Abstract
Bacterial endotoxin (lipopolysaccharide [LPS]) is a potent inducer of human dendritic cell (DC) maturation and survival. Here we show that immature DCs exposed to LPS trigger an early and sustained caspaselike activity, which can be blocked by zVAD (z-Val-Ala-Asp), in the absence of detectable caspase 8 and caspase 10 activation, or poly(ADP-ribose) polymerase (PARP)–cleaving activity. Preventing LPS-induced caspaselike activation in DC results in massive cell death. Importantly, triggering of the caspaselike activity is required for LPS-induced activation of extracellular signal-regulated kinases (ERKs) and for LPS-induced up-regulation of cFLIP (Fas-associating protein with death domain–like interleukin-1β–converting enzyme [FLICE]–like inhibitory protein). Therefore, a caspase-dependent pathway initiated by LPS controls survival of human DCs.
Introduction
Dendritic cells (DCs) are specialized leukocytes evolved to provide a link between innate and adaptive immunity.1 Due to their extremely high capability in capturing and processing macromolecules, they effectively patrol the interstitial environment of many tissues and eventually present antigens to naive T lymphocytes to initiate a specific immune response.2 In addition to acting as very efficient antigen-presenting cells, they also convey information to T cells about the presence and the nature of infection and help direct T-cell responses.3 DCs sense potential pathogens via surface pattern-recognition receptors (PRRs)4 and, upon engagement of PRRs, undergo “maturation” (ie, morphologic and functional changes that include the modulation of adhesion/costimulatory receptors, major histocompatibility complex [MHC]/peptide complexes, chemokine receptors, and cytokine production). The complexity of these responses provides the information required to activate or inhibit specific T-cell subsets, depending on the type of infection.5,6
PRRs are engaged by pathogen-associated molecular patterns (PAMPs), structures constitutively and invariantly expressed in microbes. Among PRRs, Toll-like receptors (TLRs) are characterized by extracellular leucine-rich repeats (LRRs) and by an intracellular Toll/interleukin 1 (IL1) receptor domain (TIR).7,8 TLRs form a large receptor family counting at least 10 members in humans, 9 in Drosophila melanogaster and 1 in Caenorhabditis elegans, generally implicated in microbial immunity. LRRs are involved in microbe ligand recognition, whereas the cytoplasmic TIR domain is responsible for receptor/protein interactions and downstream signal transduction. In fact, human TLR engagement and clustering results in the recruitment to the receptor of the adaptor myeloid differentiation primary response 88 (MyD88) through homophilic interaction with its TIR domain. MyD88 then interacts with the serine/threonine kinases, IRAKs (interleukin-1 receptor-associated kinase), via homophilic interaction between their respective death domains (DDs). IRAKs can also be recruited to the TLR, independently from MyD88, by Toll-interacting protein (TOLLIP).9 This is followed by IRAK's autophosphorylation, dissociation from the receptor, and association with tumor necrosis factor receptor–associated factor 6 (TRAF6). TRAF6 becomes phosphorylated, oligomerizes, and is responsible for downstream signaling leading to the activation of nuclear factor–κ B (NFκB)–dependent genes. TLRs share with the IL1 receptor (IL1R) important structural features, including the TIR domain, and most of the intracellular signal transduction machinery.
Lipopolysaccharide (LPS) is a constitutive component of the bacterial cell wall in Gram-negative bacteria and its invariant molecular pattern, lipid A, is specifically recognized by Toll-like receptor 4 (TLR4). TLR4 is expressed and functional in human DCs.10-12 To be fully competent, TLR4 also requires CD14, lipopolysaccharide-binding protein (LBP), and MD2. The recruitment to the TLR4 of the private adaptor MyD88 adapter-like protein/Toll-interleukin 1 precursor domain–containing adapter protein (MAL/TIRAP) confers to the receptor complex unique signaling specificities compared with other TLRs and with the IL1R.13,14 TLR4 indeed can signal to TRAF6 independently from MyD88 and IRAKs via TIRAP and PKR, an RNA-dependent protein kinase. LPS/TLR4 interactions in murine DCs eventually give rise to 2 distinct intracellular signaling pathways, one involving NFκB, which controls DC maturation and may proceed independently from MyD88,15 and the other involving extracellular signal-regulated kinases (ERKs), which promote DC survival.16,17 Similarly, human DC differentiation appears to involve NFκB18 and to be negatively regulated by ERK activation.19 No information is available concerning LPS-initiated intracellular signaling controlling survival in human DCs.
Caspases are a class of cystein proteases that cleave protein substrates after selected aspartate residues.20 They participate in the proteolytic modifications of several proteins involved in intracellular signaling pathways governing cellular adaptation, differentiation, proliferation, and apoptosis. They are also primarily responsible for the ordered proteolysis associated with cellular apoptosis.21 At least 11 different caspases have been described in humans and they can be grouped into different subfamilies according to structural features, substrate specificity, and function. Caspases have been shown to participate in ultraviolet B (UVB)–induced apoptotic cell death of human DCs,22 but little is known about their involvement in other aspects of DC biology. Here we show that an early and sustained caspaselike activation participates in the LPS-generated signaling in DCs and that, importantly, it is required for LPS-induced survival of human DCs.
Materials and methods
Reagents
Escherichia coli serotype 055:B5 LPS was from Sigma (St Louis, MO). Human recombinant IL1 was from Pharmingen (San Diego, CA). zVAD-fmk (z-Val-Ala-Asp(OMe)-fluoromethylketone), z-WEHD-fmk (z-Trp-Glu-His-Asp-fluoromethylketone), Ac-YVAD-cmk (Ac-Tyr-Val-Ala-Asp-chloromethylketone), cell-permeable YVAD-CHO (N-acetyl-Ala-Ala-Val-Ala-Leu-Leu-Pro-Ala-Val-Leu-Leu-Ala-Pro-Tyr-Val-Ala-Asp-CHO), and z-IETD-fmk (z-Ile-Glu(OMe)-Thr-Asp(OMe)-fluoromethylketone) were from Biomol (Plymouth Meeting, PA). The cell-permeable cathepsin B inhibitor zFA-fmk (z-Phe-Ala-fluoromethylketone) and calpain inhibitor zVF-CHO (z-Val-Phe-CHO) were from Calbiochem (San Diego, CA).
DC generation
Peripheral blood mononuclear cells (PBMCs) from different healthy donors were isolated on lymphoprep cushions and monocytes were purified by positive sorting using anti-CD14–conjugated magnetic microbeads according to manufacturer's instructions (Mylteni Biotech, Auburn, CA). The recovered cells were 99% CD14+ as determined by flow cytometry with anti-CD14 phycoerythrin (PE)–conjugated antibody (Pharmingen). Cells were then cultured for 4 to 6 days in 6-well plates (Costar, High Wycombe, United Kingdom) at the initial concentration of 5 × 105/mL in RPMI, 10% fetal calf serum (FCS), penicillin, streptomycin, glutamine, HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) 10 mM, 1% non–essential amino acids, 1% sodium pyruvate, supplemented with IL4 (2.5 ng/mL; R&D Systems, Minneapolis, MN), and granulocyte-macrophage colony-stimulating factor (GM-CSF; 50 ng/mL; a kind gift from Prof Paolo Rossi, University of Rome, Tor Vergata, Italy). Cells were routinely controlled for CD1, CD14, and CD86 expression using the respective PE-conjugated antibodies (Pharmingen) and were consistently CD14–CD1highCD86low.
Antibodies and Western blotting
Cells were washed 2 times with phosphate-buffered saline (PBS) and the pellet was resuspended in lysis buffer (150 mM NaCl, 10 mM Tris [tris(hydroxymethyl)aminomethane; pH 7.4], 1 mM EDTA [ethylenediaminetetraacetic acid], 1 mM EGTA [ethylene glycol tetraacetic acid], 1% Triton X-100, 1% Nonidet P40 [NP40]) and protease inhibitors (1 mM PMSF [phenylmethylsulfonyl fluoride], 1 μg/mL leupeptin, 1 μg/mL aprotinin, 1 μg/mL pepstatin, 0.5 μg/mL antipain). When necessary, cells were detached with scrapers in cold PBS. Samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membrane. Membranes were incubated with the following antibodies: rabbit polyclonal anti-ERK1/2 (Santa Cruz Biotechnology, Santa Cruz, CA), mouse polyclonal anti–phospho-ERK1/2 (anti–P-ERK1/2; Santa Cruz Biotechnology), mouse monoclonal anti–caspase 10 clone 4C1 (MBL, Nagoya, Japan), mouse monoclonal anti–caspase 8, clone 5F7 (Upstate Biotechnology, Lake Placid, NY), rat monoclonal anti-FLIP (Fas-associating protein with death domain–like interleukin-1β–converting enzyme [FLICE]–inhibitory protein) clone Dave 2. For detection, the appropriate secondary antibodies antimouse, antirabbit, and antirat conjugated to horseradish peroxidase were used (Santa Cruz Biotechnology) followed by enhanced chemioluminescence. Mouse monoclonal immunoglobulin G1 (IgG1) anti–human tumor necrosis factor α (TNF-α) was from BD Pharmingen.
Fluorescence-activated cell sorter (FACS) analysis
To detect caspase activity we used CaspACE (Promega, Madison, WI) and modified the original protocol. Cells were pretreated with 2 μM fluorescein isothiocyanate (FITC)–VAD-fmk for 1 hour, with or without 100 μM zVAD-fmk, and then stimulated with LPS. In some experiments, DCs were exposed to UV 5400 J/m2 to induce apoptotic cell death, as previously described.22 After the indicated times, cells were recovered, washed extensively with cold PBS, and analyzed by a FACScan cytofluorimeter (Becton Dickinson, San Jose, CA) using CellQuest software (Becton Dickinson). To detect necrosis, cells were incubated for 20 minutes with 5 μg/mL propidium iodide in PBS and analyzed by a FACScan cytofluorimeter (Becton Dickinson) using CellQuest software. In some experiments, cells were subsequently stained with FITC–annexin V in the appropriate staining buffer (Pharmingen).
In vitro translation and cleavage assay
Full-length human poly(ADP-ribose) polymerase (PARP) and pro-IL1β cDNAs, cloned in pGem vector, were used to synthesize [35S]methionine-labeled products by coupled T7 RNA polymerase-mediated transcription and translation in a reticulocyte lysate system (Promega). Cell pellets were resuspended in 100 μL lysis buffer (50 mM NaCl, 2 mM MgCl2, 40 mM β-glycerophosphate, 5 mM EGTA, and 10 mM HEPES, pH 7.0). Cleavage reactions were performed in a volume of 36 μL containing 25 mM HEPES, pH 7.5; 100 mM NaCl; 2 mM MgCl2; 5 mM dithiothreitol; 0.1% Triton X-100; 1 mM phenylmethylsulfonyl fluoride; and 2 μg/mL aprotinin, leupeptin, and pepstatin with 3 μL [35S]methionine-labeled products and 100 μg cell lysates. The reaction was incubated for 1 hour at 37°C for pro-ILβ and up to 2 hours at 37°C for PARP. Samples were analyzed by SDS-PAGE. Gels were fixed (acetic acid 60%, methanol 40%, and glycerol 5%), treated with Amplify solution (Amersham, Arlington Heights, IL), and dried. Cleavage products were visualized by autoradiography.
Results
LPS induces early caspase activation in immature DCs
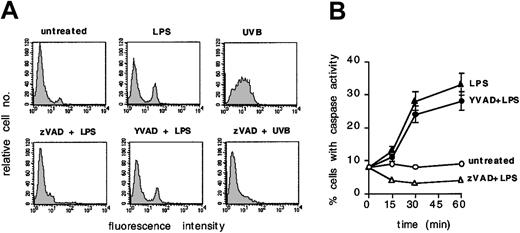
To investigate whether caspases participate in LPS-induced signaling in human DCs, we labeled immature DCs with the fluorescent caspase substrate FITC-VAD-fmk and exposed them to LPS. The tripeptide VAD can be cleaved by all known caspases. The fmk group remains irreversibly bound to the protease catalytic pockets, whereas FITC-VAD is retained intracellularly and fluorescent cells can be quantitated by FACS analysis. Figure 1A-B shows that 25% to 30% of DCs exposed to LPS trigger a caspaselike activity within 30 minutes to 1 hour, which can be completely blocked by pretreatment with the general caspase inhibitor zVAD. By way of comparison, the activation of caspases as detected after UVB exposure, an event that can also be blocked by zVAD and is associated with apoptosis of DCs,22 is also shown.
Early caspaselike activation in DCs exposed to LPS. (A) DCs were loaded with FITC-VAD and then left untreated or exposed for 30 minutes to LPS (100 ng/mL), with or without 1 hour pretreatment with zVAD (40 μM) or cell-permeable YVAD (4 μM). FITC-VAD–loaded DCs were also exposed to UVB with or without pretreatment with zVAD. Cell fluorescence intensity was analyzed by flow cytometry. (B) The percentage of FITC-positive cells at different time points is shown for each of the above mentioned conditions. Means ± 1 SD from 3 independent experiments are shown. Each experiment was performed in duplicate.
Early caspaselike activation in DCs exposed to LPS. (A) DCs were loaded with FITC-VAD and then left untreated or exposed for 30 minutes to LPS (100 ng/mL), with or without 1 hour pretreatment with zVAD (40 μM) or cell-permeable YVAD (4 μM). FITC-VAD–loaded DCs were also exposed to UVB with or without pretreatment with zVAD. Cell fluorescence intensity was analyzed by flow cytometry. (B) The percentage of FITC-positive cells at different time points is shown for each of the above mentioned conditions. Means ± 1 SD from 3 independent experiments are shown. Each experiment was performed in duplicate.
LPS is known to activate caspase 1 in myeloid cells, an activation that can be blocked by the caspase 1–like inhibitor YVAD.23 Yet, the early caspaselike activity induced in DCs by LPS could not be blocked by YVAD at doses that could inhibit the activation of caspase 1 (data not shown). This result suggests that caspases other than caspase 1 are involved in early LPS signaling.
LPS-induced caspase activation is required for DC survival
We therefore investigated the functional relevance of LPS-induced caspase activation. Immature DCs were pretreated with zVAD to prevent caspase activation and exposed to LPS. Figure 2A shows that in the presence of zVAD, LPS-stimulated DCs do not cluster and undergo massive cell death. Loss of birifrangence and cellular swelling by microscopic inspection, as well as propidium iodide exclusion and FACS analysis, revealed that cell death of LPS-stimulated DCs in which caspases are blocked by zVAD occurs by necrosis (Figure 2B).
Caspaselike activation is required for DC survival. (A) DCs were left untreated, pretreated with zVAD (1 hour, 40 μM), exposed to LPS (100 ng/mL), or pretreated with zVAD and exposed to LPS. After 16 hours cells were visualized by microscopy (Olympus IX50; Olympus Optical, Tokyo, Japan). Original magnification, × 40. Six independent experiments gave similar results. (B) DCs were left untreated, treated with zVAD (40 μM), exposed to LPS (100 ng/mL), or pretreated with zVAD and exposed to LPS. After 16 hours the cells were analyzed for propidium iodide (PI) uptake by flow cytometry. Five independent experiments gave similar results. Horizontal bars indicate necrotic cells.
Caspaselike activation is required for DC survival. (A) DCs were left untreated, pretreated with zVAD (1 hour, 40 μM), exposed to LPS (100 ng/mL), or pretreated with zVAD and exposed to LPS. After 16 hours cells were visualized by microscopy (Olympus IX50; Olympus Optical, Tokyo, Japan). Original magnification, × 40. Six independent experiments gave similar results. (B) DCs were left untreated, treated with zVAD (40 μM), exposed to LPS (100 ng/mL), or pretreated with zVAD and exposed to LPS. After 16 hours the cells were analyzed for propidium iodide (PI) uptake by flow cytometry. Five independent experiments gave similar results. Horizontal bars indicate necrotic cells.
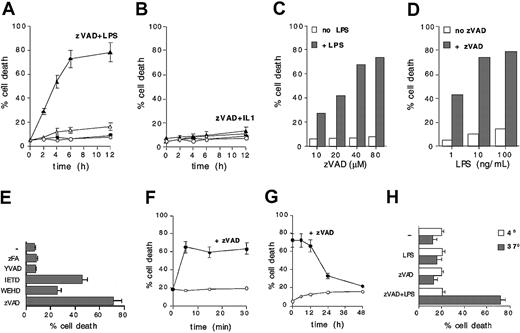
Cell death of LPS-stimulated DCs pretreated with zVAD follows a fast kinetic, since about 30% of the cells are dead by 2 hours and about 70% within 6 to 8 hours (Figure 3A). Despite the structural and signaling similarities between TLR4 and IL1R, IL1 does not induce cell death in zVAD-pretreated DCs, indicating that critical caspases are selectively activated by LPS (Figure 3B). Blocking caspases with zVAD also does not interfere with IL1-induced maturation of DCs (data not shown). LPS-induced cell death can be augmented by zVAD in a dose-dependent fashion (Figure 3C) and can be induced, in the presence of zVAD, at very low doses of LPS (Figure 3D).
LPS-induced caspaselike activation is specific, rapid, and sustained in time. (A) Untreated (○), zVAD (40 μM) treated (•), LPS (100 ng/mL) stimulated (▵), or zVAD pretreated (1 hour) and LPS-stimulated (100 ng/mL) (▴) DCs were analyzed for PI uptake by flow cytometry at the indicated time points. Means ± 1 SD from 5 different experiments are shown. (B) Untreated (○), zVAD pretreated (•), IL1 (100 ng/mL) stimulated (▵), or zVAD pretreated and IL1-stimulated (▴) DCs were analyzed for PI uptake by flow cytometry at the indicated time points. Means ± 1 SD from 3 different experiments are shown. (C) DCs were pretreated with different doses of zVAD before LPS stimulation. Cells were analyzed after 8 hours for PI uptake by flow cytometry. (D) DCs were pretreated with 40 μM zVAD and then stimulated with different doses of LPS. After 8 hours cells were analyzed for PI uptake by flow cytometry. (E) DCs were pretreated with YVAD (up to 160 μM), IETD (up to 40 μM), zFA (up to 160 μM), WEHD (up to 20 μM), or zVAD (40 μM) and exposed to LPS. Cell death was analyzed by FITC–annexin V staining and flow cytometry after 24 hours. Means ± 1 SD from 4 different experiments are shown. (F) DCs were exposed to LPS. At the indicated time points, cells were washed 3 times and put back in colture with or without zVAD. After 12 hours cells were analyzed for PI uptake by flow cytometry. Means ± 1 SD from 3 different experiments are shown. (G) DCs left without additional treatment (○) or treated with zVAD (•) at different time points after LPS stimulation. Eight hours after zVAD addition, cells were analyzed for PI uptake by flow cytometry. Means ± 1 SD from 3 different experiments are shown. (H) A typical experiment was performed in parallel at 37°C and at 4°C. Twenty-four hours after LPS exposure zVAD pretreated DCs were analyzed for PI uptake by flow cytometry. Means ± 1 SD from 3 different experiments are shown.
LPS-induced caspaselike activation is specific, rapid, and sustained in time. (A) Untreated (○), zVAD (40 μM) treated (•), LPS (100 ng/mL) stimulated (▵), or zVAD pretreated (1 hour) and LPS-stimulated (100 ng/mL) (▴) DCs were analyzed for PI uptake by flow cytometry at the indicated time points. Means ± 1 SD from 5 different experiments are shown. (B) Untreated (○), zVAD pretreated (•), IL1 (100 ng/mL) stimulated (▵), or zVAD pretreated and IL1-stimulated (▴) DCs were analyzed for PI uptake by flow cytometry at the indicated time points. Means ± 1 SD from 3 different experiments are shown. (C) DCs were pretreated with different doses of zVAD before LPS stimulation. Cells were analyzed after 8 hours for PI uptake by flow cytometry. (D) DCs were pretreated with 40 μM zVAD and then stimulated with different doses of LPS. After 8 hours cells were analyzed for PI uptake by flow cytometry. (E) DCs were pretreated with YVAD (up to 160 μM), IETD (up to 40 μM), zFA (up to 160 μM), WEHD (up to 20 μM), or zVAD (40 μM) and exposed to LPS. Cell death was analyzed by FITC–annexin V staining and flow cytometry after 24 hours. Means ± 1 SD from 4 different experiments are shown. (F) DCs were exposed to LPS. At the indicated time points, cells were washed 3 times and put back in colture with or without zVAD. After 12 hours cells were analyzed for PI uptake by flow cytometry. Means ± 1 SD from 3 different experiments are shown. (G) DCs left without additional treatment (○) or treated with zVAD (•) at different time points after LPS stimulation. Eight hours after zVAD addition, cells were analyzed for PI uptake by flow cytometry. Means ± 1 SD from 3 different experiments are shown. (H) A typical experiment was performed in parallel at 37°C and at 4°C. Twenty-four hours after LPS exposure zVAD pretreated DCs were analyzed for PI uptake by flow cytometry. Means ± 1 SD from 3 different experiments are shown.
The cathepsin B inhibitor zFA, which is structurally similar to zVAD, and the caspase 1–like inhibitor YVAD have no influence on survival of LPS-stimulated DCs (Figure 3E). The calpain inhibitor zVF causes per se extensive apoptosis of DCs (data not shown), suggesting that zVAD is not acting by inhibiting calpain in this system. However, the caspase 4– or 5–like inhibitor WEHD and, more efficiently, the caspase 8– or 10–like inhibitor IETD, significantly inhibit survival of LPS-treated DCs, further indicating the requirement for caspases during LPS signaling in DCs (Figure 3E).
A very brief exposure to LPS is sufficient to activate cytoprotective caspase activation. Washing away excess LPS as early as 5 minutes after exposure to DCs does not substantially prevent cell death if DCs are subsequently treated with zVAD (Figure 3F). Importantly, blocking caspase activation hours after the exposure to LPS still results in extensive death of DCs. Figure 3G shows that even if caspase activation is blocked by zVAD up to 12 hours after LPS exposure, more than 60% of DCs undergo cell death. When zVAD is added after 24 to 36 hours, almost no changes in cell viability can be observed, excluding the possibility of generic toxicity in LPS-treated cells (Figure 3G). Cytoprotective caspase activation requires an active metabolic state, since LPS-induced cell death in the presence of zVAD does not occur at 4°C (Figure 3H).
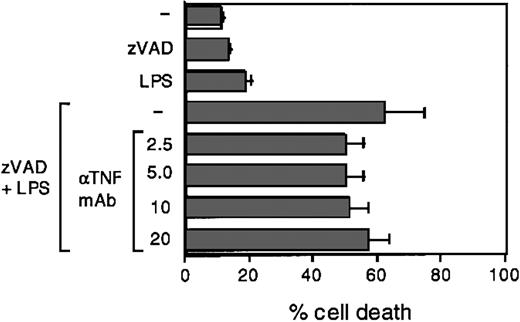
Finally, we considered the possibility that TNF, secreted in response to LPS treatment, could be responsible for the observed effects. To investigate the possible contribution of TNF, we exposed DCs to zVAD and LPS in the presence of anti-TNF–blocking antibodies, which we previously tested for the ability to completely suppress TNF-induced cell death in U937 cells (not shown). Figure 4 shows that TNF does not contribute significantly to LPS-induced caspase activation. Together these data indicate that caspase activation, which follows LPS exposure, is specific, sustained over time, and required for DC survival throughout the execution of the maturation program.
TNF does not significantly contribute to LPS-induced caspaselike activation. DCs were exposed to LPS (100 ng/mL), with or without pretreatment with zVAD (1 hour, 40 μM) and in the presence of the indicated neutralizing concentrations (2.5-20 μg/mL) of anti-TNF–blocking antibodies. After 16 hours cells were analyzed for PI uptake by flow cytometry. Means ± 1 SD from 3 different experiments are shown.
TNF does not significantly contribute to LPS-induced caspaselike activation. DCs were exposed to LPS (100 ng/mL), with or without pretreatment with zVAD (1 hour, 40 μM) and in the presence of the indicated neutralizing concentrations (2.5-20 μg/mL) of anti-TNF–blocking antibodies. After 16 hours cells were analyzed for PI uptake by flow cytometry. Means ± 1 SD from 3 different experiments are shown.
LPS does not appear to activate initiator caspases 8 and 10
In an attempt to identify the caspase(s) involved, we investigated whether the activity of caspases 8 and 10 is triggered by LPS in DCs. This was suggested by the finding that the caspase inhibitor IETD significantly affects DC viability in response to LPS (Figure 3E) and by the fact that the signal transduction machinery of TLRs allows for the recruitment of DD-containing proteins, including the adaptor FADD (Fas-associated death domain), known to, in turn, promote the activation of caspases 8 and 10. Interestingly, in fact, an apoptotic pathway initiated by TLR2 and sequentially involving MyD88, FADD, and caspase 8 has been described.24 Moreover, although initiator caspases 8 and 10 have primarily been attributed to apoptotic pathways, some evidence suggests that they can participate in entirely different programs.21,25 We therefore analyzed whether caspases 8 and 10 were responsible for LPS-induced caspase activity in DCs. Figure 5A shows that both caspases are expressed and functional in immature human DCs, since they can be triggered by staurosporine exposure, but they are not activated after LPS exposure as detectable by Western blot analysis of proteolytic products in DC lysates. Moreover, cell lysates from LPS-stimulated DCs do not contain proteolytic activity able to cleave in vitro–translated PARP, a suitable in vitro substrate for caspases 8 and 10, whereas PARP-cleaving activity can be recovered from DCs exposed to staurosporine (Figure 5B). By contrast, LPS can readily trigger caspase 1 activity in DCs, as detected by the ability of DC lysates to cleave in vitro–translated pro-IL1β (Figure 5C). These data indicate that caspase 8 and 10 are not likely to play a significant role in this process. Moreover, since we also observed a limited effect of the WEHD inhibitor on survival of LPS-treated DCs (Figure 3E), we tested the possible activation of caspases 4 and 5 in response to LPS. Both caspases are expressed in DCs but no convincing evidence for their activation could be obtained (not shown).
Caspases 8 and 10 are not activated by LPS. (A) DCs were exposed to LPS (100 ng/mL) or staurosporine (10 μM), with or without pretreatment with zVAD (1 hour, 40 μM), and activation of caspases 8 and 10 was analyzed by Western blotting at the indicated time points. Three independent experiments gave similar results. (B) DCs were exposed to LPS (100 ng/mL) or staurosporine (1 μM) for the indicated time points. Cell lysates were then incubated with in vitro–translated [35S]PARP, with or without 100 μM zVAD, and the products of the cleavage reaction were analyzed by autoradiography. Three independent experiments gave similar results. (C) DCs were left untreated or exposed to LPS (100 ng/mL) for 2 hours. Cell lysates were then incubated with in vitro–translated pro-IL1β, with or without 100 μM zVAD or YVAD, and the products of the cleavage reaction were analyzed by autoradiography. Two independent experiments gave similar results.
Caspases 8 and 10 are not activated by LPS. (A) DCs were exposed to LPS (100 ng/mL) or staurosporine (10 μM), with or without pretreatment with zVAD (1 hour, 40 μM), and activation of caspases 8 and 10 was analyzed by Western blotting at the indicated time points. Three independent experiments gave similar results. (B) DCs were exposed to LPS (100 ng/mL) or staurosporine (1 μM) for the indicated time points. Cell lysates were then incubated with in vitro–translated [35S]PARP, with or without 100 μM zVAD, and the products of the cleavage reaction were analyzed by autoradiography. Three independent experiments gave similar results. (C) DCs were left untreated or exposed to LPS (100 ng/mL) for 2 hours. Cell lysates were then incubated with in vitro–translated pro-IL1β, with or without 100 μM zVAD or YVAD, and the products of the cleavage reaction were analyzed by autoradiography. Two independent experiments gave similar results.
Caspase activation is required for LPS-induced ERK activation and cFLIP induction
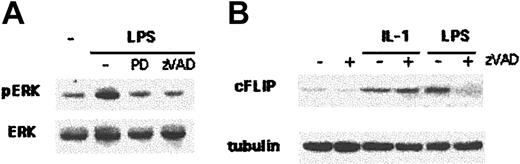
Signals that promote DC maturation and survival may follow different pathways. LPS induces an early ERK activation, which appears necessary for DC survival.16,17 It has been shown moreover that LPS exposure rapidly up-regulates the expression of cFLIP in human DCs, an event that confers significant survival advantage to mature DCs.22,26 We therefore investigated whether caspase-dependent survival signals controlled ERK activation and cFLIP induction. Figure 6A shows that 30 minutes after LPS exposure, ERK phosphorylation can be detected in immature DCs by Western blot analysis of cellular lysates. LPS-induced ERK activation is completely blocked by preventing caspase activation with zVAD, as well as by pretreating DCs with the specific ERK inhibitor PD98059, suggesting that LPS-activated caspases control ERK activation. Similarly, blocking caspase activation with zVAD prevented cFLIP up-regulation observed in DCs 2 hours after LPS exposure. Importantly, zVAD could not block IL1-induced cFLIP up-regulation (Figure 6B), again indicating that caspase activation specifically belongs to LPS-dependent signaling. LPS-induced caspase activation therefore controls molecular pathways that critically affect survival of human DCs.
Caspaselike activation is required for the generation of LPS-induced survival signals. (A) DCs were left untreated, stimulated with LPS (100 ng/mL), pretreated for 1 hour with PD98059 (30 μM) and stimulated with LPS, or pretreated for 1 hour with zVAD (40 μM) and stimulated with LPS. Thirty minutes after LPS stimulation, cell lysates were analyzed by Western blotting with a specific anti–P-ERK antibody. Anti-ERK antibody was used to control loading. Three independent experiments gave similar results. (B) DCs were left untreated or stimulated with IL1 (100 ng/mL) or LPS, with or without zVAD pretreatment. After 2 hours, cell lysates were analyzed by Western blotting with an anti-FLIP antibody. Antitubulin antibody was used to control loading. Five independent experiments gave similar results.
Caspaselike activation is required for the generation of LPS-induced survival signals. (A) DCs were left untreated, stimulated with LPS (100 ng/mL), pretreated for 1 hour with PD98059 (30 μM) and stimulated with LPS, or pretreated for 1 hour with zVAD (40 μM) and stimulated with LPS. Thirty minutes after LPS stimulation, cell lysates were analyzed by Western blotting with a specific anti–P-ERK antibody. Anti-ERK antibody was used to control loading. Three independent experiments gave similar results. (B) DCs were left untreated or stimulated with IL1 (100 ng/mL) or LPS, with or without zVAD pretreatment. After 2 hours, cell lysates were analyzed by Western blotting with an anti-FLIP antibody. Antitubulin antibody was used to control loading. Five independent experiments gave similar results.
Discussion
Here we provide evidence that a caspaselike activity triggered by LPS is required for survival of human DCs and critically controls the LPS-induced activation of ERKs and the LPS-induced up-regulation of cFLIP.
Proteolytic processing by caspases is a defined modification of proteins that enables specific cleavage products to activate/lose functions, acquire new functions, or simply act as physical signal transducers. As key players in signal generation and transduction, caspases have been shown to participate in diverse cellular programs from cell differentiation to adaptation, proliferation, and apoptosis. During apoptosis, they also accomplish most of the ordered degradation of structural cellular proteins, which is required for the making of apoptotic bodies. Caspases are expressed as inactive zymogens, or procaspases, and require proteolytic processing to allow for the generation of active subunits and assembly of functional enzymes. Specific structural features in the prodomain of the zymogens allow the grouping of different caspases into distinct subfamilies, which may correspond to the ability to carry out specialized tasks. The presence of protein interaction death effector domains (DEDs), for instance, distinguishes “initiator” caspases, such as caspases 8 and 10. Initiator caspases become activated after oligomerization induced by DED-mediated clustering with the adaptor FADD, which in turn is recruited via its death domain (DD) to DD-expressing surface receptors. Procaspases 8 and 10 are therefore activated in membrane-associated multiprotein signaling complexes and initiate receptor-dependent signaling.
Evidence has shown that caspases can be activated following the engagement of TLRs. Caspase 1 can be activated in human monocytoid THP1 cells via engagement of TLR2 by bacterial lipoproteins (BLP), resulting in pro-IL1 proteolytic processing and IL1 release.25 More importantly, a proapoptotic pathway can also be initiated by TLR2 in cycloheximide-treated THP1 cells. This pathway may sequentially involve MyD88, FADD, and caspase 8 and be counteracted by a parallel MyD88-dependent pathway, which includes TRAF6 and leads to NFκB activation.24,27 LPS, a ligand for TLR4, can induce apoptosis in multiple cellular systems. A TLR4-mediated apoptotic pathway can be triggered by LPS in cycloheximide-treated endothelial cells and can be blocked by zVAD.28,29 Concomitantly, LPS triggers survival pathways in several cell types, including human DCs, which result in the up-regulation of cFLIP, an inhibitor of the proapoptotic initiator caspases.22,26 Suppression of the protein synthesis–dependent up-regulation of cFLIP greatly enhances LPS-induced apoptotic cell death,30 indicating that apoptotic signaling by TLRs runs in parallel with survival pathways, the final outcome depending on reciprocal inhibition and general integration.
Proapoptotic caspases can therefore be triggered by microbial products via TLRs, together with molecular strategies designed to block their activation. Quite differently, our data show that LPS, the primary component of the outer membrane of Gram-negative bacteria, triggers a caspaselike activity that is required for survival of human DCs. No information is currently available concerning the involvement of caspases in TLR-dependent survival pathways in mammals.
The fruit fly Drosophila melanogaster produces a number of antibiotic peptides to counteract microbial infections. The genes coding for these peptides are under the control of 2 different pathways.31 The Toll pathway, required for the immune response to Gram-positive bacteria and fungi, is initiated by surface Toll receptors. Similarly to mammalian TLRs, Toll interacts with either dMyD88 via TIR or with the DD-containing adaptor Tube. The DDs of dMyD88 or Tube allow for further interaction with Pelle, a serine/threonine kinase homologous to IRAK. This is followed by the downstream phosphorylation of Cactus, an NFκB inhibitor (IκB) homolog expressing ankirin repeats, and its degradation and dissociation from dorsal-related immunity factor (DIF), a transcription factor of the Rel family. Once rid of Cactus, DIF translocates to the nucleus where it acts as the major transactivator of genes coding for anti–Gram-positive and antifungal peptides.32
The pathway controlling the peptide response to Gram-negative bacteria in Drosophila is less understood. Recently, PGRP-LC, a member of the family of peptidoglycan recognition surface receptors (PGRPs), has been shown to control anti–Gram-negative bacterial responses.33-35 Genetic analysis has revealed that this pathway requires immune deficiency (IMD), a DD-containing adaptor homologous to mammalian receptor interacting protein (RIP).36 IMD acts upstream of dFADD, a DD-containing adaptor.37,38 dFADD may allow the recruitment of DREDD (death-related CED-3/NEDD2-like protein), a caspase 8 homolog and the only caspase found so far involved in microbial defense in Drosophila.39 Downstream of IMD is also dTAK1 (Drosophila homolog of transforming growth factor-β–activated kinase 1), a mitogen-activated protein 3 (MAP3) kinase, homolog to human TAK1. dTAK1 is probably involved in the activation of the IκB kinase (IKK) complex required for the nuclear translocation of the transcription factor Relish.40 Relish, another member of the Rel family, is activated by phosphorylation and by proteolytic cleavage necessary to release an ankirin repeat–rich Cactus-like domain and allow for nuclear translocation. Relish controls the transactivation of genes coding for anti–Gram-negative bacterial peptides. Importantly, the PGRP-IMD-Relish pathway is also triggered by LPS.35,41
In mammals, peptidoglycans use TLR2, whereas LPS uses TLR4 to induce DC maturation.42,43 Following LPS/TLR4 interactions, the DD of MyD88 is in principle available for interactions with FADD allowing further recruitment and activation of initiator as well as apoptotic caspases.24 However no apoptotic caspase activation is detectable during LPS signaling in DCs. Survival is therefore critically dependent on LPS-induced cytoprotective pathways, including cFLIP expression. Intriguingly, we find that LPS-induced cFLIP up-regulation itself requires the activation of a caspaselike activity. Moreover, our data indicate that the early ERK activation, which also contributes to DC survival, is under the control of upstream caspases.
It is remarkable that caspase activation is relevant for LPS but not for IL1-dependent signaling in DCs. Blocking caspases, in fact, does not prevent IL1-induced cFLIP up-regulation, maturation, and survival. Similarly to TLR4, the IL1 receptor signals via MyD88 or TOLLIP to IRAKs and TRAF6. However, MAL/TIRAP-dependent pathways are unique to TLR4.13,14 Early caspase activation may therefore contribute to the specificity of LPS-induced signaling.
The identity of the critical caspase(s) is currently missing, since it does not seem to correspond to canonical initiator caspases 8 or 10, despite its very early activation during LPS signaling. Accordingly, the caspase 8 homolog DREDD does not seem to be implicated in early signaling along the PGRP-Relish pathway, as defined by epistatic analysis.40 An attractive candidate could be the human paracaspase MLT/MALT1 (mucosa-associated lymphoid tissue lymphoma translocation gene 1),44-46 which possesses a DD highly homologous to the DD of MyD88 and IRAKs as well as to Drosophila Pelle and Tube.47 Further investigation is required to identify the relevant caspase(s) involved in LPS signaling in DCs and to better clarify the intimate connections between survival and death decisions governing cellular adaptation to microbial organisms.
Prepublished online as Blood First Edition Paper, June 26, 2003; DOI 10.1182/blood-2003-03-0967.
Supported by grants from Associazione Italiana Ricerca sul Cancro, Ministero Istruzione Universita' e Ricerca, Agenzia Spaziale Italiana, and European Commission V Framework Program.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors acknowledge Dario Serio for technical help and all lab members for insightful discussions.


![Figure 5. Caspases 8 and 10 are not activated by LPS. (A) DCs were exposed to LPS (100 ng/mL) or staurosporine (10 μM), with or without pretreatment with zVAD (1 hour, 40 μM), and activation of caspases 8 and 10 was analyzed by Western blotting at the indicated time points. Three independent experiments gave similar results. (B) DCs were exposed to LPS (100 ng/mL) or staurosporine (1 μM) for the indicated time points. Cell lysates were then incubated with in vitro–translated [35S]PARP, with or without 100 μM zVAD, and the products of the cleavage reaction were analyzed by autoradiography. Three independent experiments gave similar results. (C) DCs were left untreated or exposed to LPS (100 ng/mL) for 2 hours. Cell lysates were then incubated with in vitro–translated pro-IL1β, with or without 100 μM zVAD or YVAD, and the products of the cleavage reaction were analyzed by autoradiography. Two independent experiments gave similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/8/10.1182_blood-2003-03-0967/6/m_h82035089005.jpeg?Expires=1769297473&Signature=0qMdJY~r7bJDKSdq1M4sFGxx3TMY9qnLVZvxaAgfJWDINW1IPV86MXkKrkvZ~EQwVDfEYTvNNf3lmB7D2hos-5PJgo3yV1jOL6OHXpsX5l0GtUR19cKL1OLhsHKk5w28YpjpvP0G4z8Mdoe-sK8~Q0xRYFGnOWT3FRhxfSbPoxyKDyuTkppYUIlR~w77E5FKcJxyzfa39gVBujPHxLTn9dM7bGPrNRMdW9kKjjq9TUX9Oz-XD2mklgT~eoiM6sGfxB9~A7IQh~mJbG~ijWsridb40BZPTkkYXU0CFxXQV0Tew-kr-2yFvXcEU5qITmOT0Wh~WKKZa4-LvHC6Pv0L1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal