Abstract
We describe large B cells, many of which have a stellate or dendritic morphology, found in the interfollicular T-cell–rich areas of human peripheral lymphoid tissue. These B cells probably require CD40/CD40 ligand signaling for their generation and have a unique phenotype and a post–germinal center pattern of immunoglobulin gene mutation. The only comparable B cell is the “asteroid” cell of the thymic medulla. Their anatomic location and pattern of gene mutation suggest that these cells may represent the cell of origin of some human large-cell lymphomas. Studies in experimental animals should help to elucidate the role of these cells in the immune response.
Introduction
The maturation of human B-lymphoid cells has been the subject of extensive studies over many years.1-7 B-cell precursors in the bone marrow undergo rearrangement of their immunoglobulin genes and initially generate a form of immunoglobulin M (IgM) (confined largely to the cytoplasm) in which μ-chain is associated with “pseudolight chains” (variable preB [VpreB] and λ5). Subsequently, B cells leave the marrow to become naive circulating and mantle zone cells, carrying IgM and IgD as their surface antigen receptor, physically associated with signal transduction molecules (eg, CD79, Fc receptor–γIIB [FcRγIIB]). The antigen-binding repertoire of the surface immunoglobulin is already unique for each clone, as a result of selective usage in each cell of different variable (V), diversity (D), and joining (J) region sequences, accompanied by the generation of random-linking N sequences (via the action of terminal transferase).
B cells then enter the germinal centers of secondary lymphoid follicles and undergo a process of random hypermutation in theirV immunoglobulin region genes, under the influence of activation-induced cytidine deaminase (AID), resulting in yet more diversity in the antigen-binding repertoire of their surface immunoglobulin. B cells within the germinal center then perish through spontaneous apoptosis unless their surface immunoglobulin binds with high affinity to antigen (present within immune complexes) on follicular dendritic cells. The small numbers of surviving cells then differentiate further to become either plasma cells or memory cells.1,8-10
The pattern of somatic mutations in the V regions of immunoglobulin heavy (H) chain genes acquired in the germinal center has been used to assign B-cell populations (and lymphomas of B-cell origin) to 2 broad categories: naive B cells harboring unmutated IgH genes; and germinal center and post–germinal center B cells carrying somatically mutated IgH genes.1 This distinction has been of major value in understanding the physiology of the human immune response and in correlating categories of human B-cell neoplasia with a specific maturation stage.
In this study, we report a population of human B cells, often with a stellate or dendritic morphology, that are found as scattered large cells in T-cell–rich areas of peripheral lymphoid tissue. Interfollicular large B cells have been reported in mice as extrafollicular plasmablasts.11-15 However, little attention has been paid to interfollicular large B cells in humans, with the exception of cytoplasmic immunoglobulin-positive immunoblast-like B cells seen in reactive lymph nodes.16 The nearest recognized equivalent to the large interfollicular B cells that we describe in this report is probably the large “asteroid” B cell seen in the thymic medulla.17-19
Further studies are therefore needed to determine the relationship of the large interfollicular B cells that we describe in this report (many of which are in cell cycle) to other B cells. It will also be of interest if equivalent cells can be identified in experimental animals. The anatomic location and the molecular features of these T-cell–associated large B cells suggest that they may represent the cell of origin of some human lymphomas.
Materials and methods
Tissue samples
Formalin-fixed, paraffin-embedded, and frozen samples of lymph node (7 cases), tonsil (2 cases), and thymus (2 cases) were obtained from the files of the Histopathology Department, John Radcliffe Hospital (Oxford, United Kingdom). Paraffin-embedded samples of lymph nodes from 2 patients with hyper-IgM syndrome,20 frozen samples of 2 tonsils and 2 lymph nodes, as well as 1 sample of ectopic thymus were obtained from the Institute of Pathology of Brescia University (Brescia, Italy). There was no clinical history of tumors or infectious diseases among the cases we analyzed. The lymph nodes were removed during surgery for different conditions (eg, appendectomy, carotid endarterectomy, mastectomy), and they showed quiescent or only mild reactive features.
Immunohistochemistry
First, 5-μm–thick paraffin-embedded tissue sections on poly-L-lysine–treated slides were dewaxed, rehydrated, and then submitted to antigen retrieval by heating in 0.01 M Tris-EDTA (tris(hydroxymethyl)aminomethane–ethylenediaminetetraacetic acid) (pH 9.0) in a microwavable pressure cooker (A. Menarini Diagnostics, Wokingham, United Kingdom) at 900 W for 3 minutes. After cooling, the slides were placed in Tris-buffered saline (TBS) for 5 minutes. Endogenous peroxidase was blocked by means of a commercial peroxidase-blocking reagent (DakoCytomation, Glostrup, Denmark). Slides were then incubated for 30 minutes at room temperature with antibodies to the following: CD3, CD20, CD21, CD23, CD30, CD34, CD35, CD45, CD68, B-cell leukemia 6 (BCL-6), caspase-3, IgD, IgM, κ- and λ-light chains, MIB-1 (Ki-67), S-100, terminal deoxynucleotidyl transferase (TdT), and VS38c (p63) (DakoCytomation); CD5, CD8, CD75, CD79a, and BCL-2 (D.Y.M.'s laboratory, Oxford, United Kingdom); J-chain (Biogenex, San Ramon, CA); CD4, CD10, CD27, CD38, MSH2, and p27/KIP1 (Novocastra, Newcastle upon Tyne, United Kingdom); CD40, CD80, and CD86 (Sixth HLDA[human leukocyte differentiation antigens] Workshop, Kobe, Japan, 1996); multiple myeloma oncogene protein 1 (MUM-1) (interferon regulatory factor 4 [IRF-4]) (Prof B. Falini's laboratory, Perugia, Italy); CD1a, paired box 5 (PAX-5), and PU.1 (BD Biosciences, San Diego, CA); BOB-1/OCA-B/OBF-1, and OCT-2 (Santa Cruz Biotechnology, Santa Cruz, CA), CD208 (dendritic cell–lysosome-associated member protein [DC-LAMP]) (Beckman Coulter France, Roissy, France). All antibodies were diluted with human serum (deactivated for 15 minutes at 50°C) to block possible cross-reactivities of the antibodies via Fc receptors. Sections were stained in parallel without primary antibody to provide a negative control for each reaction. Antibody binding was then detected by the peroxidase-based EnVision (DakoCytomation) method,21 and sections were counterstained with hematoxylin.
Double immunoenzymatic labeling
Double immunoenzymatic labeling of paraffin sections following the described pretreatment was carried out by means of the EnVision peroxidase and alkaline phosphatase kits (DakoCytomation). Primary antibodies were incubated for 30 minutes at room temperature, and a diaminobenzidine (DAB) substrate (DakoCytomation) was then used for detection of antibody binding. Sections were then incubated for 30 minutes with the second antibody, and then for an additional 30 minutes with the alkaline phosphatase EnVision kit (DakoCytomation). The second reaction was detected by means of a Vector blue alkaline phosphatase substrate kit (Vector Laboratories, Peterborough, United Kingdom). The sections were washed in tap water and mounted in aquamount (Merck, Poole, United Kingdom).
Double immunofluorescence
Sections were pretreated as described above and subsequently incubated for 45 minutes at room temperature with pairs of primary antibodies that were either from different species (eg, mouse and rabbit) or of differing immunoglobulin isotypes/subclasses (eg, IgG1, IgG2a, IgM), as previously described.22 Sections were washed in phosphate-buffered saline (PBS) for up to 5 minutes and then incubated in the dark for 45 minutes with pairs of species-, isotype-, or subclass-specific secondary antibodies, conjugated to Alexa Fluor 488 (green) or Alexa Fluor 594 (red) (Molecular Probes, Leiden, The Netherlands). The slides were washed in PBS for up to 5 minutes, mounted in fluorescence mounting medium (DakoCytomation) containing 1 μg/mL DAPI (4,6-diamidino-2-phenylindole), and examined by means of an E800 Eclipse fluorescence microscope (Nikon, Kingston-upon-Thames, United Kingdom). Images were captured with an Axiocam charge-coupled device (CCD) camera and Axiovision software (Imaging Associates, Bicester, United Kingdom), and then opened by means of Photoshop (Adobe, San Jose, CA). Slides for viewing by confocal microscopy were immunostained in the same way, with the exception that Alexa Fluor 568–conjugated secondary antibodies were used in place of Alexa Fluor 594 conjugates, and counterstaining was performed by adding T0T03 solution (Molecular Probes) at a dilution of 1:1000 to the mounting medium. Sections were viewed with a Zeiss LSM510 confocal microscope (Thornwood, NY). Frozen sections from one lymph node and one tonsil were also tested by double immunofluorescence to confirm that the negative results we obtained when staining paraffin-embedded sections were not due to denaturation of epitopes. Furthermore, for all paraffin section immunostaining, positive cells were present (eg, normal B cells), providing a positive control.
Combined immunoperoxidase and immunofluorescence labeling
Paraffin and frozen sections of reactive tonsils and lymph nodes were immuno-stained by the indirect immunoperoxidase technique (as described) for OCT-2 (monoclonal) (kindly provided by Dr Lynn M Corcoran, Royal Melbourne Hospital, Melbourne, Australia) and then washed in TBS for 5 minutes before the performance of double immunofluorescence labeling for CD20 and CD27 (as described). Brightfield and fluorescent images were captured with an E800 Eclipse fluorescence microscope, equipped with an Axiocam CCD camera and Axiovision software as described. The immunoperoxidase image was viewed by transmitted light, and corresponding gray-scale images were inverted and pasted into the blue channel of the red-green-blue (RGB) Photoshop image. By means of this technique, cells expressing OCT-2 were visualized in blue, whereas immunofluorescence labeling of the other 2 markers was detected in red and green.
Isolation by microdissection of single interfollicular large B cells
First, 9-μm-thick frozen tissue sections from 2 lymph node and 2 tonsil biopsies were air-dried overnight, fixed in acetone for 10 minutes, and then stained by the alkaline phosphatase anti–alkaline phosphatase (APAAP) technique for CD20 or by double immunoenzymatic staining for CD20 and OCT-2 (as described). Interfollicular large B cells were then isolated by means of manipulation capillaries as previously described23 and transferred into single polymerase chain reaction (PCR) tubes (Roche Diagnostics, Mannheim, Germany). Isolated T cells and aliquots of the overlaying buffer were also collected (as negative controls) after each interfollicular large B cell was isolated. In addition, single cells from germinal centers and from the mantle zone were analyzed as positive controls.
Single-cell PCR and cycle sequencing
IgH variable region gene sequences were amplified from individual interfollicular large B cells by means of a fully nested PCR. Family-specific framework 1 (FW1) primers for the first amplification, and family-specific FW2 primers for the reamplification, were employed, in combination with 2-nested primers for the immunoglobulin heavy-chain gene (JH) joining region, as previously described.24 PCR was performed on a GeneAmp PCR System 9700 (Applied Biosystems, Warrington, Cheshire, United Kingdom) in a 100 μL reaction volume as previously described.24 The PCR products were visualized with ethidium bromide under ultraviolet (UV) light and purified from a 6% polyacrylamide gel.
Cycle sequencing was performed with the dideoxy chain termination method25 with the use of the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit V2.0 (Applied Biosystems) and the ABI PRISM 3100 GeneticAnalyzer (Applied Biosystems), and the same pairs of primers were used as for the second round of PCR.
To identify somatic hypermutations, the nucleotide sequences obtained were compared with the germ line of the V region of the human IgH chain gene, accessed from gene databanks (GenBank, VBASE). Nucleotide sequences were compared with each other and with published databank sequences to exclude PCR contamination. Each VH sequence comprising the full complementarity-determining region 2 (CDR2) and FW3 region was analyzed for somatic mutations, and the ratio of replacement-to-silent mutations was evaluated in the CDR2 and FW3 regions as previously described.24
Antigen selection
In each sequence, somatic mutations were analyzed for compatibility with antigen selection by 2 methods. Method 1: the ratio of replacement-to-silent mutations (R/s) in the CDR2 and FW3 regions was considered to be antigen dependent when the R/s ratio was higher than 2.9 in the CDR2 and lower than 1.5 in the FW3.26 Method 2: the R/s ratio was evaluated only in the FW3 region and was regarded as being antigen-selected when the ratio was lower than 1.6.27
Sequence accession numbers
Results
Tissue sections from 2 tonsil and 7 lymph node samples were immunostained for CD20, and all showed scattered large CD20+ cells lying in the T-cell–rich interfollicular areas (Figure 1). They had abundant cytoplasm and medium to large nuclei (in which nucleoli were sometimes prominent). Many cells showed dendritic processes (Figure 1) that penetrated between the closely packed surrounding T cells. Interfollicular B cells usually show a spectrum of morphologic features, but, on average, large cells (whose diameter was at least twice that of small lymphocytes) with prominent dendritic projections accounted for at least a quarter of all B cells seen in interfollicular areas.
Detection of large B cells of dendritic morphology in interfollicular regions of lymph node and tonsil, and in thymus. (A) Lymph node: Sections of 2 lymph nodes immunostained for CD20. The low-power views (× 10) on the left show scattered CD20+ B cells in the T-cell–rich areas that lie between and around lymphoid follicles (Foll). The boxed areas in these low-power views are seen at higher power (× 40) to the right.Arrows indicate processes extending from interfollicular large B cells. (B) Tonsil: Confocal images from a section stained for CD20 (red) and OCT-2 (green). In the upper half of the picture, 2 adjacent cells are illustrated, seen as a single optical section (left) or as multiple sections flattened into a single image (right). A flattened image of a second cell is seen in the lower part of the image. Magnification for all images, × 60. Note the striking dendritic morphology of the cells visualized by this technique. (C) Hyper-IgM syndrome lymph node: CD20 staining (magnification, × 10) shows prominent primary follicles (Foll) but only one interfollicular large B cell (an example is indicated by an arrow and shown at higher magnification [× 40]). (D) Thymus: CD20 staining (magnification, × 10) of large B cells in the thymus, lying principally in the medulla around a Hassall corpuscle (HC). In the inset, high magnification (× 40) of these cells shows morphology similar to cells seen in lymph node. (E) Thymus: Double labeling for CD20 (red) and CD3 (green) (magnification, × 20) shows the differing numbers of cells in the medulla and the cortex (left panel), and two B cells in the cortex, with processes penetrating between surrounding T cells (right panel and inset). Inset magnification, × 40.
Detection of large B cells of dendritic morphology in interfollicular regions of lymph node and tonsil, and in thymus. (A) Lymph node: Sections of 2 lymph nodes immunostained for CD20. The low-power views (× 10) on the left show scattered CD20+ B cells in the T-cell–rich areas that lie between and around lymphoid follicles (Foll). The boxed areas in these low-power views are seen at higher power (× 40) to the right.Arrows indicate processes extending from interfollicular large B cells. (B) Tonsil: Confocal images from a section stained for CD20 (red) and OCT-2 (green). In the upper half of the picture, 2 adjacent cells are illustrated, seen as a single optical section (left) or as multiple sections flattened into a single image (right). A flattened image of a second cell is seen in the lower part of the image. Magnification for all images, × 60. Note the striking dendritic morphology of the cells visualized by this technique. (C) Hyper-IgM syndrome lymph node: CD20 staining (magnification, × 10) shows prominent primary follicles (Foll) but only one interfollicular large B cell (an example is indicated by an arrow and shown at higher magnification [× 40]). (D) Thymus: CD20 staining (magnification, × 10) of large B cells in the thymus, lying principally in the medulla around a Hassall corpuscle (HC). In the inset, high magnification (× 40) of these cells shows morphology similar to cells seen in lymph node. (E) Thymus: Double labeling for CD20 (red) and CD3 (green) (magnification, × 20) shows the differing numbers of cells in the medulla and the cortex (left panel), and two B cells in the cortex, with processes penetrating between surrounding T cells (right panel and inset). Inset magnification, × 40.
These cells showed no clear spatial relationship to lymphoid follicles or to high endothelial venules. In both lymph node biopsies from hyper-IgM syndrome patients, CD20+ interfollicular large B cells were essentially absent (Figure 1). Similar cells were also found in the medulla (and, at much lower numbers, in the cortex) of normal human thymus (3 samples) (Figure 1), but not in sections of normal bone marrow (3 samples).
Phenotype of interfollicular large B cells
The immunophenotype of these cells is summarized in Table 1 and illustrated in Figures 2, 3, 4, 5. The majority of the results were obtained by double-immunostaining techniques in which labeling for CD20 was combined with labeling for a second marker.
Phenotype of interfollicular (IF) large B cells and other mature B cells
. | . | Other B-cell subtypes . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Markers . | IF large B cells . | Naive/mantle zone . | Germinal center . | Monocytoid . | Marginal zone of the spleen . | Memory . | Plasma cells . | |||||
| B-cell surface | ||||||||||||
| CD20 | Pos | Pos | Pos | Pos | Pos | Pos | Neg | |||||
| CD75 | Pos | Pos (weak) | Pos | Pos | Neg | Pos | Neg | |||||
| CD79a | Pos (cyt) | Pos | Pos | Pos | Pos | Pos | Pos (cyt) | |||||
| J-chain | Neg/(Pos) | Neg | Neg/(Pos) | Neg | Neg | No data | Neg/Pos | |||||
| B-cell—associated transcription factors | ||||||||||||
| PAX-5 | Pos | Pos | Pos | Neg/(Pos) | Pos | No data | Neg | |||||
| BOB-1 | Pos | Neg/(Pos) | Pos | Pos | Pos | Pos | Neg | |||||
| OCT-2 | Pos | Pos | Pos | Pos | Pos | Pos | Pos | |||||
| PU.1 | Neg | Pos | Pos | Pos | Pos | No data | Neg | |||||
| Mantle zone | ||||||||||||
| IgD | Neg | Pos | Neg | Neg | Neg/(Pos) | Neg/Pos* | Neg/(Pos) | |||||
| CD5 | Neg | Pos (weak) | Neg | Neg | Neg | Neg/Pos | Neg | |||||
| CD23 | Neg | Pos | Neg | Neg | Neg | Neg | Neg | |||||
| Germinal center | ||||||||||||
| CD10 | Neg | Neg | Pos | Neg | Neg | Neg | Neg | |||||
| BCL-6 | Neg | Neg/(Pos) | Pos | Neg | Neg | Neg | Neg | |||||
| Memory B cells | ||||||||||||
| CD27 | Neg/(Pos) | Neg/(Pos) | Neg/(Pos) | Neg | Pos | Pos | Pos | |||||
| IgM | Neg/(Pos) | Pos | Pos | Neg | Pos | Pos | Neg/Pos | |||||
| Plasma cells | ||||||||||||
| VS38c (p63) | Neg | Neg | Neg | Neg | Neg | Neg | Pos | |||||
| MUM-1/IRF-4 | Pos (50%) | Neg | Neg/(Pos)† | Neg | Neg | Neg | Pos | |||||
| CD38 | Neg | Neg | Pos | Pos (m/w) | Neg | Neg | Pos | |||||
| CD138 | Neg | Neg | Neg | Neg | Neg | Neg | Pos | |||||
| Activation-related | ||||||||||||
| CD30 | Neg/(Pos)‡ | Neg | Neg/(Pos) | Neg | Neg | Neg | Neg | |||||
| Costimulatory | ||||||||||||
| CD40 | Pos (weak) | Pos | Pos | Pos (weak) | Pos | Pos | Pos (weak) | |||||
| CD80 | Neg | Neg | Pos | NA§ | Pos | Pos | Neg | |||||
| CD86 | Neg | Neg | Pos | NA§ | Pos | Pos | Pos | |||||
| Proliferation-related | ||||||||||||
| MIB-1 | Pos (50%) | Neg | Neg/Pos∥ | Low (10%-15%) | Low (5%-10%) | Neg/Pos | Neg/Pos | |||||
| p27/KIP1 | Neg | Pos | Neg/Pos¶ | Pos | Pos | Pos | Pos | |||||
| Apoptosis-associated | ||||||||||||
| BCL-2 | Neg | Pos | Neg/(Pos) | Neg | Pos | Pos | Pos | |||||
| Caspase-3 | Pos | Neg | Pos | Pos | ND | Pos | Pos | |||||
| B-cell precursor | ||||||||||||
| TdT | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |||||
| CD34 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |||||
| Complement receptor | ||||||||||||
| CD21 | Neg | Pos (weak) | Pos | Neg | Pos | Pos | Neg/(Pos) | |||||
| CD35 | Neg | Neg | Pos | Neg | Pos | Pos | Neg/(Pos) | |||||
| Dendritic cell | ||||||||||||
| CD208 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |||||
| Miscellaneous | ||||||||||||
| MSH2 | Pos | Neg/(Pos) | Pos | ND | ND | No data | Neg | |||||
| S-100 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |||||
| CD68 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |||||
. | . | Other B-cell subtypes . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Markers . | IF large B cells . | Naive/mantle zone . | Germinal center . | Monocytoid . | Marginal zone of the spleen . | Memory . | Plasma cells . | |||||
| B-cell surface | ||||||||||||
| CD20 | Pos | Pos | Pos | Pos | Pos | Pos | Neg | |||||
| CD75 | Pos | Pos (weak) | Pos | Pos | Neg | Pos | Neg | |||||
| CD79a | Pos (cyt) | Pos | Pos | Pos | Pos | Pos | Pos (cyt) | |||||
| J-chain | Neg/(Pos) | Neg | Neg/(Pos) | Neg | Neg | No data | Neg/Pos | |||||
| B-cell—associated transcription factors | ||||||||||||
| PAX-5 | Pos | Pos | Pos | Neg/(Pos) | Pos | No data | Neg | |||||
| BOB-1 | Pos | Neg/(Pos) | Pos | Pos | Pos | Pos | Neg | |||||
| OCT-2 | Pos | Pos | Pos | Pos | Pos | Pos | Pos | |||||
| PU.1 | Neg | Pos | Pos | Pos | Pos | No data | Neg | |||||
| Mantle zone | ||||||||||||
| IgD | Neg | Pos | Neg | Neg | Neg/(Pos) | Neg/Pos* | Neg/(Pos) | |||||
| CD5 | Neg | Pos (weak) | Neg | Neg | Neg | Neg/Pos | Neg | |||||
| CD23 | Neg | Pos | Neg | Neg | Neg | Neg | Neg | |||||
| Germinal center | ||||||||||||
| CD10 | Neg | Neg | Pos | Neg | Neg | Neg | Neg | |||||
| BCL-6 | Neg | Neg/(Pos) | Pos | Neg | Neg | Neg | Neg | |||||
| Memory B cells | ||||||||||||
| CD27 | Neg/(Pos) | Neg/(Pos) | Neg/(Pos) | Neg | Pos | Pos | Pos | |||||
| IgM | Neg/(Pos) | Pos | Pos | Neg | Pos | Pos | Neg/Pos | |||||
| Plasma cells | ||||||||||||
| VS38c (p63) | Neg | Neg | Neg | Neg | Neg | Neg | Pos | |||||
| MUM-1/IRF-4 | Pos (50%) | Neg | Neg/(Pos)† | Neg | Neg | Neg | Pos | |||||
| CD38 | Neg | Neg | Pos | Pos (m/w) | Neg | Neg | Pos | |||||
| CD138 | Neg | Neg | Neg | Neg | Neg | Neg | Pos | |||||
| Activation-related | ||||||||||||
| CD30 | Neg/(Pos)‡ | Neg | Neg/(Pos) | Neg | Neg | Neg | Neg | |||||
| Costimulatory | ||||||||||||
| CD40 | Pos (weak) | Pos | Pos | Pos (weak) | Pos | Pos | Pos (weak) | |||||
| CD80 | Neg | Neg | Pos | NA§ | Pos | Pos | Neg | |||||
| CD86 | Neg | Neg | Pos | NA§ | Pos | Pos | Pos | |||||
| Proliferation-related | ||||||||||||
| MIB-1 | Pos (50%) | Neg | Neg/Pos∥ | Low (10%-15%) | Low (5%-10%) | Neg/Pos | Neg/Pos | |||||
| p27/KIP1 | Neg | Pos | Neg/Pos¶ | Pos | Pos | Pos | Pos | |||||
| Apoptosis-associated | ||||||||||||
| BCL-2 | Neg | Pos | Neg/(Pos) | Neg | Pos | Pos | Pos | |||||
| Caspase-3 | Pos | Neg | Pos | Pos | ND | Pos | Pos | |||||
| B-cell precursor | ||||||||||||
| TdT | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |||||
| CD34 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |||||
| Complement receptor | ||||||||||||
| CD21 | Neg | Pos (weak) | Pos | Neg | Pos | Pos | Neg/(Pos) | |||||
| CD35 | Neg | Neg | Pos | Neg | Pos | Pos | Neg/(Pos) | |||||
| Dendritic cell | ||||||||||||
| CD208 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |||||
| Miscellaneous | ||||||||||||
| MSH2 | Pos | Neg/(Pos) | Pos | ND | ND | No data | Neg | |||||
| S-100 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |||||
| CD68 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |||||
The data on interfollicular large B cells come from the present study. The data on other B-cell types comprise immunohistologic results from the literature and from the authors' own observations. The data on memory cells refer to studies of intraepithelial and peripheral blood cells. Pos indicates all cells positive; Neg, all cells negative; Pos (cyt), cytoplasmic positivity; Neg/(Pos), only scattered positive cells; Neg/Pos, less than 50% of cells positive; Pos (m/w), all cells show moderate to weak positivity; NA, not available; and ND, not done.
As described by Klein et al.2
MUM-1 is expressed by scattered cells in the light zone of germinal center.
Large interfollicular cells expressing this marker were often present but the great majority are CD20-.
Data are not available as CD80 and CD86 antibodies worked on frozen sections only.
The majority of the cells in the dark zone of germinal centers are MIB-1+.
Some p27+ B cells were found in the light zone of germinal centers.
Immunostaining of interfollicular large B cells for pan–B-cell markers and B-cell–associated transcription factors. (A) Staining of pan–B-cell markers. Upper left panel: By the immunoperoxidase technique scattered CD79+ (brown) large cells are seen in an interfollicular region (and shown at higher magnification [× 40] in the inset). Right panel: Immunofluorescence staining for CD20 (red) in conjunction with CD79a (green). The upper row illustrates the staining pattern of the 2 markers separately and together in the same area at low magnification (× 10). The 2 cells in the interfollicular area (upper row, arrows), one of which shows only weak expression of CD79a, are seen at higher magnification (× 40) in the middle row. The bottom row shows other interfollicular large B cells expressing CD20 and CD79a to illustrate the intracellular localization of the latter marker (magnification, × 60). Lower left panel: CD75 strongly labels B-cell follicles (magnification, × 20) and interfollicular large B cells (as shown at higher magnification [× 40] in the inset). (B) Staining of B-cell–associated transcription factors. Double immunoenzymatic staining for CD20 (blue) and a transcription factor (brown). In each image, lymphoid follicles, or part of a follicle, are seen, containing cells expressing the transcription factor. The CD20+ interfollicular large B cells express 3 B-cell–associated transcription factors (as shown at higher magnifications in the insets), but lack PU-1. Magnification is × 20 for all main images and × 40 for the insets.
Immunostaining of interfollicular large B cells for pan–B-cell markers and B-cell–associated transcription factors. (A) Staining of pan–B-cell markers. Upper left panel: By the immunoperoxidase technique scattered CD79+ (brown) large cells are seen in an interfollicular region (and shown at higher magnification [× 40] in the inset). Right panel: Immunofluorescence staining for CD20 (red) in conjunction with CD79a (green). The upper row illustrates the staining pattern of the 2 markers separately and together in the same area at low magnification (× 10). The 2 cells in the interfollicular area (upper row, arrows), one of which shows only weak expression of CD79a, are seen at higher magnification (× 40) in the middle row. The bottom row shows other interfollicular large B cells expressing CD20 and CD79a to illustrate the intracellular localization of the latter marker (magnification, × 60). Lower left panel: CD75 strongly labels B-cell follicles (magnification, × 20) and interfollicular large B cells (as shown at higher magnification [× 40] in the inset). (B) Staining of B-cell–associated transcription factors. Double immunoenzymatic staining for CD20 (blue) and a transcription factor (brown). In each image, lymphoid follicles, or part of a follicle, are seen, containing cells expressing the transcription factor. The CD20+ interfollicular large B cells express 3 B-cell–associated transcription factors (as shown at higher magnifications in the insets), but lack PU-1. Magnification is × 20 for all main images and × 40 for the insets.
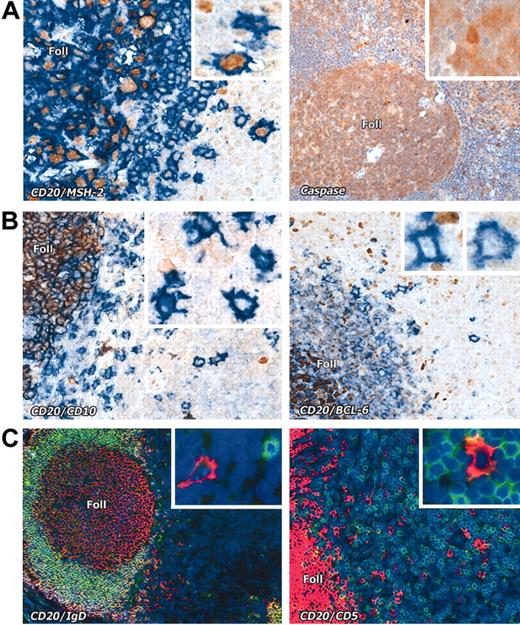
Interfollicular large B cells stained with various markers. (A) Left panel: Nuclear expression (brown) of the mismatch repair enzyme MSH2 is seen in CD20+ (blue) interfollicular large B cells (seen at higher magnification in the inset). Right panel: Interfollicular large B cells express caspase-3 (brown); inset shows a caspase-3+ interfollicular large B cell at higher magnification. (B) The germinal center–associated markers CD10 (brown) and BCL-6 (brown) are absent from CD20+ (blue) interfollicular large B cells. Insets show the staining pattern of the interfollicular large B cells at higher magnification. Note expression of these markers in cells within lymphoid follicles (Foll). (C) Interfollicular large B cells do not express IgD or CD5 (double immunofluorescence labeling, with CD20 in red and the other markers in green, as also shown at higher magnification in the insets). Magnification is × 20 for all main images and × 40 for the insets.
Interfollicular large B cells stained with various markers. (A) Left panel: Nuclear expression (brown) of the mismatch repair enzyme MSH2 is seen in CD20+ (blue) interfollicular large B cells (seen at higher magnification in the inset). Right panel: Interfollicular large B cells express caspase-3 (brown); inset shows a caspase-3+ interfollicular large B cell at higher magnification. (B) The germinal center–associated markers CD10 (brown) and BCL-6 (brown) are absent from CD20+ (blue) interfollicular large B cells. Insets show the staining pattern of the interfollicular large B cells at higher magnification. Note expression of these markers in cells within lymphoid follicles (Foll). (C) Interfollicular large B cells do not express IgD or CD5 (double immunofluorescence labeling, with CD20 in red and the other markers in green, as also shown at higher magnification in the insets). Magnification is × 20 for all main images and × 40 for the insets.
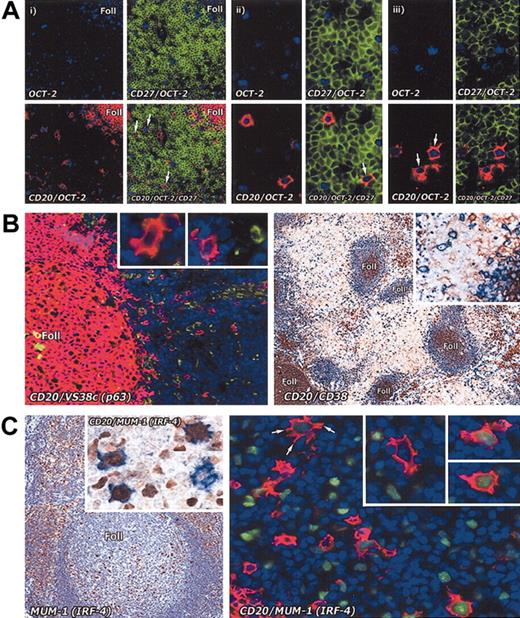
Immunostaining of interfollicular large B cells for memory and plasma cell markers. (A) Staining for memory markers. The memory cell marker CD27 analyzed on interfollicular large B cells in the tonsil by combining immunoenzymatic (OCT-2 blue) and double immunofluorescence staining (CD27 green; CD20 red). (i) Low-power view (paraffin section; magnification, × 20) shows that interfollicular large B cells are OCT-2+ and CD20+, but are CD27– (arrows). In contrast, T cells (green) are strongly CD27+. (ii) Two OCT-2+ and CD20+ interfollicular large B cells in the same section are shown at higher magnification (× 40): one of them (arrow) appears to be weakly CD27+. (iii) Two OCT-2+ and CD20+ (arrows) interfollicular large B cells in a frozen tissue section are clearly CD27–. Magnification, × 40. (B) The plasma cell–associated markers p63 (antibody VS38c) (as also shown at higher magnification in the insets) and CD38 are not expressed by interfollicular large B cells. Left panel: double immunofluorescence staining (CD20 red; VS38c green). Magnification, × 20. Right panel: double immunoenzymatic staining (CD20 blue; CD38 brown). Magnification, × 10. (C) In contrast, MUM-1 (IRF-4), a transcription factor found in many plasma cells and in scattered cells in the light zone of germinal centers, is seen in some interfollicular large B cells (left panel; magnification, × 20). Double immunoenzymatic staining (left panel, inset) shows 3 out of 4 cells coexpressing CD20 (blue) and MUM-1 (brown). Magnification, × 40. On the right, double immunofluorescence labeling (CD20 red; MUM-1 green) of an interfollicular area confirms that a proportion of CD20+ cells coexpress MUM-1 (as also shown at higher magnification in the insets). Note the markedly dendritic profile of the arrowed cell. Magnification is × 40 for all insets.
Immunostaining of interfollicular large B cells for memory and plasma cell markers. (A) Staining for memory markers. The memory cell marker CD27 analyzed on interfollicular large B cells in the tonsil by combining immunoenzymatic (OCT-2 blue) and double immunofluorescence staining (CD27 green; CD20 red). (i) Low-power view (paraffin section; magnification, × 20) shows that interfollicular large B cells are OCT-2+ and CD20+, but are CD27– (arrows). In contrast, T cells (green) are strongly CD27+. (ii) Two OCT-2+ and CD20+ interfollicular large B cells in the same section are shown at higher magnification (× 40): one of them (arrow) appears to be weakly CD27+. (iii) Two OCT-2+ and CD20+ (arrows) interfollicular large B cells in a frozen tissue section are clearly CD27–. Magnification, × 40. (B) The plasma cell–associated markers p63 (antibody VS38c) (as also shown at higher magnification in the insets) and CD38 are not expressed by interfollicular large B cells. Left panel: double immunofluorescence staining (CD20 red; VS38c green). Magnification, × 20. Right panel: double immunoenzymatic staining (CD20 blue; CD38 brown). Magnification, × 10. (C) In contrast, MUM-1 (IRF-4), a transcription factor found in many plasma cells and in scattered cells in the light zone of germinal centers, is seen in some interfollicular large B cells (left panel; magnification, × 20). Double immunoenzymatic staining (left panel, inset) shows 3 out of 4 cells coexpressing CD20 (blue) and MUM-1 (brown). Magnification, × 40. On the right, double immunofluorescence labeling (CD20 red; MUM-1 green) of an interfollicular area confirms that a proportion of CD20+ cells coexpress MUM-1 (as also shown at higher magnification in the insets). Note the markedly dendritic profile of the arrowed cell. Magnification is × 40 for all insets.
Immunostaining of interfollicular large B cells for different markers. (A) Left panel: Some interfollicular large B cells express the proliferation-associated marker MIB-1 (Ki-67) (double immunofluorescence staining for CD20 in red and MIB-1 [Ki-67] in green). Note the numerous proliferating cells in a lymphoid follicle and also scattered interfollicular MIB-1+CD20– cells (magnification, × 20). In the inset, 3 different interfollicular large B cells, 2 of which express MIB-1, can be seen (magnification, × 40). Center panel: Interfollicular large B cells lack p27 (inset; magnification, × 40), as shown by double immunoenzymatic staining for this molecule (brown) and CD20 (blue; magnification, × 20). In the germinal center of a follicle most B cells are p27–. Right panel: Double immunofluorescence labeling (CD20 red; BCL-2 green) shows that interfollicular large B cells lack BCL-2. Magnification, × 20 for the main panel and × 40 for the inset. (B) Left panel: Double immunofluorescence staining reveals that interfollicular large B cells are CD20+ (red) but CD30– (green). The inset shows 4 examples of CD20+ interfollicular large B cells lying near CD30+ cells. Magnification is × 40 for all images. Center and right panels: No labeling of interfollicular large B cells is seen in sections stained for the complement receptors CD21 (center panel) and CD35 (right panel) (immunoperoxidase staining). Magnification is × 20 for both images. (C) Double immunofluorescence labeling for CD20 (green) and CD208 (DC-LAMP) (red) shows that interfollicular large B cells do not express the latter dendritic cell–associated marker, which labels a clearly distinct population of cells (magnification, × 20). In the high-power view (× 40) on the right, a typical interfollicular large B cell is indicated by an arrow. The inset shows a CD208– interfollicular large B cell at higher magnification. (D) Left panel: Double immunofluorescence staining shows no tendency of CD8+ T cells (green) to associate with interfollicular large B cells (red) (magnification, × 20). Right panel: High-power views (× 40) show a single example of an interfollicular large B cell (asterisk) in intimate contact with a CD8+ cell. Note the extensive dendritic processes extending from 2 other interfollicular large B cells (arrowed). The inset shows a CD8– interfollicular large B cell at higher magnification.
Immunostaining of interfollicular large B cells for different markers. (A) Left panel: Some interfollicular large B cells express the proliferation-associated marker MIB-1 (Ki-67) (double immunofluorescence staining for CD20 in red and MIB-1 [Ki-67] in green). Note the numerous proliferating cells in a lymphoid follicle and also scattered interfollicular MIB-1+CD20– cells (magnification, × 20). In the inset, 3 different interfollicular large B cells, 2 of which express MIB-1, can be seen (magnification, × 40). Center panel: Interfollicular large B cells lack p27 (inset; magnification, × 40), as shown by double immunoenzymatic staining for this molecule (brown) and CD20 (blue; magnification, × 20). In the germinal center of a follicle most B cells are p27–. Right panel: Double immunofluorescence labeling (CD20 red; BCL-2 green) shows that interfollicular large B cells lack BCL-2. Magnification, × 20 for the main panel and × 40 for the inset. (B) Left panel: Double immunofluorescence staining reveals that interfollicular large B cells are CD20+ (red) but CD30– (green). The inset shows 4 examples of CD20+ interfollicular large B cells lying near CD30+ cells. Magnification is × 40 for all images. Center and right panels: No labeling of interfollicular large B cells is seen in sections stained for the complement receptors CD21 (center panel) and CD35 (right panel) (immunoperoxidase staining). Magnification is × 20 for both images. (C) Double immunofluorescence labeling for CD20 (green) and CD208 (DC-LAMP) (red) shows that interfollicular large B cells do not express the latter dendritic cell–associated marker, which labels a clearly distinct population of cells (magnification, × 20). In the high-power view (× 40) on the right, a typical interfollicular large B cell is indicated by an arrow. The inset shows a CD208– interfollicular large B cell at higher magnification. (D) Left panel: Double immunofluorescence staining shows no tendency of CD8+ T cells (green) to associate with interfollicular large B cells (red) (magnification, × 20). Right panel: High-power views (× 40) show a single example of an interfollicular large B cell (asterisk) in intimate contact with a CD8+ cell. Note the extensive dendritic processes extending from 2 other interfollicular large B cells (arrowed). The inset shows a CD8– interfollicular large B cell at higher magnification.
Many B-cell markers (eg, CD20; CD75; CD79a; the transcription factors PAX-5, OCT-2 and BOB-1; the mismatch repair gene product MSH2; and caspase-3) were shared between the interfollicular large B cells and the majority of germinal center B cells (Figures 1, 2, 3). However, the interfollicular cells lacked BCL-6 and CD10 (Figure 3B), and the transcription factor PU.1 (Figure 2B), all of which are strongly expressed by germinal center B cells.
Interfollicular large B cells were also IgD–, whereas, as expected, mantle zone B cells were IgD+ (Figure 3C). Mantle zone B cells also expressed CD5, whereas interfollicular large B cells were negative for this marker (Figure 3C) and also for the plasma cell markers p63 (antibody VS38c) and CD38 (Figure 4B). In contrast, the transcription factor MUM-1 (IRF-4), usually expressed by plasma cells and by a minority of B cells in the light zone of germinal centers, was found in approximately 45% of interfollicular large B cells (Figure 4C).
We also investigated the expression of CD27, which has been widely reported to be a reliable marker of memory B cells.2,28-34 For this purpose, we performed triple labeling (on both paraffin-embedded and frozen tonsil sections) for CD27, CD20, and OCT-2 (a transcription factor constitutionally expressed by B cells, including interfollicular large B cells). In all sections, the strong labeling of plasma cells and T lymphocytes provided a positive control for the CD27 staining. The results (Figure 4A) indicated that the great majority of CD20+ and OCT-2+ interfollicular large B cells were CD27– and none of the small minority that were positive showed CD27 labeling comparable to that of plasma cells and T cells.
The proliferation marker MIB-1 (Ki-67) (Figure 5A) was found in approximately 50% of interfollicular large B cells. These cells were negative for p27/KIP1 (Figure 5A), a cyclin-dependent kinase inhibitor35 that is expressed in normal lymphoid tissue mainly by quiescent lymphocytes, for example, by mantle B cells but not by proliferating B cells in germinal centers.36
Interfollicular large B cells were negative for the apoptosis inhibitor BCL-2 (Figure 5A). The activation marker CD30 has long been noted on scattered interfollicular large lymphoid cells, but double staining with CD20 clearly showed that CD30 was absent from the interfollicular large B cells tested (Figure 5B). Interfollicular large B cells expressed CD40 weakly but showed no evidence of the dendritic cell–associated markers CD80, CD86, or CD208 (DC-LAMP), and double immunofluorescence staining for CD208 and CD20 showed that dendritic cells in interfollicular areas were clearly distinct from large B cells (Figure 5C). In tissue sections double stained for CD20 in combination with CD8 (Figure 5D), there was no evidence that CD8+ T cells were physically associated with the CD20+ interfollicular large B cells.
Immunoglobulin gene mutation in microdissected cells
Interfollicular large B cells were isolated from frozen sections of normal lymph nodes and tonsils (2 samples of each tissue). Immunoglobulin heavy-chain gene sequences were amplified from 12 of 53 cells and sequenced. IgH sequence analysis of the 12 interfollicular large B cells showed the presence of somatic mutations in all instances with an average mutation frequency of 9% (range, 2%-13%) (Table 2). The immunoglobulin genes showed in-frame rearrangements in 11 of the 12 sequences analyzed. No clonally related sequences were observed among the single isolated interfollicular large B cells, and it was therefore not possible to comment on whether ongoing mutations occur in this cell population. No IgH amplification products were detected from each individually isolated small CD20– interfollicular T cell (in total, 45) or from the aliquots of the buffer overlaying the tissue section (in total, 53).
Patterns of somatic mutations in the rearranged immunoglobulin genes of B-cell populations
. | Average immunoglobulin mutation incidence, % . | Ongoing mutations . | Reference no. . |
|---|---|---|---|
| Mantle zone cells | 0 | No | 37 |
| Germinal center cells | 7.9 | Yes | 37 |
| Memory cells | 2, 27, 32, 38-40 | ||
| CD27+IgM+IgD+ | 5 | No | |
| IgM+ only | 1.5-6 | No | |
| IgG+ | 4-12.6 | No | |
| Marginal zone cells | 5 | ||
| Splenic | 4.2 | Possible | |
| Nodal | 2.6 | Possible | |
| Monocytoid B cells | 0-3.8* | No | 4, 5, 41 |
| Plasma cells | 8.5-9.0 | No | 42 |
| Interfollicular large B cells | 9 | NA† | This study |
. | Average immunoglobulin mutation incidence, % . | Ongoing mutations . | Reference no. . |
|---|---|---|---|
| Mantle zone cells | 0 | No | 37 |
| Germinal center cells | 7.9 | Yes | 37 |
| Memory cells | 2, 27, 32, 38-40 | ||
| CD27+IgM+IgD+ | 5 | No | |
| IgM+ only | 1.5-6 | No | |
| IgG+ | 4-12.6 | No | |
| Marginal zone cells | 5 | ||
| Splenic | 4.2 | Possible | |
| Nodal | 2.6 | Possible | |
| Monocytoid B cells | 0-3.8* | No | 4, 5, 41 |
| Plasma cells | 8.5-9.0 | No | 42 |
| Interfollicular large B cells | 9 | NA† | This study |
Data on known B-cell populations are from previously published studies. NA indicates not available.
According to Stein et al,4 only a fraction of monocytoid B cells (26%) carry mutated IgH rearrangements, whereas the majority (74%) are unmutated. Other studies have identified only mutated immunoglobulin genes.5,41
It was impossible to establish whether the cells show ongoing mutations, because the derived IgH rearrangements were not clonally related in any case examined.
Antigen selection
Two different methods were used to determine whether the VH mutation patterns in the interfollicular large B-cell sequences were indicative of antigen selection. When the R/s ratio was calculated according to method 1 (“Materials and methods”), 3 (25%) of the 12 sequences were compatible with antigen selection. When method 2 was used, 5 (42%) of the 12 sequences showed signs of antigen selection. Interestingly, 3 of these 5 sequences corresponded to the ones identified by method 1.
Discussion
Hematopathologists have in the past noted large B cells in the interfollicular T-cell–rich zones of human lymph nodes,16,43 but no studies have characterized the immunophenotype of these cells in detail or analyzed the mutational status of their immunoglobulin gene variable regions. Furthermore, hematopathologists studying interfollicular large B cells have tended to focus on hyperreactive conditions (eg, viral infection) and have suggested that these cells represent an interfollicular B-cell reaction (possibly germinal center–independent) leading to the generation of plasma cells.44,45
In the present study, we investigated normal lymph nodes, rather than samples showing histologic evidence of an immune reaction, and the interfollicular T-cell–associated large B cells we describe can therefore be considered a basal cellular element present in normal human lymphoid tissue. However, it remains to be established whether these cells develop only following exposure to pathogens since we have not studied samples from infants.
Interfollicular large B cells show morphologic resemblance to the scattered large B lymphocytes previously described in the thymus,17 sometimes referred to as asteroid cells,18,19 which also contain low to medium levels of immunoglobulin heavy-chain gene mutations.46,47 In addition, as previously observed for thymic large B cells,48 interfollicular large B cells are CD40+ but lack CD80 and CD86.
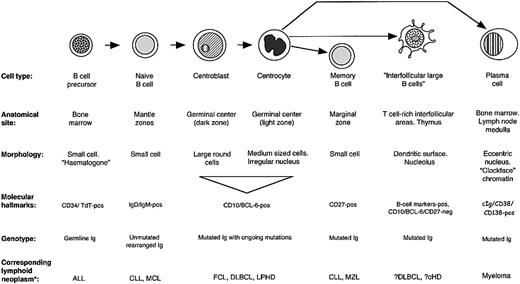
The analysis of interfollicular large B-cell phenotype indicated that they differ from other B-cell populations in their pattern of molecular expression (Figure 6). Their physical location and the presence of mutations in their IgH genes might be taken as evidence that they are errant germinal center B cells that have migrated into the T-cell–rich interfollicular region. However, they differ in phenotype from the 2 major categories of B cells found in germinal centers: the proliferating centroblasts in the dark zone, and their progeny, the centrocytes of the light zone. For example, they lack the hallmark of centroblasts, BCL-6, a transcription factor believed to be causally involved in controlling gene expression in centroblasts before they switch to a late/post–germinal center maturation stage (Figure 3B).49 Equally, interfollicular large B cells differ from centrocytes in terms of expression of molecules such as p27 and the proliferation marker MIB-1 (Table 1), and also in their morphology. About 50% of interfollicular large B cells express the MUM-1 (IRF-4) transcription factor, a transcription factor found in scattered cells in the light zones of germinal centers (and also in most plasma cells). However, it is also expressed by activated T cells,50 so that its presence in interfollicular large B cells does not constitute conclusive evidence that they represent late germinal center cells.
Molecular characteristics of interfollicular large B cells and other B cells.
The phenotype of the interfollicular large B cells shows some similarities to monocytoid cells (Table 1), a B-cell type occasionally observed in reactive lymph nodes (eg, following infection by Toxoplasma gondii),51,52 but monocytoid B cells differ in several respects (eg, they express PU-1, p27, and CD38, but not PAX-5).53 Furthermore, they are typically located in subcapsular or trabecular sinuses at the periphery of lymph nodes (unaccompanied by T cells), rather than in interfollicular areas, and they usually carry unmutated (74% of the cases) immunoglobulin genes.4
Interfollicular large B cells show some phenotypic similarities to memory B cells (Table 1), but they lack molecules such as CD21, CD80, CD86, and p27. Of particular note is that we were unable to demonstrate CD27 on the great majority of interfollicular large B cells (Figure 4). Although there is agreement that this molecule is a reliable marker of memory B cells,2,28-34 its expression on other B-cell types is controversial. Some flow cytometric studies have reported that CD27 is present homogeneously on germinal center B cells,28-30,34 whereas immunohistologic labeling of frozen tissue sections, in the present paper and in published reports,30,33,54,55 has shown that only a variable number of germinal center cells are CD27+, and many of these may be CD27+ T cells.56 Theoretically, these discrepancies might be due to a lower sensitivity of immunohistochemistry in comparison with flow cytometric analysis, and we therefore cannot exclude the possibility that interfollicular large B cells express CD27 at levels too low to be detected by immunohistology. Attempts to quantitate CD27 expression on the interfollicular large B cells by flow cytometry would be technically difficult, since interfollicular large B cells are relatively rare and lack a specific marker to distinguish them from other B cells.
One marker that we did not evaluate in interfollicular large B cells was surface immunoglobulin. These cells appeared to show variable expression of IgM, but we could not evaluate the expression of surface IgG (the isotype that, as switched post–germinal center cells, they would be expected to express) since background immunoglobulin makes it difficult to detect this marker on cell membranes in tissue sections. However, it is of interest that many of the cells expressed CD79a within their cytoplasm rather than on the cell surface (Figure 2A). Since immunoglobulin is inserted in the cell surface membrane in physical association with CD79 dimer, the intracellular accumulation of CD79a may possibly indicate that interfollicular large B cells lack surface immunoglobulin. There is indirect evidence that the similar large B cells found in the thymic medulla are surface immunoglobulin–negative,57,58 that is, mediastinal B-cell lymphoma is a tumor believed to arise from these cells and lacks surface immunoglobulin.
In view of these findings, the interfollicular large B cells we have characterized in this report appear to represent a unique B-cell population. To investigate their stage of maturation, we analyzed their immunoglobulin variable region gene status and found a pattern suggesting that they are post–germinal center cells (since it is highly unlikely that they have undergone immunoglobulin gene mutation independently of a germinal center). However, these cells clearly differ from the 2 well-recognized descendants of germinal centers, namely, memory cells and plasma cells (Table 1). As a technical point, it may be added that the failure to amplify immunoglobulin gene sequences from 41 of the microdissected cells was to be expected and does not indicate that they are not of B-cell origin, since it is well recognized that the amplification rate from microdissected single B cells varies greatly and is always less than 100%. This reflects both the quality of the frozen sections analyzed (and consequently of the DNA) and also the fact that somatic mutations in the rearranged immunoglobulin genes influence the capacity of primers to bind to the VH region sequences. For this reason, the reported percentage of IgH PCR products obtainable from mantle zone cells (which carry little or no evidence of somatic mutations) is close to 60%, whereas this figure decreases to nearly 30% for B cells such as follicle center cells (carrying a high load of somatic mutations), and to approximately 40% for memory cells or plasma cells (T.M., unpublished data, January 2002). The primers used in this study had been validated in extensive previous studies of microdissected lymphoid cells.23,24,59 The dendritic surface processes, which extended, sometimes for considerable distances, from the large interfollicular B cells, raise the possibility that these cells play an antigen-presenting role, but it may be noted that they lacked the markers associated with dendritic cells: CD80, CD86, and CD208 (DC-LAMP).60
The identification of a population of scattered large B cells in T-cell–rich interfollicular zones raises the question of whether they give rise to any category of human lymphoma. A potential candidate is classical Hodgkin disease, a B-cell neoplasm24,61 that tends preferentially to involve interfollicular T-cell areas in lymph nodes62-65 and also the thymus,63,66 where the morphologically similar asteroid B cells are found. The neoplastic cells in Hodgkin disease (Reed-Sternberg cells) have an intimate association with T cells, often being surrounded by rosettes of adherent T cells,67 an interaction that survives the preparation of cell suspensions, and they also share with interfollicular large B cells the presence of dendritic surface projections (as revealed by immunostaining for fascin)68 and the presence of mutated immunoglobulin genes.24,69 In addition, Reed-Sternberg cells contain an integrated Epstein-Barr virus (EBV) genome in about 50% of Hodgkin disease cases.70 Interestingly, normal B lymphocytes infected with this virus (eg, in acute infectious mononucleosis) are located predominantly in interfollicular regions, as are the cells we describe in this study.71-76 One can speculate that an interfollicular large B cell that continues to harbor the virus following EBV infection can transform to create a clone of Reed-Sternberg cells, following the occurrence of further mutagenic events.
However, there are arguments against this hypothesis. The fact that the phenotypes of the 2 cell types are very different (eg, in their expression of CD20 and CD30) does not exclude a direct relationship, given the recent evidence that a major alteration in gene expression creates an abnormal “lost” B-cell phenotype in Reed-Sternberg cells (involving reduced expression of many genes).77 However, Reed-Sternberg cells in about 25% of Hodgkin disease cases show crippling mutations in their immunoglobulin genes: If this is causally relevant to malignant transformation (eg, if a second acquired genetic abnormality affecting apoptosis prevented the elimination of cells in which these mutations have arisen), it would argue strongly that the neoplasm originates within germinal centers during immunoglobulin gene hypermutation.78,79
Thus, further studies are needed to decide if interfollicular large B cells give rise to a category of human lymphoma. Their phenotypic profile (negative for CD10 and BCL-6) suggests that they might represent a precursor of the group of activated B-cell–like diffuse large B-cell lymphomas identified in gene expression microarray studies.80,81 The rarity of interfollicular large B cells is currently an obstacle to the study of their mRNA content. However, the production of new antibodies to molecules expressed in different categories of diffuse large B-cell lymphoma (defined by gene profiling) may help in the future to identify the neoplastic counterpart of interfollicular large B cells.
In conclusion, we describe a population of interfollicular large B cells in human lymphoid tissue with unique features that differentiate them from known B-cell populations. It will be of interest if the same cells can be identified in mouse peripheral lymphoid tissue, since insight into their origin may then be obtained by studying knock-out animals in which normal B-cell maturation is abrogated (eg, strains lacking germinal centers because of inactivation of BCL-6, CD40, BOB-1, TNF, or NFκ-B genes).
Supported by Leukaemia Research Fund, United Kingdom, grant 9970 (D.Y.M.), and Oxfordshire Health Services Research Committee grant OHSRC 720 (D.Y.M. and T.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, June 26, 2003; DOI 10.1182/blood-2003-03-0692.
We thank Professor Jim Wainscoat and Dr Jackie Boultwood for providing laboratory facilities for molecular biologic techniques. We also thank Bridget Watson for her assistance in the drafting of the manuscript; Paul Allen for his technical help; Nick White for help with confocal microscopy (Figure 1); Sundeep Bhandari from Nikon UK for technical support; Rifat A. Hamoudi for his help in performing part of the sequencing technique; and Margaret Abel for help in preparing capillaries. We thank Dr Lynn M. Corcoran (The Walter and Eliza Hall Institute of Medical Research, Immunology Division, Royal Melbourne Hospital, Melbourne, Australia) for kindly providing the anti–OCT-2 monoclonal antibody.

![Figure 1. Detection of large B cells of dendritic morphology in interfollicular regions of lymph node and tonsil, and in thymus. (A) Lymph node: Sections of 2 lymph nodes immunostained for CD20. The low-power views (× 10) on the left show scattered CD20+ B cells in the T-cell–rich areas that lie between and around lymphoid follicles (Foll). The boxed areas in these low-power views are seen at higher power (× 40) to the right.Arrows indicate processes extending from interfollicular large B cells. (B) Tonsil: Confocal images from a section stained for CD20 (red) and OCT-2 (green). In the upper half of the picture, 2 adjacent cells are illustrated, seen as a single optical section (left) or as multiple sections flattened into a single image (right). A flattened image of a second cell is seen in the lower part of the image. Magnification for all images, × 60. Note the striking dendritic morphology of the cells visualized by this technique. (C) Hyper-IgM syndrome lymph node: CD20 staining (magnification, × 10) shows prominent primary follicles (Foll) but only one interfollicular large B cell (an example is indicated by an arrow and shown at higher magnification [× 40]). (D) Thymus: CD20 staining (magnification, × 10) of large B cells in the thymus, lying principally in the medulla around a Hassall corpuscle (HC). In the inset, high magnification (× 40) of these cells shows morphology similar to cells seen in lymph node. (E) Thymus: Double labeling for CD20 (red) and CD3 (green) (magnification, × 20) shows the differing numbers of cells in the medulla and the cortex (left panel), and two B cells in the cortex, with processes penetrating between surrounding T cells (right panel and inset). Inset magnification, × 40.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/8/10.1182_blood-2003-03-0692/6/m_h82035074001.jpeg?Expires=1767872414&Signature=EBIgZ5aT94fyLOZd7ZWxmHC0NHITXlG7FkNfXZlvEPux-SYaKNBbhJIzIA1b63yT13K08svJNgg43aO3BZJZHDhcXlyfMKY4dNeqi4U~3MEX3iMg2gBSTtwd-eN-Sn82ax3UMuDZlwFlERlqZbhpckHJX21RVG1cxyTiUkWBn~o3FOyzTYicbdXdreB4r~xYGl1ihV2qGTq4dPoEuW4v1xW4r-gfLwDfeN7~giYkABKDn23eLPrWC-X6LsIdn0XL54UBIKdCTn~UMORdBlmhXQUvKvae2~nEZvhWTh70vMFFuogunehHngziFru830ZxTbFQV1~zrd5-UA-OPjGkFA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Immunostaining of interfollicular large B cells for pan–B-cell markers and B-cell–associated transcription factors. (A) Staining of pan–B-cell markers. Upper left panel: By the immunoperoxidase technique scattered CD79+ (brown) large cells are seen in an interfollicular region (and shown at higher magnification [× 40] in the inset). Right panel: Immunofluorescence staining for CD20 (red) in conjunction with CD79a (green). The upper row illustrates the staining pattern of the 2 markers separately and together in the same area at low magnification (× 10). The 2 cells in the interfollicular area (upper row, arrows), one of which shows only weak expression of CD79a, are seen at higher magnification (× 40) in the middle row. The bottom row shows other interfollicular large B cells expressing CD20 and CD79a to illustrate the intracellular localization of the latter marker (magnification, × 60). Lower left panel: CD75 strongly labels B-cell follicles (magnification, × 20) and interfollicular large B cells (as shown at higher magnification [× 40] in the inset). (B) Staining of B-cell–associated transcription factors. Double immunoenzymatic staining for CD20 (blue) and a transcription factor (brown). In each image, lymphoid follicles, or part of a follicle, are seen, containing cells expressing the transcription factor. The CD20+ interfollicular large B cells express 3 B-cell–associated transcription factors (as shown at higher magnifications in the insets), but lack PU-1. Magnification is × 20 for all main images and × 40 for the insets.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/8/10.1182_blood-2003-03-0692/6/m_h82035074002.jpeg?Expires=1767872414&Signature=oI43JVKbaDCCw~KIVfkKcFObc-FQaYrkwsSx9krzEjAivrCXCiEwi6ZT7YmHwHheQKqUHGV1hTdAeTU9vul8WaG3WsgAynf6x9IK3dr3hvVP64U2s00U-Bysb8x79OPcfHbmDqXTGj-CTihye9VTDYtPZYM70Xn2vKq~XyOi0xWnvrLv4bJy0w78hy4LCkSSuRemumsvbvywAkP8SV8nmr2Y4mWajtdAeuJZEIx1Wi5w5KIsRS9iLhbp1k4YVo66cQBDsF74v2Phl0N6b2GvtjS1SuWjHH4BgFM3upwT8ulVLsPVcDT5RjPJLdV4RTQSVg~9pfkfyUs2jNRVqEw9wg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Immunostaining of interfollicular large B cells for different markers. (A) Left panel: Some interfollicular large B cells express the proliferation-associated marker MIB-1 (Ki-67) (double immunofluorescence staining for CD20 in red and MIB-1 [Ki-67] in green). Note the numerous proliferating cells in a lymphoid follicle and also scattered interfollicular MIB-1+CD20– cells (magnification, × 20). In the inset, 3 different interfollicular large B cells, 2 of which express MIB-1, can be seen (magnification, × 40). Center panel: Interfollicular large B cells lack p27 (inset; magnification, × 40), as shown by double immunoenzymatic staining for this molecule (brown) and CD20 (blue; magnification, × 20). In the germinal center of a follicle most B cells are p27–. Right panel: Double immunofluorescence labeling (CD20 red; BCL-2 green) shows that interfollicular large B cells lack BCL-2. Magnification, × 20 for the main panel and × 40 for the inset. (B) Left panel: Double immunofluorescence staining reveals that interfollicular large B cells are CD20+ (red) but CD30– (green). The inset shows 4 examples of CD20+ interfollicular large B cells lying near CD30+ cells. Magnification is × 40 for all images. Center and right panels: No labeling of interfollicular large B cells is seen in sections stained for the complement receptors CD21 (center panel) and CD35 (right panel) (immunoperoxidase staining). Magnification is × 20 for both images. (C) Double immunofluorescence labeling for CD20 (green) and CD208 (DC-LAMP) (red) shows that interfollicular large B cells do not express the latter dendritic cell–associated marker, which labels a clearly distinct population of cells (magnification, × 20). In the high-power view (× 40) on the right, a typical interfollicular large B cell is indicated by an arrow. The inset shows a CD208– interfollicular large B cell at higher magnification. (D) Left panel: Double immunofluorescence staining shows no tendency of CD8+ T cells (green) to associate with interfollicular large B cells (red) (magnification, × 20). Right panel: High-power views (× 40) show a single example of an interfollicular large B cell (asterisk) in intimate contact with a CD8+ cell. Note the extensive dendritic processes extending from 2 other interfollicular large B cells (arrowed). The inset shows a CD8– interfollicular large B cell at higher magnification.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/8/10.1182_blood-2003-03-0692/6/m_h82035074005.jpeg?Expires=1767872414&Signature=ZFG1hWPDph5wUCJBCRuVk0oQBP9VXkUd6JEHYD4RSvjSWH~wC7VrBLyx7E~BpsuLxt-YmfCkxVcU19ABOTuopHJegb6McS3y5c3HlZFUQDz1fAwRejZ3rmRoFOHoYvgAgBlCsjnjz-xB1VMR6FZrXElbyFH-Uqr-APtECvYgIPin3Yu0GKKTs4peo-B80Yvb9zGeVf~ELz95mJRCZCLqNbq6he~IpHx5F3ezOZfvuXuuUhuNp3QzRXLgs6IIn5YCJFLvjHPlwkOdSSnS3sCGbfw2A41cSMY10YDBHKdSbtAGEbmqfNYdIj5qfaEJgQhne1udHv~grtaO1nyu5MtI6Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal