCGAGTTAGTCAGGGCCC-3′, respectively. Mutations introduced to generate stop codons are underlined. PCR products were digested with NotI and XhoI to replace the normal fragment from pcDNA3-β3.
cDNAs encoding [608Ala]β3, [614Ala]β3, [617Ala]β3, [663Ala]β3, and [687Ala]β3 mutants were prepared by the splicing by overlap extension PCR procedure using the oligonucleotide primers β3-sense (1670-1689) and β3-AS-(2319-2296)-XhoI: 5′-TGATAATGACTCGAGGATGACTGC-3′, and the overlapped pairs of the following primers: [608Ala]β3-S: 5′-CCAGATGCCGCCACCTTTAAG-3′ and [608Ala]β3-AS: 5′-CTTAAAGGTGGCGGCATCTGG-3′; [614Ala]β3-S: 5′-AAGAAAGAAGCTGTGGAGTGT-3′ and [614Ala]β3-AS: 5′-ACACTCCACAGCTTCTTTCTT-3′; [617Ala]β3-S: 5′-TGTGTGGAGGCTAAGAAGTTTGAC-3′ and [617Ala]β3-AS: 5′-GTCAAACTTCTTAGCCTCCACACA-3′; [663Ala]β3-S: 5′-GAGGATGACGCTGTCGTCAGA-3′ and [663Ala]β3-AS: 5′-TCTGACGACAGCGTCATCCTC-3′; [687Ala]β3-S: 5′-GAGCCAGAGGCTCCCAAGGGC-3′ and [687Ala]β3-AS: 5′-GCCCTTGGGAGCCTCTGGCTC-3′. The [608Ala]β3, [614Ala]β3, [617Ala]β3, and [663Ala]β3 cDNAs were used as template to generate [608Ala/655Ala]β3, [614Ala/635Ala]β3, [617Ala/631Ala]β3, and [663Ala/687Ala]β3 mutant cDNAs, respectively, and the overlapped pairs of primers: [655Ala]β3-S: 5′-GCAGTGAATGCTACCTATAAG-3′ and [655Ala]β3-AS: 5′-CTTATAGGTAGCATTCACTGC-3′; [635Ala]β3-S: 5′-AACCGTTACGCCCGTGACGAG-3′ and [635Ala]β3-AS: 5′-CTCGTCACGGGCGTAACGGTT-3′; [631Ala]β3-S: 5′-GACGAAAATACCGCCAACCGTTAC-3′ and [631Ala]β3-AS: 5′-GTAACGGTTGGCGGTATTTTCGTCATG-3′; and [687Ala]β3-S and [687Ala]β3-AS, respectively. Final PCR products carrying the desired mutations were digested with XhoI and MluI and ligated into pcDNA3 carrying normal β3 cDNA digested with XhoI and partially digested with MluI.
β3(685-690del), β3(654-690del), and β3(616-690del) were obtained by PCR amplification from the plasmid pBJ1-β3, with antisense primers located just before the region to delete: β3-AS-(2055-2030): 5′-TGGCTCTTCTACCACATACGGATGG-3′, β3-AS-(1959-1938): 5′-CACTGCATCCTTGCCAGTGTCC-3′ and β3-AS-(1845-1819): 5′-CACACATTCTTTCTTAAAGGTGCAGGC-3′, respectively, and the sense primer β3-S-(2071-2092): 5′CCTGACATCCTGGTGGTCCTGC-3′, located after the fragment to be removed. The PCR products were phosphorylated and ligated to obtain pBJ1-β3(685-690del), pBJ1-β3(654-690del), and pBJ1-β3(616-690del) that were used as template for another PCR performed with oligonucleotides β3-S-(1670-1689) and β3-AS-(2319-2296)-XhoI. The resulting PCR products were digested with NotI and XhoI to replace the normal fragment from pcDNA3-β3.
A β3-β7 chimera was obtained as follows. The 2078-2323 fragment of β7 was amplified by PCR with the oligonucleotides β7-S-(2078-2095): 5′-ACACCATGGCTAGCACACCGGGAC-3′ and β7-AS-(2323-2298): 5′-CGTGTGGTATCGATCCTTTTCTTGG-3′, containing a NheI and a ClaI site, respectively. The PCR product was digested with NheI and ClaI and ligated to pcDNA3-β3 where the homologous fragment had been deleted. For this, NheI and ClaI were previously introduced in the β3 sequence using the overlapped oligonucleotide primers β3-S (2137-2160)-ClaI: 5′-TGTCCCAAGGATCGATACATCCTG-3′ and β3-AS-(2160-2137)-ClaI: 5′-CAGGATGTATCGATCCTTGGGACA-3′. The cDNA so obtained was used as a template to introduce the NheI site with the following overlapped oligonucleotides: β3-S (1918-1939)-NheI: 5′-TGTGTGGAGCTAGCGAAGTTTGAC-3′ and β3-AS (1939-1918)-NheI: 5′-GTCAAACTTCGCCTAGCTCCACACA-3′. The final PCR product carrying the NheI and ClaI sites was digested with XhoI and MluI and ligated into pCDNA3-β3 cDNA digested with XhoI and partially digested with MluI. The DNA sequence of each construct was verified. The αIIb cDNA was cloned into the HindIII site of pcDNA3, as previously described.16
Cell culture and transfection
CHO cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS). Cells were transiently cotransfected by the diethylaminoethyl (DEAE)-dextran method17 with normal or mutated pcDNA3-β3 constructs and, when indicated, with pcDNA3-αIIb. Forty-eight hours after transfection the cells were harvested and the surface expression of αIIbβ3 complexes was assessed by flow cytometry analysis or immunoprecipitation.
CHO cell lines stably expressing β3 or β3(616-690del) were established by transfection with 15 μg pcDNA3-β3 or pcDNA3-β3(616-690del), using the calcium phosphate precipitation procedure. The transfected cells were fed with medium containing 800 μg/mL G-418 every 3 to 4 days, and clones of cells stably expressing β3 were selected by flow cytometry analysis. Cells stably expressing β3 or β3(616-690del) were transfected with pcDNA3-αIIb, and cells expressing αIIb were cloned by cell sorting with fluorescence-activated cells sorting (FACS Vantage; Becton Dickinson) with argon laser tuned to 488 nm of excitation and specific fluorescence signals collected by a 530-bp filter.
Adhesion of CHO cell lines to immobilized fibrinogen and vitronectin
The 96-well flat-bottomed plates were coated with 10 μg/mL fibrinogen in phosphate-buffered saline (PBS) pH 7.2 (100 μL/well) for 2 hours at 37°C and then washed with PBS, blocked with 200 μL 1% bovine serum albumin (BSA) for 1 hour at 37°C, and washed with PBS. CHO cell lines were labeled with 10 μM calcein-am (Molecular Probes, Leiden, The Netherlands) for 10 minutes, washed by centrifugation, and resuspended in serum-free DMEM, plated onto dishes in duplicate at a density of 4 × 104 cells/well and incubated for different periods of time at 37°C. Nonadherent cells were removed by carefully washing twice with 200 μL PBS. Cell adhesion was quantitated by cytofluorometry. Basal adherence to BSA (cell binding to BSA-coated wells was always < 1%) was subtracted from attachment values. To study adhesion of cell lines to vitronectin, cytomatrix cell adhesion strips with human vitronectin (Chemicon) were used following the manufacturer's recommendations. At the end of the assays, adherent cells were examined with a phase-contrast Nikon TMS microscope (Barcelona, Spain) and micrographs were taken with a digital camera.
Soluble fibrinogen-dependent aggregation of CHO cell lines
CHO cells were incubated with 1 mg/mL fibrinogen for 15 minutes at room temperature at a density of 2.5 × 106 cells/mL. Then, 250 μL cell suspension was plated onto wells of a 24-well culture dish precoated with 1 mg/mL BSA. Ten to 15 minutes after plating, cell aggregates were examined by phase-contrast microscopy as described. In some cases, cells were preincubated with 10 μg anti-β3 antibody H1a or 10 mM EDTA (ethylenediaminetetraacetic acid).
Flow cytometric analysis of αIIb and β3
Transfected cells were harvested using 0.5 mM EDTA in PBS, washed with PBS, resuspended at a density of 106 cells/100 μL, and incubated for 20 minutes at 4°C with mAbs specific to αIIb (2bc1), β3 (H1a), or αvβ3 (MAB1976). Next, cells were washed and exposed to FITC-F(ab′)2 fragment of rabbit antimouse Ig (Dako, Glostrup, Denmark) at 4°C for 20 minutes, and the surface fluorescence was analyzed in a Coulter flow cytometer, model EPICS XL (Miami, FL).
Binding of fibrinogen and PAC-1 to transfected CHO cells
Human fibrinogen was labeled with FITC as described previously.16 CHO cells (5 × 105) coexpressing normal or mutated β3, and either human αIIb or endogenous αv were resuspended in Tyrode buffer (5 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 2 mM MgCl2, 0.3 mM NaH2PO4, 3 mM KCl, 134 mM NaCl, 12 mM NaHCO3, 0.1% glucose, 0.1% BSA, and 1 mM CaCl2, pH 7.0) with 10 μg FITC-fibrinogen and incubated for 25 minutes at room temperature. After washing, cells were resuspended and analyzed by flow cytometry. When indicated, cells were preincubated for 30 minutes at 4°C with mAbs directed against activated αvβ3 (WOW-1) or activated αIIbβ3 (PAC-1), 2 mM MnCl2, or 10 mM EDTA.
To assess the binding of PAC-1, cells were resuspended in Tyrode buffer, incubated at room temperature for 15 minutes with FITC-PAC-1, and then cell fluorescence was determined by flow cytometry.
Metabolic labeling and immunoprecipitation analysis of αIIbβ3 complexes
Metabolic labeling of transfected CHO cells was performed 48 hours after transfection. First, cells were incubated in medium without methionine and cysteine for 30 minutes and, then, with [35S]-methionine/cysteine (400 μCi/mL; 14.8 MBq) for 3 hours. Cells were washed 3 times with PBS and extracted in 0.5 mL lysis buffer (50 mM Tris [tris(hydroxymethyl)aminomethane], pH 7.4, 150 mM NaCl, 2 mM phenylmethylsulfonyl fluoride [PMSF], 1% Triton X-100, 0.05% Tween 20, and 0.03% sodium azide). Precleared lysates were immunoprecipitated with either anti-β3 or anti-αIIb. The immunoprecipitates were then bound to protein A-Sepharose CL-4B beads (Amersham Pharmacia Biotech, Uppsala, Sweden), washed, and eluted by incubating 10 minutes at 100°C in 50 μL reducing loading buffer (50 mM Tris-HCl, pH 6.8, 10% glycerol, 5% β-mercaptoethanol, 4% sodium dodecyl sulfate [SDS], and 0.002% bromophenol blue) and, finally, resolved by electrophoresis. The gels were vacuum dried and exposed to hypersensitive x-ray film for 48 hours.
Results
Role of transmembrane and cytosolic domains of β3 on the surface exposure of heterodimers
We have recently reported a nonsense mutation producing a truncated form of β3 (β3Δ616) associated with thrombasthenic phenotype (type I Glanzmann thrombasthenia).15 The underlying molecular mechanism for the thrombasthenic phenotype was the failure of β3Δ616 to complex αIIb. This observation appeared to be in conflict with previous reports in which β3-truncated proteins complex α subunits14,18,19 and the mutated complexes reached the cell surface.14 Because the apparent discrepancy could be due to differences in the lengths of the deleted fragments, we performed a transient transfection analysis of progressive carboxy-terminal or internally deleted forms of β3. Figure 1 depicts the deletion mutants used for transfection analysis (Figure 1A) as well as some structural features of the carboxy-terminal ectodomain of β3 (Figure 1B) based on recent studies on the crystal structure of heterodimeric αv and β3 ectodomains.20
Schematic representations of deletion mutants and disulfide linkages. (A) Schematic representation of the deletion mutants of β3 used for transfection analysis. PSI indicates plexin-semaphorin-integrin domain; EGF, epidermal growth factor domain; βTD, β tail domain; TM, transmembrane domain; and CT, cytoplasmic domain. (B) Schematic representation of βTD and disulfide linkages, according to Xiong et al.20
Schematic representations of deletion mutants and disulfide linkages. (A) Schematic representation of the deletion mutants of β3 used for transfection analysis. PSI indicates plexin-semaphorin-integrin domain; EGF, epidermal growth factor domain; βTD, β tail domain; TM, transmembrane domain; and CT, cytoplasmic domain. (B) Schematic representation of βTD and disulfide linkages, according to Xiong et al.20
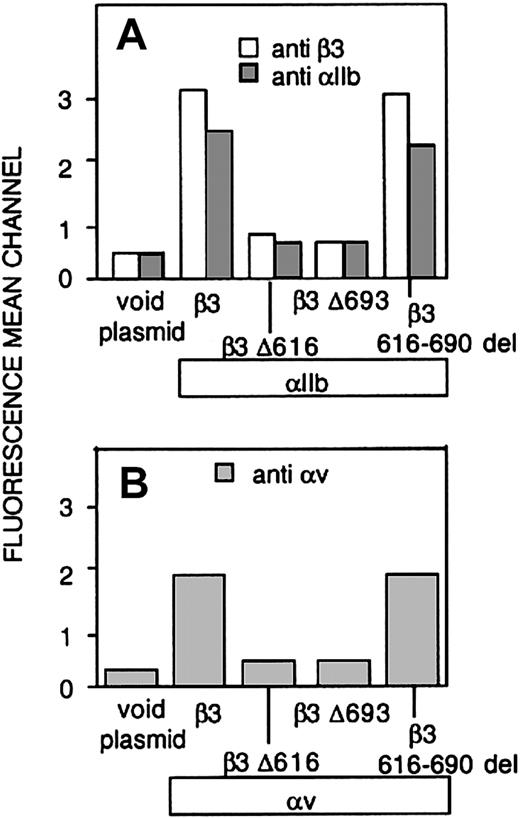
Whether the carboxy-terminal-deleted constructs were transfected alone or with αIIb, no detectable surface expression of β3 or β3 heterodimers was appreciated by flow cytometry (Figure 2). In contrast, deletion of the 616-690 carboxy-terminal region of the β3 ectodomain (616-690del) did not prevent the surface expression of αvβ3 or αIIbβ3 heterodimers. According to these observations, a normal level of surface exposure of these receptors demands the presence of the transmembrane and cytosolic regions of β3.
Surface exposure of αIIbβ3 and αvβ3 complexes in CHO cells transfected with αIIb and either normal or mutated forms of β3 (A) CHO cells were transiently cotransfected with cDNAs encoding normal αIIb and either normal or mutant forms of β3, and the level of surface expression of αIIbβ3 complexes was determined by flow cytometry using a mAb directed against αIIb (▦) or β3 (□). (B) Cells were transfected with either normal or mutant forms of β3, and expression of surface complexes with endogenous αv was evaluated using a mAb directed against αvβ3. The results are expressed as means ± SEMs of at least 4 independent experiments performed in duplicate.
Surface exposure of αIIbβ3 and αvβ3 complexes in CHO cells transfected with αIIb and either normal or mutated forms of β3 (A) CHO cells were transiently cotransfected with cDNAs encoding normal αIIb and either normal or mutant forms of β3, and the level of surface expression of αIIbβ3 complexes was determined by flow cytometry using a mAb directed against αIIb (▦) or β3 (□). (B) Cells were transfected with either normal or mutant forms of β3, and expression of surface complexes with endogenous αv was evaluated using a mAb directed against αvβ3. The results are expressed as means ± SEMs of at least 4 independent experiments performed in duplicate.
Immunoprecipitation analysis of metabolically labeled cells cotransfected with αIIbβ3 were performed (Figure 3). When anti-β3 was used, immunoprecipitates of the expected size for the normal and mutated forms of β3 were obtained. All the anti-β3 immunoprecipitates contain products with apparent molecular size of pro-αIIb, whereas mature heavy chains of αIIb were detected only in cells transfected with either normal β3 or β3(616-690del). When anti-αIIb was used, pro-αIIb was precipitated in all cases, and αIIb and β3 were only coprecipitated in cells transfected with either normal β3 or β3(616-690del). Because the proteolytic cleavage of pro-αIIb occurs only when the subunit is complexed to β3, the absence of the mature heavy chain of GPIIb suggests absent or very low rate of pro-αIIb-β3 complex formation. Immunoprecipitation of concentrated 48-hour-conditioned medium with either anti-β3 or anti-αIIb did not yield detectable amounts of truncated β3. Thus, Δβ3 forms are not secreted to the extracellular medium, either alone or associated with α heavy chain, at a significant rate; however, it cannot be excluded that the Δβ3 or the αIIbΔβ3 complexes could have been rapidly degraded.
Immunoprecipitation analysis of αIIbβ3 complexes from CHO cells transiently cotransfected with αIIb and either normal or mutant forms of β3. CHO cells were transiently transfected with αIIb and either normal β3 or β3(616-690del), β3Δ616, β3Δ638, β3Δ657, β3Δ675, or β3Δ693 cDNA constructs. Cells were incubated with [35S]-methionine-cysteine for 3 hours. Cell lysates were immunoprecipitated with anti-β3 or anti-αIIb and processed as described in “Materials and methods.”
Immunoprecipitation analysis of αIIbβ3 complexes from CHO cells transiently cotransfected with αIIb and either normal or mutant forms of β3. CHO cells were transiently transfected with αIIb and either normal β3 or β3(616-690del), β3Δ616, β3Δ638, β3Δ657, β3Δ675, or β3Δ693 cDNA constructs. Cells were incubated with [35S]-methionine-cysteine for 3 hours. Cell lysates were immunoprecipitated with anti-β3 or anti-αIIb and processed as described in “Materials and methods.”
Functional features of αvβ3(616-690del) andαIIbβ3(616-690del) complexes
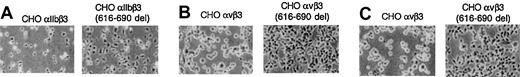
CHO cells expressing recombinant αIIbβ3 receptors adhere spontaneously to solid-phase fibrinogen. Thus, we found it of interest to examine whether the mutant αIIbβ3(616-690del) complexes exhibited a distinct capacity of adhesion onto ligand-coated plates. The CHO-αIIbβ3(616-690del) cells were larger than cells expressing normal complexes and showed enhanced fibrinogen concentration-dependent adherence onto ligand-coated plates (Figure 4A). Because CHO cells express endogenous αv, we stably transfected normal β3 or β3(616-690del) and studied the adherence of normal CHO-αvβ3 and CHO-αvβ3(616-690del) cells to plates coated with either vitronectin (Figure 4B) or fibrinogen (Figure 4C). In both conditions the cells expressing the mutant complexes showed enhanced adherence to coated plates.
Adhesion of CHO cells stably expressing normal or mutant αIIbβ3 or αvβ3 complexes to solid-phase fibrinogen or vitronectin. Approximately 4 × 105 cells were added to the wells of microtiter plates coated with ligands and incubated at 37°C.Adhesion was examined by phase-contrast microscopy as described in “Materials and methods.” (A) CHO-αIIbβ3 or CHO-αIIbβ3(616-690del) cells were seeded onto plates coated with 10 μg/mL fibrinogen and incubated for 10 minutes. (B-C) CHO-αvβ3 and CHO-αvβ3(616-690del) cells were seeded onto microtiter plates coated with 10 μg/mL vitronectin (B) or 10 μg/mL fibrinogen (C) and incubated for 30 minutes. Original magnification, × 100.
Adhesion of CHO cells stably expressing normal or mutant αIIbβ3 or αvβ3 complexes to solid-phase fibrinogen or vitronectin. Approximately 4 × 105 cells were added to the wells of microtiter plates coated with ligands and incubated at 37°C.Adhesion was examined by phase-contrast microscopy as described in “Materials and methods.” (A) CHO-αIIbβ3 or CHO-αIIbβ3(616-690del) cells were seeded onto plates coated with 10 μg/mL fibrinogen and incubated for 10 minutes. (B-C) CHO-αvβ3 and CHO-αvβ3(616-690del) cells were seeded onto microtiter plates coated with 10 μg/mL vitronectin (B) or 10 μg/mL fibrinogen (C) and incubated for 30 minutes. Original magnification, × 100.
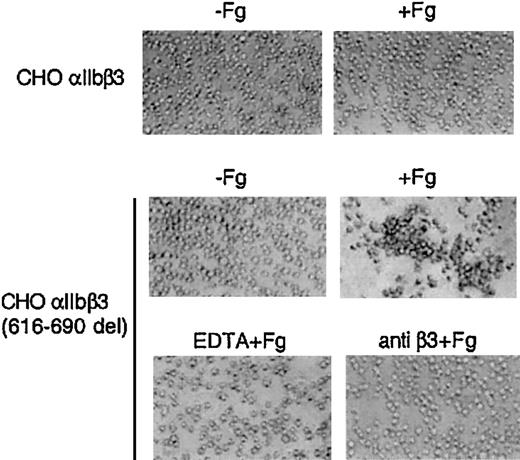
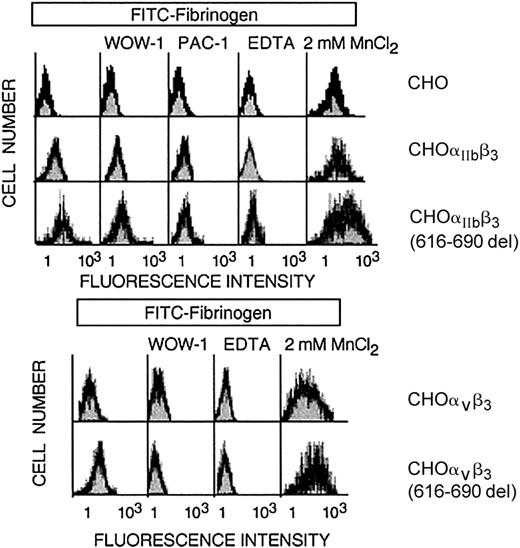
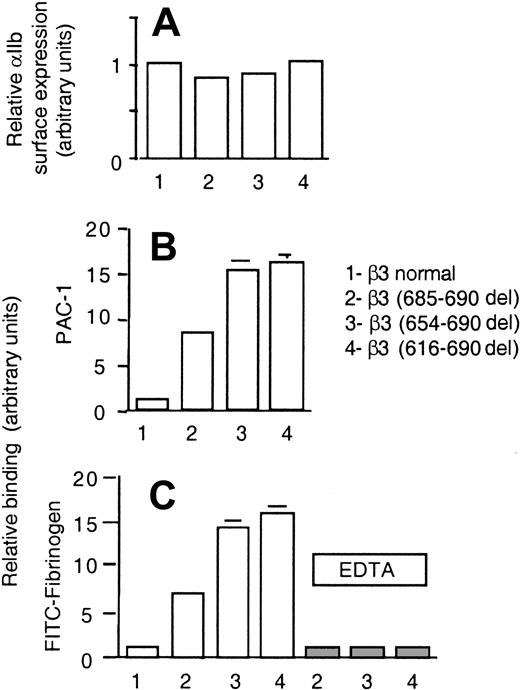
Normal resting CHO-αIIbβ3 cells in suspension do not bind to soluble fibrinogen at significant rates and, therefore, as expected, no spontaneous aggregation was observed (Figure 5). In contrast, CHO-αIIbβ3(616-690del) cells in suspension underwent receptor and soluble fibrinogen-dependent spontaneous aggregation and this effect was prevented by pretreatment with either EDTA or an anti-β3 function blocking mAb (Figure 5). The fibrinogen-dependent aggregation of CHO-αIIbβ3(616-690del) cells suggested that the mutant receptor was constitutively active. To further investigate this point, we studied the spontaneous binding to soluble FITC-labeled fibrinogen of cells expressing either normal or mutated complexes. As shown in Figure 6, CHO-αIIbβ3(616-690del) but not CHO-αIIbβ3 cells bound to soluble fibrinogen. Fibrinogen (Fg) is a ligand for both αIIbβ3 and αvβ3. Thus, to assess the binding of FITC-Fg to each integrin, the cells were incubated with function-blocking, activation-dependent mAbs directed against either αIIbβ3 (PAC-1) or αvβ3 (WOW-1). As observed in Figure 6, the binding of FITC-Fg was partially prevented by both WOW-1 and PAC-1. In agreement with their different sites of action, the combined administration of WOW-1 and PAC-1 showed additive effects, so that the binding of cells to FITC-Fg was virtually abolished (data not shown). The presence of Mn2+ enabled CHO-αvβ3 or CHO-αIIbβ3 cells to bind to soluble fibrinogen. Cells expressing the β3(616-690del) subunit showed spontaneous binding to FITC-Fg that was further stimulated by Mn2+ (Figure 6 lower panels). In all conditions, the binding of cells to FITC-Fg was completely abolished by preincubation with EDTA. We next tried to determine whether β3 subunits containing deletions shorter than 616-690del, such as 654-690del or 685-690del, would confer receptor activity. All the constructs yielded normal rates of cell surface expression of αIIbβ3 complexes (Figure 7A). The cells carrying αIIbβ3 receptors with internally deleted β3 subunit, even one as small as 5 residues, β3(685-690del), exhibited enhanced binding to PAC-1 and FITC-Fg (Figure 7B-C). EDTA prevented the binding of cells to FITC-Fg (Figure 7C).
Soluble fibrinogen-dependent aggregation of CHO-αIIbβ3 (616-690del) cells. Cells (2.5 × 106/mL) were incubated without or with fibrinogen (1 mg/mL) for 15 minutes at room temperature in a final volume of 250 μL and then plated onto BSA-precoated wells. When indicated, cells were preincubated for 5 to 15 minutes with either 10 mM EDTA or 10 μg/mL anti-β3 antibody H1a. An irrelevant mAb was used as negative control (not shown). Aggregate formation was visualized by phase-contrast microscopy as described in “Materials and methods.” Original magnification, × 100.
Soluble fibrinogen-dependent aggregation of CHO-αIIbβ3 (616-690del) cells. Cells (2.5 × 106/mL) were incubated without or with fibrinogen (1 mg/mL) for 15 minutes at room temperature in a final volume of 250 μL and then plated onto BSA-precoated wells. When indicated, cells were preincubated for 5 to 15 minutes with either 10 mM EDTA or 10 μg/mL anti-β3 antibody H1a. An irrelevant mAb was used as negative control (not shown). Aggregate formation was visualized by phase-contrast microscopy as described in “Materials and methods.” Original magnification, × 100.
Flow cytometric analysis of binding of CHO cells expressing either normal or mutated β3 receptors to FITC-labeled fibrinogen. CHO cells stably expressing heterodimers of normal or mutant β3(616-690del) with endogenous αv or with transfected αIIb were incubated with soluble FITC-fibrinogen alone or in the presence of mAbs directed against activated αvβ3 (WOW-1) or activated αIIbβ3 (PAC-1), 2 mM MnCl2,or 5mM EDTA. Bound fluorescence was determined by flow cytometric analysis.
Flow cytometric analysis of binding of CHO cells expressing either normal or mutated β3 receptors to FITC-labeled fibrinogen. CHO cells stably expressing heterodimers of normal or mutant β3(616-690del) with endogenous αv or with transfected αIIb were incubated with soluble FITC-fibrinogen alone or in the presence of mAbs directed against activated αvβ3 (WOW-1) or activated αIIbβ3 (PAC-1), 2 mM MnCl2,or 5mM EDTA. Bound fluorescence was determined by flow cytometric analysis.
Binding of CHO cells, transiently transfected with αIIb plus either normal or deleted β3 mutants, to fibrinogen or PAC-1. (A) The surface expression of αIIbβ3 complexes was determined by flow cytometry using a mAb directed against αIIb, as described in “Materials and methods.” (B-C) Transfected CHO cells were incubated with FITC-PAC-1 (B) and FITC-Fg (C) as described in “Materials and methods.” Nonspecific fibrinogen binding was determined in the presence of 10 mM EDTA (▦). Data are expressed in arbitrary units. The values are means ± SEMs of 4 independent experiments performed in duplicate.
Binding of CHO cells, transiently transfected with αIIb plus either normal or deleted β3 mutants, to fibrinogen or PAC-1. (A) The surface expression of αIIbβ3 complexes was determined by flow cytometry using a mAb directed against αIIb, as described in “Materials and methods.” (B-C) Transfected CHO cells were incubated with FITC-PAC-1 (B) and FITC-Fg (C) as described in “Materials and methods.” Nonspecific fibrinogen binding was determined in the presence of 10 mM EDTA (▦). Data are expressed in arbitrary units. The values are means ± SEMs of 4 independent experiments performed in duplicate.
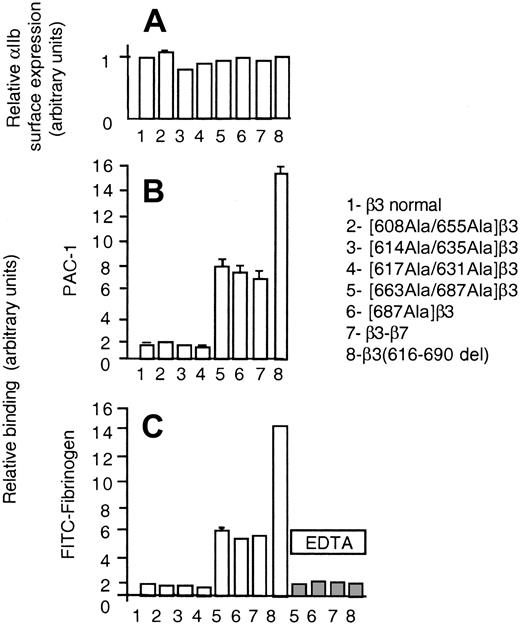
A recent crystallographic study on the αvβ3 integrin described a new fold in the carboxy-terminal end 606-690 of the β3 ectodomain, named β tail domain (βTD), that shows a weak homology to the cytostatin fold and contains 4 disulfide bridges (608Cys-655Cys, 614Cys-635Cys, 617Cys-631Cys, and 663Cys-687Cys).20 Since the smaller deletion conferring receptor activity, β3(685-690del), disrupts the 663-687 disulfide bridge, we next investigated the functional effect of disrupting each of the disulfide bridges of the βTD, by replacing the cysteine residues by alanine. None of these mutations altered the normal levels of surface expression of αIIbβ3 complexes (Figure 8A); however, disruption of the 663-687 disulfide bridge enabled the cells to bind spontaneously to soluble fibrinogen and PAC-1 (Figure 8B-C). To further verify the role of this disulfide bridge, we mutated only the 687C>A. The β3(685-690del) construct as well as [663Ala-687Ala]β3 and [687Ala]β3 mutants were less efficient than β3(616-690del) in enhancing the receptor activity (approximately 50%). The latter observation suggested that both shortening of the carboxy-terminal ectodomain of β3 and disulfide bridge disruption could have contributed to confer constitutive activation to β3(616-690del). To further investigate this point, we prepared a chimeric β3 subunit in which the 616-690 fragment was replaced with the homologous domain of β7 that lacks the cysteines found in β3 at positions 663 and 687. Figure 8B-C shows that heterodimers of the β3-β7 chimera bound spontaneously to PAC-1 and fibrinogen to a similar extent as the 663Ala-687Ala and 687Ala β3 mutants, that is, less than β3(616-690del). This observation supports the idea that both disruption of the 663Cys-687Cys disulfide bridge and shortening of the carboxy-terminal ectodomain enhance the activity of β3 heterodimers by different yet additive mechanisms. Although the importance of the βTD in maintaining the basal, nonactive state of the β3 receptors is evident under our experimental conditions, the physiologic significance of these findings is difficult to ascertain. The possibility exists that a normal mechanism of receptor activation could involve the sliding of the subunits with the result of a relative shortening of the β3. On the other hand, molecular genetic defects in the βTD of β3 might be associated with constitutive active β3 receptors and subsequent pathologic disorders.
Binding of CHO cells, transiently transfected with αIIb plus either normal or mutant forms of β3, to FITC-Fg or PAC-1. Experimental procedures and data expression were the same as described in Figure 7. To facilitate the comparison, data from CHO cells expressing heterodimers of mutant β3(616-690del), shown in Figure 7, have been included.
Binding of CHO cells, transiently transfected with αIIb plus either normal or mutant forms of β3, to FITC-Fg or PAC-1. Experimental procedures and data expression were the same as described in Figure 7. To facilitate the comparison, data from CHO cells expressing heterodimers of mutant β3(616-690del), shown in Figure 7, have been included.
Discussion
Role of the carboxy-terminal domains of β3 on the assembly and surface exposure of αIIbβ3 complexes
We have recently reported a case of Glanzmann thrombasthenia associated with a point mutation that changed Glu616 to a termination codon resulting in a truncated β3 protein that lacks the transmembrane and cytoplasmic regions.15 The lack of surface exposure of αIIbβ3Δ616 was caused by a failure of the truncated protein to complex αIIb. This observation appeared to be in conflict with a previous report indicating that a truncated β3 lacking the transmembrane and cytoplasmic domains (β3Δ693) allowed assembly and surface exposure of αIIbΔβ3 complexes.14 Moreover, truncated forms of recombinant αIIb and β3 seem to form soluble ΔαIIb-Δβ3 heterodimers capable of binding to ligands.21 To determine whether the apparent discrepancy could be due to the extension of the β3 truncation in each experimental condition, we analyzed the ability of β3-truncated (Δβ3) forms of different lengths to complex αIIb. We failed to demonstrate association of any of the Δβ3 forms tested with αIIb. The proteolytic cleavage of pro-αIIb to yield αIIb heavy (αIIbH) and light (αIIbL) chains requires a previous association of α and β subunits; therefore, the lack of coprecipitation of αIIbH with Δβ3 indicates that pro-αIIb failed to form stable complexes with any of the Δβ3 forms analyzed. This assertion agrees with the observation that the Iraqi-Jewish thrombasthenic phenotype associated with the β3Δ650 mutation shows detectable platelet pro-αIIb22 and absence of platelet αIIbβ3 and αvβ3 receptors23,24 ; moreover, the recombinant β3Δ657 does not complex αIIb.25 However, truncated β3 preserving the transmembrane domain and 1 or 8 of the 47 cytoplasmic amino acids have been reported to reach the cell surface.26,27 On the other hand, the αIIb and β3 ectodomains can form heterodimers that bind ligands and exhibit immunochemical properties similar to the full-length receptor inserted in the plasma membrane.21,28 On these grounds, it seems plausible to conclude that the cell surface exposure of detectable levels of αIIbβ3 demands the presence of the transmembrane region of β3.
State of activation of recombinant αvβ3(616-690del) and αIIbβ3(616-690del) receptors
Platelet αIIbβ3 receptors, about 80 000/cell,8 as well as their main ligands, fibrinogen and von Willebrand factor, are present in blood at high concentrations. Thus, to prevent intravascular aggregation, the receptors must be maintained in a low-affinity state for their ligands. The activation of platelets by physiologic agonists leads to increased fibrinogen binding to αIIbβ3 and the ligand-bound receptors propagate signals into the cell resulting in cytoskeletal organization and receptor clustering.8-10 Domains within the cytoplasmic tails of α and β subunits seem to be involved in the bidirectional signaling through αIIbβ3.29,30 The precise molecular mechanisms of receptor activation have not been yet elucidated, although early studies on this matter postulated that ligand affinity changes could be the result of conformational changes.11 In agreement with this postulate, recent crystallographic studies of the ectodomains of β3 revealed that the binding of the ligand mimetic RGD peptide is associated with precise conformational changes31-34 and that clasping of the α and β tails prevents the integrin activation.35 Moreover, analysis of peptide fragments from resting and activated αIIbβ312 and 3-dimensional modeling of αIIbβ3 based on electron microscopy and x-ray crystallographic studies13 reported the existence of domain-specific conformational changes in receptors from activated platelets. Studies of binding of platelets to monoclonal antibodies also suggest specific agonist-induced conformational changes in αIIbβ3.36 Furthermore, point mutations in β3 leading to conformational changes are associated with changes in the state of activation of αIIbβ3.37-42 Thus, a variety of experimental approaches support the contention that integrin activation is accompanied by conformational changes; however, the mechanisms by which the putative conformational changes increase the ligand binding affinity remain unclear.35 The same comment applies to the mechanisms involved in locking the fibrinogen receptor in a low-affinity state. Our observation that deletion of residues 616 to 690 in β3 endows constitutive activity to the αIIbβ3(616-690del) receptor suggested the presence within this region of a not-previously-identified domain essential to maintain a resting, nonactivated state. Further mutation analysis revealed that disruption of the 663Cys-687Cys β3 disulfide bridge is sufficient to confer constitutive activity. This finding may provide a molecular basis for the postulate that disulfide swapping could play a role in integrin activation.43 A chimeric receptor in which the region 616-690 of β3 was replaced by its homologous region of β7 that lacks cysteines 663 and 687 showed a constitutive activity similar to that observed when the 663Cys-687Cys disulfide bridge was disrupted, that is, less than 50% of the binding capacity of the β3(616-690del) complexes. Moreover, the degree of αIIbβ3 activation was a function of the length of the carboxy-terminal deletion of β3. These observations suggest that shortening of the β3 carboxy-terminal ectodomain or disruption of the 663-687 disulfide bridge acted by different mechanisms in activating αIIbβ3. The role played by the 663-687 disulfide bridge of β3 in activating αIIbβ3 adds to the list of natural38-41 or experimental41,44 mutations of β3 cysteines associated with receptor activation. According to these observations, receptor activation may be achieved by different patterns of protein folding due to loss or rearrangement of disulfide links. Regardless of the mechanism by which the mutated β3 subunits confer activity to β3 receptors, signals must be propagated to elicit these changes in the ligand binding domain. In principle, 2 possibilities could be considered: first, conformational changes in the βTD are transmitted directly to the ligand-binding domain; second, structural changes in the βTD could force the α and β cytoplasmic tails to adopt a position unable to maintain the proper association with regulatory or cytoskeletal proteins. The first possibility finds support in the observation that a correlation exists between the length of the deleted β3 fragment and the state of activation, suggesting that in the native complex shortening the β3 stalk could make more accessible the ligand to the binding site in the amino-terminal globular domain. On the other hand, the observed changes in size and shape of CHO-αIIbβ3(616-690del) cells support the second possibility. In this regard, impairment of actin polymerization induces fibrinogen binding to platelets, suggesting that cytoskeletal interaction may contribute to maintain the αIIbβ3 receptor in a resting state.45
The divalent manganese ion increases the ligand-binding affinity to integrins and promotes cell adherence to immobilized ligands.46,47 It is worth noting that Mn2+ was able to further enhance the ligand binding in cells expressing mutated αvβ3 or αIIbβ3 receptors, suggesting that Mn2+ and mutations in the βTD of β3 may act in activating β3 integrins by different mechanisms. This and other observations36 indicating alternative mechanisms of activation suggest that, under physiologic conditions, the state of activation of platelets may be the result of the combinatorial action of several positive and negative modulators.
The vitronectin and fibrinogen receptors share the β3 subunit as well as some ligands. Nevertheless, their patterns of tissue expression as well as their pathophysiologic roles are markedly different. In most cell lines and in all adherent cells the vitronectin receptor seems to be in a permanent state of activation28,48 ; however, in platelets and some lymphoid cells, the ligand affinity is subjected to regulation by physiologic agonists49-51 and, most likely, the receptor occupancy generates cellulipetal (outside-in) signals of regulatory importance.52,53 In principle, the ligand specificity and affinity of αvβ3 and αIIbβ3 receptors should be determined by their distinct α subunits; but the knowledge about the mechanisms controlling this process is rather limited. Our data indicate that the βTD of β3 plays a previously unnoticed role in maintaining both receptors in a resting, nonactive state.
To conclude, the 616-690 region located in the carboxy-terminal tail of the β3 ectodomain, so-called βTD, is not essential for cell surface expression of β3 receptors. However, either deletions within this region or disruption of the C663-C687 disulfide bridge, confers constitutive activity to β3 integrins. Thus, the normal conformation of this β3 domain contributes to restrain β3 integrins in a resting, low ligand-affinity state.
Prepublished online as Blood First Edition Paper, June 19, 2003; DOI 10.1182/blood-2003-01-0213.
Supported in part by grants from the Dirección General de Investigación (SAF 2000-0127, PB97-1240, and BMC2002-01053), Fondo de Investigaciones Sanitarias (FIS-PI021263), and Comunidad de Madrid no. 08,4/0015.1/2001. N.B. is a recipient of tenure track grant Ramon y Cajal from the Spanish Ministry of Science. S.L. and M.F. were supported by posdoctoral fellowships from the Comunidad de Madrid (08.4/0031/1998 and 02/0446/01).
N.B. and E.G.A.S. contributed equally to this paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr S. Shattil (The Scripps Research Institute, La Jolla, CA) for the gift of PAC-1 and WOW-1 Fabs.


![Figure 3. Immunoprecipitation analysis of αIIbβ3 complexes from CHO cells transiently cotransfected with αIIb and either normal or mutant forms of β3. CHO cells were transiently transfected with αIIb and either normal β3 or β3(616-690del), β3Δ616, β3Δ638, β3Δ657, β3Δ675, or β3Δ693 cDNA constructs. Cells were incubated with [35S]-methionine-cysteine for 3 hours. Cell lysates were immunoprecipitated with anti-β3 or anti-αIIb and processed as described in “Materials and methods.”](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/7/10.1182_blood-2003-01-0213/6/m_h81935000003.jpeg?Expires=1765983398&Signature=VSbwQrFTKHNdDhfm206mochpZKzz8mQYCjrbhS8pveOvKF4WXQGoziJRHIMEUZ2~sQzwx~DUBLK62Xhe95QNZk4nyON1EwAFrRzfvN2fWqkOtdMcILEf-b0vADlJqm~DhvNI5cgcD5BwNfy2rbxMXxniri6mZ8EB9zNFvMDjZ4~izEBa4JsF~OzgQCAlSJ3wljgZgHjqqxUFZ4wTxMHJeXmI2rtYbJedwRQJq9pbQPq~ZaZTNi5mhdPYABSyI1CmLQs80FSWujFIAAI1Hn02F2NehVlN67H8nNkoTUTPjQRBsS3Yu93jAgrVwUf~wBVDUyL4DSxgBYlixAmPCI3K6A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal