Abstract
The clinical activity of rituximab has been evaluated in a phase 2 study in both untreated and relapsed mucosa-associated lymphoid tissue (MALT) lymphomas. Treatment consisted of 4 standard (375 mg/m2) weekly doses. Thirty-five patients were enrolled, and 34 completed the treatment program. The primary lymphoma location was stomach in 15 patients, and extragastric in 20. Eleven patients had previously been treated with chemotherapy. At study entry 12 patients had Ann Arbor stage IE, 3 had stage IIE, and 20 had stage IV disease. The overall response rate was 73% (95% confidence interval, 56%-87%), with 15 complete responses and 10 partial responses, and the response rate was significantly higher in the chemotherapy-naive patients, who had an 87% response rate compared with 45% of the previously treated patients (P = .03). The median response duration was 10.5 months. At a median follow-up of 15 months, 9 patients (26%) relapsed. The median time to treatment failure was 14.2 months in the whole series, but it was significantly longer (22 versus 12 months) in the chemotherapynaive patients compared with those who had prior chemotherapy (P = .001). Most adverse events were of mild to moderate severity with no grade 4 toxicity. This study indicates that rituximab is safe with significant activity in MALT lymphomas.
Introduction
Mucosa-associated lymphoid tissue (MALT) lymphomas, first described in the early 1980s, represent approximately 8% of all non-Hodgkin lymphomas.1,2 This condition has been included in the World Health Organization (WHO) classification of neoplastic diseases of the hematopoietic and lymphoid tissues as a separate entity, namely the “extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue.”3
This type of lymphoma usually has an indolent course, with survival rates at 5 years from diagnosis ranging from 80% up to 95%, but the progression-free survival is relatively short, particularly for patients presenting with advanced stage or unfavorable International Prognostic Index.1,2,4-6
There is increasing evidence that eradication of Helicobacter pylori with antibiotics can be effectively employed as the sole initial treatment of localized gastric MALT lymphoma.1,2,7-9 However, it is still unknown whether H pylori eradication will definitively cure the lymphoma.7-9
For the patients who fail to respond to antibiotics, for the subset of gastric MALT lymphomas in which no evidence of H pylori infection can be found, and for nongastric or disseminated lymphoma, a therapeutic choice can be made from conventional oncologic modalities including chemotherapy, radiotherapy, and surgery, alone or in combination.1,2,4-6,10-15 Unfortunately, no published randomized studies assist in the decision, and the treatment strategy has to be “patient-tailored,” taking the site, the stage, and the clinical characteristics of the individual patient into account.1,2
The clinical efficacy of rituximab, a chimeric monoclonal antibody directed against the B-cell–specific antigen CD20, was first demonstrated in follicular lymphomas,16 but the use of the antibody has been extended over the last few years to other subtypes of non-Hodgkin lymphomas with promising results, as single agent16-18 or in combination with chemotherapy.19-22
CD20 antigen is expressed on the surface of neoplastic cells in virtually all MALT lymphomas; therefore, it is reasonable to predict that rituximab may be active in this disease. Nevertheless, available information on its use in these lymphomas has been anecdotal.23
Thus, the International Extranodal Lymphoma Study Group (IELSG) conducted a phase 2 study to prospectively evaluate the antitumor activity and tolerability of a 4-weekly-doses (375 mg/m2 each) schedule of rituximab in previously untreated and treated adult patients with MALT lymphomas. The results of this trial are reported in this study.
Patients, materials, and methods
Study design and end points
The primary objective of the trial was to assess the overall response rate (ORR) to rituximab in MALT lymphoma patients. The secondary aim was to assess the toxicity profile of rituximab in this population. In this nonrandomized phase 2 study the sample size was based on the primary end point of ORR. The number of patients required was therefore calculated on the assumption that a response rate of at least 30% would be sufficient to qualify the treatment as promising for further investigation. Accordingly, at least 30 patients are required to provide a single-arm study with 80% power at an overall 5% significance level.24
Study sites
The trial was conducted between January 2000 and May 2001 at 7 centers in Italy, France, and Switzerland and was performed according to the principles of the Declaration of Helsinki with its current amendments. All patients gave written informed consent. The protocol and informed consent forms were approved by the local institutional review boards and ethics committees of each participating institution.
Patient population and pretreatment evaluation
Patients were eligible if they were more than 18 years of age and had untreated, relapsed or refractory, biopsy-proven, CD20+ extranodal marginal zone B-cell lymphoma of MALT type, arising at any extranodal site and diagnosed according to the Revised European-American Lymphoma (REAL)/WHO classification criteria.3 All cases underwent central histologic review by expert lymphoma pathologists (M.P., E.P., G.P.); in all cases measurable or evaluable disease, defined according to the National Cancer Institute (NCI) international workshop criteria,25 was required. All patients were clinically staged according to the Ann Arbor criteria. The International Prognostic Index (IPI)26 was used to determine the prognostic risk. Patients were required to have a performance status of 0 to 2, according to the criteria of the Eastern Clinical Oncology Group (ECOG), and no prior lymphoma treatment for at least 4 weeks. Patients were not eligible where there was evidence of histologic transformation into aggressive lymphoma, central nervous system involvement, previous or concomitant malignancy with the exception of nonmelanoma skin cancer, or clinically relevant cardiac disease. Patients with a positive serologic test for the human immunodeficiency virus were also excluded. All patients underwent staging procedures including history; physical examination; complete blood counts; chemistry profile; computed tomography of the chest, abdomen, and pelvis; and bone marrow aspiration/biopsy within the 4 weeks prior to treatment. Endoscopic investigations with multiple mucosal biopsies were performed in case of gastric lymphoma involvement.
Treatment
All patients were treated with weekly doses of rituximab (Mabthera; Roche Pharma, Basel, Switzerland; 375 mg/m2) administered on 4 consecutive weeks by slow intravenous infusion following standard guidelines. Administration of dexamethasone was not permitted. If serious infusion-related events occurred, infusion of rituximab was to be discontinued and then be restarted at a lower infusion rate. The rate of infusion was then to be re-escalated until a maximum tolerated infusion rate was reached.
Assessment of response
Patients were assessed for response at 2, 6, and 12 months after the start of the 4-week course of rituximab. Restaging included a repeat of all previously abnormal staging tests. Tumor responses were classified as complete response (CR), partial response (PR), stable disease (SD), or relapsing/progressive disease (PD) according to the standardized response criteria for non-Hodgkin lymphomas.25 ORR was defined as the sum of CR and PR rates. All patients who completed 4 weeks of therapy were considered evaluable for response.
For primary gastric localizations, documentation of histologic lymphoma regression was required. Definition of complete histologic regression was obtained when the posttreatment biopsies showed an empty lamina propria with small basal clusters of lymphocytes and scattered plasma cells and no sign of remaining lymphoma. Partial histologic regression is defined as posttreatment biopsy samples revealing either focal atypical lymphoid cells or focal lymphoepithelial lesions and an empty lamina propria as signs of lymphoma regression.
Assessment of toxicity
Patients who received at least one dose of rituximab were included in the analysis of toxicity. This was evaluated and graded using the NCI Common Toxicity Criteria.27
Statistical considerations
Statistical analysis was conducted using the STATA 5.0 software package (Stata, College Station, TX). The median follow-up was computed by the reverse Kaplan-Meier method.28 Response duration and time to treatment failure were defined according to the NCI workshop criteria.25 Response duration was calculated from time to best response to the date of relapse/progression or last follow-up; time to treatment failure was calculated from time of entry into trial to the date of treatment failure or last follow-up. Survival curves were determined using the Kaplan-Meier method, and differences were analyzed using the log-rank test.29 Binomial exact 95% confidence intervals (95% CI) were calculated for remission rates. Associations in 2-way tables were tested for statistical significance using the Fisher exact test (2-tailed).30
Results
Patient characteristics
Thirty-five patients with a median age of 57 years (range, 27-85 years) were enrolled. Patient characteristics at time of study entry are listed in Table 1. The primary site of lymphoma localization was defined as the clinically dominant extranodal component requiring diagnostic investigations, and the site to which treatment was directed at time of study entry. Stomach was the primary site in 15 patients; 20 patients had a primary nongastric localization.
Patients' clinical characteristics at study entry
Features . | No. of patients (%) . |
|---|---|
| Sex | |
| Male | 11 (31) |
| Female | 24 (69) |
| Ann Arbor stage | |
| IE | 12 (34) |
| IIE | 3 (9) |
| IV | 20 (57) |
| B symptoms | 2 (6) |
| Serum LDH level | |
| Elevated | 3 (9) |
| Unknown | 1 (3) |
| Serum β2-microglobulin level | |
| Elevated | 6 (17) |
| Unknown | 5 (14) |
| More than 1 extranodal site | 7 (20) |
| Nodal involvement | 13 (37) |
| Bone marrow involvement | 9 (26) |
| Intermediate/high to high-risk IPI score | 7 (20) |
| Previous chemotherapy | 11 (31) |
| Primary site* | |
| Stomach | 15 (43) |
| skin/subcutaneous tissue | 7 (20) |
| Salivary gland | 4 (11) |
| Lung | 4 (11) |
| Orbit | 3 (9) |
| Breast | 1 (3) |
| Liver | 1 (3) |
Features . | No. of patients (%) . |
|---|---|
| Sex | |
| Male | 11 (31) |
| Female | 24 (69) |
| Ann Arbor stage | |
| IE | 12 (34) |
| IIE | 3 (9) |
| IV | 20 (57) |
| B symptoms | 2 (6) |
| Serum LDH level | |
| Elevated | 3 (9) |
| Unknown | 1 (3) |
| Serum β2-microglobulin level | |
| Elevated | 6 (17) |
| Unknown | 5 (14) |
| More than 1 extranodal site | 7 (20) |
| Nodal involvement | 13 (37) |
| Bone marrow involvement | 9 (26) |
| Intermediate/high to high-risk IPI score | 7 (20) |
| Previous chemotherapy | 11 (31) |
| Primary site* | |
| Stomach | 15 (43) |
| skin/subcutaneous tissue | 7 (20) |
| Salivary gland | 4 (11) |
| Lung | 4 (11) |
| Orbit | 3 (9) |
| Breast | 1 (3) |
| Liver | 1 (3) |
In 7 of these cases multiple mucosal sites were involved.
Previous H pylori infection
Thirteen of the 15 patients with a primary gastric localization had H pylori infection at diagnosis, which was eradicated with antibiotic treatment, and at the time of study onset all these 15 patients were negative for H pylori infection. Among the 13 patients with a prior evidence of H pylori infection, the median time since the H pylori infection eradication to study entry was 25 months (range, 5-89 months).
Previous treatments
The median time from diagnosis to study entry was 24 months (range, 1-119 months). Among the 20 previously treated patients, 9 with a primary gastric localization had undergone the sole anti–H pylori antibiotic therapy, and 11 patients received a systemic chemotherapy—in 7 cases a single-alkylating agent, a CVP (cyclophosphamide, vincristine, prednisone) program, or a purine analog (cladribine or fludarabine) and in 4 cases an anthracycline-containing program, (ie, CHOP [cyclophosphamide, doxorubicin, vincristine, prednisone] or a CHOP-like regimen). Two patients received chemotherapy after a primary radiotherapy program and 4 after an initial anti–H pylori antibiotic treatment. In 7 patients with a primary gastric localization the lymphoma did not regress after Hpylori eradication. The remaining 13 pretreated patients (7 with gastric and 6 with nongastric lymphoma) were relapsing after a response to prior therapy (2 after antibiotic alone, 4 after antibiotic plus chemotherapy, 7 after chemotherapy or chemoradiotherapy).
Response to rituximab therapy
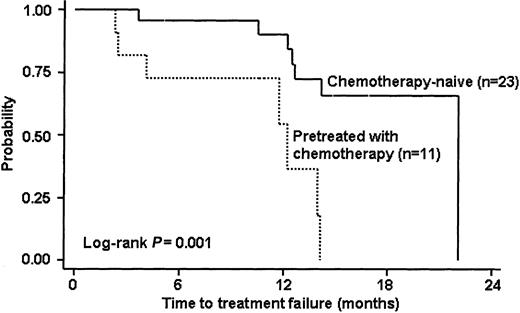
Only one patient (with primary gastric disease) did not complete the treatment program: after the second infusion of rituximab she experienced a grade III infection after which, despite a full recovery, she refused to complete the treatment program and was lost to follow-up. All the remaining 34 patients received the 4 scheduled courses of rituximab and were evaluable for response. Twenty-five (73%; 95% CI, 56%-87%) of these 34 patients had objective responses with 15 (44%; 95% CI, 27%-62%) CRs and 10 (29%; 95% CI, 15%-47%) PRs. Responses were observed either in gastric and nongastric MALT lymphomas. Four of the 7 patients refractory to previous antibiotic therapy had an objective response to treatment in the study (1 CR and 3 PRs), in 2 other refractory patients a stabilization of disease was reported, and the remaining case was not evaluable for response. Table 2 summarizes the treatment results by previous treatment and primary site of lymphoma involvement. The median time to best response was 2.2 months (range, 1.6-6 months), whereas in 6 (18%; 95% CI, 7%-35%) patients SD was observed and 3 patients experienced a disease progression, in one case related to the histologic transformation into an aggressive histotype. After a median follow-up of 15 months (range, 1-23 months), all patients were alive and 10 patients were disease free. Nine of the 25 responding patients have relapsed so far: 5 after a CR and 4 after a PR. Two patients in whom an SD was documented at the end of study treatment later experienced a disease progression. The observed median response duration was 10.5 months (range, 3-20 months). The median time to treatment failure was 14.2 months in the whole series and was nearly identical in gastric and nongastric lymphoma, but it was significantly longer (22 versus 12 months) in the chemotherapy-naive patients in compared with those who had prior chemotherapy (Figure 1).
Best response to rituximab according to the primary site of lymphoma localization and previous chemotherapy in 34 evaluable patients
Response . | No prior chemotherapy, n = 23 . | Prior chemotherapy, n = 11 . | Primary gastric site, n = 14* . | Primary nongastric site, n = 20 . | All patients, n = 34 . |
|---|---|---|---|---|---|
| ORR (%) | 20 (87)† | 5 (45)† | 9 (64) | 16 (80) | 25 (73) |
| CR (%) | 11 (48) | 4 (36) | 4 (29) | 11 (55) | 15 (44) |
| PR (%) | 9 (39) | 1 (9) | 5 (35) | 5 (25) | 10 (29) |
| SD (%) | 2 (9) | 4 (36) | 4 (29) | 2 (10) | 6 (18) |
| PD (%) | 1 (4) | 2 (18) | 1 (7) | 2 (10) | 3 (9) |
Response . | No prior chemotherapy, n = 23 . | Prior chemotherapy, n = 11 . | Primary gastric site, n = 14* . | Primary nongastric site, n = 20 . | All patients, n = 34 . |
|---|---|---|---|---|---|
| ORR (%) | 20 (87)† | 5 (45)† | 9 (64) | 16 (80) | 25 (73) |
| CR (%) | 11 (48) | 4 (36) | 4 (29) | 11 (55) | 15 (44) |
| PR (%) | 9 (39) | 1 (9) | 5 (35) | 5 (25) | 10 (29) |
| SD (%) | 2 (9) | 4 (36) | 4 (29) | 2 (10) | 6 (18) |
| PD (%) | 1 (4) | 2 (18) | 1 (7) | 2 (10) | 3 (9) |
One patient with primary gastric disease did not complete the treatment and was not evaluable for response.
The difference in ORR between chemotherapy-naive and pretreated patients was statistically significant (2-sided Fisher exact test, P = .03).
Survival curve of time to treatment failure according to prior receipt of chemotherapy (34 evaluable patients).
Survival curve of time to treatment failure according to prior receipt of chemotherapy (34 evaluable patients).
Prognostic factors
Despite the small number of patients, several variables, including sex, age, stage, performance status, lactate dehydrogenase (LDH) and β2-microglobulin level, IPI, B symptoms, previous treatment, and lymph node, spleen and bone marrow involvement, were analyzed for their possible association with response. The one variable significantly associated with a lower probability of response was prior chemotherapy (P = .03). Only 5 of 11 (45%; 95% CI, 17%-77%) patients who relapsed after prior chemotherapy achieved a response compared with 20 responses observed in the 23 chemotherapy-naive patients (87%; 95% CI, 66%-97%).
Toxicity
Fifteen patients did not experience any adverse event. In the 138 courses assessed for toxicity, 20 patients experienced 29 adverse events (most commonly the expected infusion-related side effects, including hypotension, angioedema, bronchospasm, skin rash, fever). Most of these events were of mild to moderate severity and self-limiting. Most adverse events occurred during the first infusion, and both incidence and frequency declined with subsequent infusions. Gastric bleeding occurred 24 hours after the first infusion in one patient with a primary bulky gastric disease. The episode did not require any transfusional support, and the patient rapidly recovered, completing the study treatment according to the plan. No grade IV toxicity and only 3 grade III events were seen (1 infection, 1 bronchospasm, and 1 glottis edema). In only one case did an infection (pneumonia), observed after the second rituximab infusion, require hospitalization.
Discussion
Our study aimed to evaluate the clinical activity and toxicity of rituximab in either untreated or relapsed MALT lymphomas. The treatment was generally well tolerated, with most toxicity being directly infusion related (most commonly the first infusion). Although further follow-up is required, the demonstration of minimal toxicity and considerable activity of this biologic agent represents a substantial step toward more specific, less toxic treatment for this group of indolent lymphomas. Indeed, the drug had significant activity, especially in previously untreated patients, and the observed ORR (73%) is similar to that reported in a study of untreated follicular and small lymphocytic lymphomas31 that received the same 4-week treatment followed by maintenance rituximab courses at 6-month intervals. In our study, however, no maintenance treatment was given and the median duration of response (10.5 months) was similar to duration of response previously reported in series of mainly pretreated follicular16 and mantle cell lymphomas17 that received only the standard 4 weekly doses.
Despite abundant literature on histologic, clinical, and biologic features of MALT lymphoma, results of controlled trials to define optimal therapy have not yet been published. There are few published studies specifically reporting treatment outcome for MALT lymphoma.1,2 The more recent studies predominantly refer to retrospective series of patients not uniformly staged and treated.1,2
With the exception of anti–H pylori therapy in localized primary gastric MALT lymphoma with H pylori infection,1 no established guidelines exist for the management of MALT lymphoma patients.1,2,7 The efficacy of conventional strategies including chemotherapy, radiotherapy, and surgery, alone or in combination, has been reported1,2,4-6,10-15 with no significant difference in survival.12 For localized disease, local treatment (either radiotherapy or surgery) can usually achieve good disease control4,5,14,15,32 and the use of radiotherapy is becoming increasingly popular. However, a variety of radiation doses and fields have been employed, data on long-term toxicity remain unreported, and it is difficult to define a standard radiation therapy approach. Moreover, local therapy is inadequate in at least a quarter of cases, which present with disseminated disease, usually with involvement of multiple mucosal sites.2,10,11
Chemotherapy has never been adequately evaluated in MALT lymphoma because it was most often administered after either surgery or radiotherapy. Some scanty data suggesting the efficacy of chlorambucil in low-grade gastric lymphoma can be found in the literature.1,13 The indication for more aggressive chemotherapy regimens has never been properly investigated in clinical trials and remains controversial in MALT lymphoma, which usually displays an indolent course.1,2,12
While overall survival of patients with MALT lymphoma seems excellent regardless of treatment,12 their optimal therapy remains to be determined and the poor progression-free survival in advanced-stage patients supports the need to develop novel systemic treatment strategies for this disease.1,2,4-6
This study is the first phase 2 study of rituximab performed in MALT lymphomas and showed a significant activity of the compound. Prior receipt of chemotherapy resulted in a lower probability of response and also in a shorter time to treatment failure, but the small number of previously treated patients prevents any definitive conclusion about the activity of rituximab in this setting.
Interestingly, in MALT lymphoma different nonrandom chromosomal translocations have been reported, which may identify different pathogenetic pathways2,7,33-35 and may predict the response to antibiotic therapy in gastric lymphomas.2,7,36 Whether these cytogenetic alterations may have a role in determining the response to chemotherapy and to rituximab remains to be elucidated.
The high relapse rate (36%) observed in our series suggests that the 4-weekly-doses regimen used may not represent the best schedule for administering rituximab in MALT lymphoma, and increasing evidence has already been provided in other lymphoma subtypes that higher doses, maintenance treatment, and combination with conventional chemotherapy may all be of benefit.20-22,31,37-40
Further studies are also required to optimize maintenance rituximab therapy or its combination with chemotherapy for the treatment of MALT lymphoma. Based on the results of this study and on the potential synergistic action between rituximab and chemotherapy,20-22,37,38 the IELSG is conducting a randomized clinical trial to evaluate the combination of chlorambucil and rituximab in MALT lymphomas.
Prepublished online as Blood First Edition Paper, July 3, 2003; DOI 10.1182/blood-2002-11-3496.
Supported in part by Roche Pharma (Schweiz) AG.
B.C. is a member of the advisory board (regarding rituximab) of F. Hoffman-La Roche Ltd.
A.C. and G.M. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This study was conducted on behalf of the International Extranodal Lymphoma Study Group (IELSG).
We thank Mrs Cristina Morinini for her expert assistance in data management and Dr Dimitrios O. Papadopoulos (Roche Pharma AG) for his valuable cooperation. We also thank Roche Pharma (Schweiz) AG for partially supporting this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal