Abstract
Although the migratory property of lymphocytes is critical for protective immunity, tissue infiltration of lymphocytes sometimes causes harmful immune responses. DOCK2 plays a critical role in lymphocyte migration by regulating actin cytoskeleton through Rac activation, yet the mechanism by which DOCK2 activates Rac remains unknown. We found that DOCK2 associates with engulfment and cell motility (ELMO1) through its Srchomology 3 (SH3) domain. When DOCK2 was expressed in T-hybridoma cells lacking endogenous expression of DOCK2, Rac activation and actin polymerization were induced. However, such responses were not elicited by the DOCK2 mutant lacking the region required for ELMO1 binding. On the other hand, we found that the expression of ELMO1 induces Rac activation in the plasmacytoma cells expressing DOCK2 but not ELMO1. These results indicate that the association of DOCK2 with ELMO1 is critical for DOCK2-mediated Rac activation, thereby suggesting that their association might be a therapeutic target for immunologic disorders caused by lymphocyte infiltration.
Introduction
DOCK2 is a new member of the CDM family proteins—Caenorhabditis elegans CED-5, human DOCK180, and Drosophila melanogaster myoblast city—that are known to regulate actin cytoskeleton by functioning upstream of Rac.1-5 While DOCK180 is expressed in cells other than lymphocytes, the expression of DOCK2 is largely restricted to lymphocytes.6 By generating DOCK2-deficient mice, we have earlier reported that DOCK2 plays a critical role in lymphocyte migration by regulating actin cytoskeleton through Rac activation.6 Therefore, an important question is how DOCK2 activates Rac in lymphocytes. Regarding this, it was recently reported that DOCK2 associates with Crk-like (CrkL) and activates Rac.7 However, in the light of the large size of DOCK2, it seems likely that DOCK2 interacts with multiple partners to regulate Rac activation.
ELMO1 is a mammalian homolog of CED-12 that is known to interact with CED-5. Recently, it has been demonstrated that ELMO1 associates with DOCK180 and cooperatively regulates Rac activation.8,9 The “Docker” domain required for Rac binding is conserved between DOCK2 and DOCK180.9 Therefore, as suggested by Reif and Cyster,10 a similar mechanism may govern DOCK2-mediated Rac activation. In this study, we have examined whether ELMO1 functions in DOCK2-mediated Rac activation.
Study design
Northern blot analysis
RNA prepared from each cell line was hybridized with the cDNA probe.
Plasmids
The PBJ1 vector encoding full-length DOCK2 (PBJ1-DOCK2) has been described.6 The genes encoding DOCK2 and DOCK2 deletion mutants (DOCK2, DOCK2delC, DOCK2N, and DOCK2delN) with a hemagglutinin (HA) tag were created by polymerase chain reaction (PCR) with the use of PBJ1-DOCK2 as a template and introduced into PcDNA/His max (Invitrogen, Carlsbad, CA). The genes encoding ELMO1, ELMO1 deletion mutants (ELMO1del1, ELMO1del8, and ELMO1del10), and CrkL were created by PCR from cDNA prepared from mouse tissues and introduced into PcDNA V5-His (Invitrogen) or PBJ1. The details of each construct are summarized in Figure 1A. The mutations at DOCK2 Src homology 3 (SH3) domain were introduced with the use of the QuickChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA).
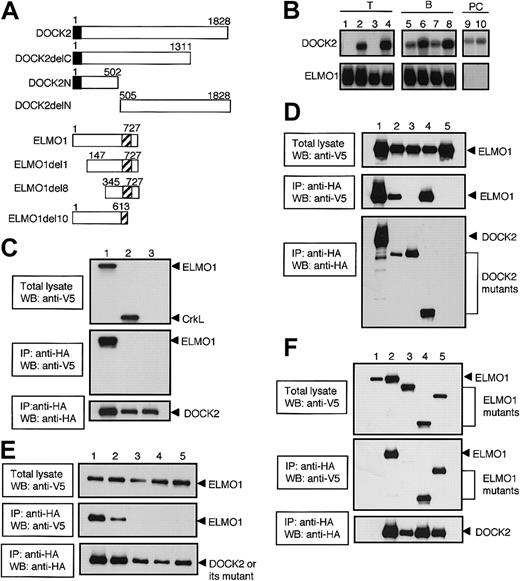
Association of DOCK2 with ELMO1 through its SH3 domain. (A) Schematic representation of DOCK2- and ELMO1-deletion mutants used in this study. The closed or hatched box indicates SH3 domain of DOCK2 or PH-like domain of ELMO1, respectively. (B) Total cellular RNA (10 μg) or polyA RNA (2 μg) was prepared from BW5147α–β– (lane 1), BW5147 (lane 2), BEα16-3 (lane 3), N3-5 (lane 4), 70Z/3 (lane 5), WEHI231 (lane 6), L1210 (lane 7), M12C3 (lane 8), J558 (lane 9), and NS1 (lane 10), and hybridized with DOCK2 or ELMO1 cDNA probe. (C) After either ELMO1 (lane 1), CrkL (lane 2), or empty vector (lane 3) was expressed with DOCK2 (lanes 1-3) in 293T cells, cell extracts were immunoprecipitated with anti-HA affinity matrix (to precipitate DOCK2) and analyzed by immunoblotting with the use of anti-V5 antibody (to detect ELMO1 and CrkL) or anti-HA antibody (to detect DOCK2). The reactivity to anti-V5 antibody before (top panel) or after (middle panel) immunoprecipitation, and the reactivity to anti-HA antibody after immunoprecipitation (bottom panel) are shown. IP indicates immunoprecipitation; WB, Western blot. (D) After either DOCK2 (lane 1), DOCK2delC (lane 2), DOCK2delN (lane 3), DOCK2N (lane 4), or empty vector (lane 5) was expressed with ELMO1 (lanes 1-5) in 293T cells, cell extracts were immunoprecipitated with anti-HA affinity matrix (to precipitate DOCK2 or its deletion mutants) and analyzed by immunoblotting with the use of anti-V5 antibody (to detect ELMO1) or anti-HA antibody (to detect DOCK2 or its deletion mutants). The reactivity to anti-V5 antibody before (top panel) or after (middle panel) immunoprecipitation, and the reactivity to anti-HA antibody after immunoprecipitation (bottom panel) are shown. (E) After either DOCK2 (lane 1), DOCK2L27E (lane 2), DOCK2G32E (lane 3), DOCK2P60E (lane 4), or DOCK2F63E (lane 5) was expressed with ELMO1 (lanes 1-5) in 293T cells, cell extracts were immunoprecipitated with anti-HA affinity matrix (to precipitate DOCK2 or its SH3 mutants) and analyzed by immunoblotting with the use of anti-V5 antibody (to detect ELMO1) or anti-HA antibody (to detect DOCK2 or its SH3 mutants). The reactivity to anti-V5 antibody before (top panel) or after (middle panel) immunoprecipitation, and the reactivity to anti-HA antibody after immunoprecipitation (bottom panel) are shown. (F) After DOCK2 (lanes 2-5) or empty vector (lane 1) was expressed with ELMO1 (lanes 1-2), ELMO1del10 (lane 3), ELMO1del8 (lane 4), or ELMO1del1 (lane 5) in 293T cells, cell extracts were immunoprecipitated with anti-HA affinity matrix (to precipitate DOCK2) and analyzed by immunoblotting with the use of anti-V5 antibody (to detect ELMO1 or its deletion mutants) or anti-HA antibody (to detect DOCK2). The reactivity to anti-V5 antibody before (top panel) or after (middle panel) immunoprecipitation, and the reactivity to anti-HA antibody after immunoprecipitation (bottom panel) are shown.
Association of DOCK2 with ELMO1 through its SH3 domain. (A) Schematic representation of DOCK2- and ELMO1-deletion mutants used in this study. The closed or hatched box indicates SH3 domain of DOCK2 or PH-like domain of ELMO1, respectively. (B) Total cellular RNA (10 μg) or polyA RNA (2 μg) was prepared from BW5147α–β– (lane 1), BW5147 (lane 2), BEα16-3 (lane 3), N3-5 (lane 4), 70Z/3 (lane 5), WEHI231 (lane 6), L1210 (lane 7), M12C3 (lane 8), J558 (lane 9), and NS1 (lane 10), and hybridized with DOCK2 or ELMO1 cDNA probe. (C) After either ELMO1 (lane 1), CrkL (lane 2), or empty vector (lane 3) was expressed with DOCK2 (lanes 1-3) in 293T cells, cell extracts were immunoprecipitated with anti-HA affinity matrix (to precipitate DOCK2) and analyzed by immunoblotting with the use of anti-V5 antibody (to detect ELMO1 and CrkL) or anti-HA antibody (to detect DOCK2). The reactivity to anti-V5 antibody before (top panel) or after (middle panel) immunoprecipitation, and the reactivity to anti-HA antibody after immunoprecipitation (bottom panel) are shown. IP indicates immunoprecipitation; WB, Western blot. (D) After either DOCK2 (lane 1), DOCK2delC (lane 2), DOCK2delN (lane 3), DOCK2N (lane 4), or empty vector (lane 5) was expressed with ELMO1 (lanes 1-5) in 293T cells, cell extracts were immunoprecipitated with anti-HA affinity matrix (to precipitate DOCK2 or its deletion mutants) and analyzed by immunoblotting with the use of anti-V5 antibody (to detect ELMO1) or anti-HA antibody (to detect DOCK2 or its deletion mutants). The reactivity to anti-V5 antibody before (top panel) or after (middle panel) immunoprecipitation, and the reactivity to anti-HA antibody after immunoprecipitation (bottom panel) are shown. (E) After either DOCK2 (lane 1), DOCK2L27E (lane 2), DOCK2G32E (lane 3), DOCK2P60E (lane 4), or DOCK2F63E (lane 5) was expressed with ELMO1 (lanes 1-5) in 293T cells, cell extracts were immunoprecipitated with anti-HA affinity matrix (to precipitate DOCK2 or its SH3 mutants) and analyzed by immunoblotting with the use of anti-V5 antibody (to detect ELMO1) or anti-HA antibody (to detect DOCK2 or its SH3 mutants). The reactivity to anti-V5 antibody before (top panel) or after (middle panel) immunoprecipitation, and the reactivity to anti-HA antibody after immunoprecipitation (bottom panel) are shown. (F) After DOCK2 (lanes 2-5) or empty vector (lane 1) was expressed with ELMO1 (lanes 1-2), ELMO1del10 (lane 3), ELMO1del8 (lane 4), or ELMO1del1 (lane 5) in 293T cells, cell extracts were immunoprecipitated with anti-HA affinity matrix (to precipitate DOCK2) and analyzed by immunoblotting with the use of anti-V5 antibody (to detect ELMO1 or its deletion mutants) or anti-HA antibody (to detect DOCK2). The reactivity to anti-V5 antibody before (top panel) or after (middle panel) immunoprecipitation, and the reactivity to anti-HA antibody after immunoprecipitation (bottom panel) are shown.
Transfection and stable transfectants
The stable transfectants were developed by introducing the plasmid DNA into T-cell hybridoma BEα16-3 or plasmacytoma (PC) cell line NS1 by electroporation. The following BEα16-3 transfectants were used in this study: 25-7 expressing full-length DOCK2 with HA tag; 17-11 expressing full-length DOCK2 without HA tag; and 84-3 expressing DOCK2delN with HA tag. Transient transfection was done with the calcium phosphate method.
Immunoprecipitation, pull down, and immunoblotting
The antibody used to detect DOCK2 expression has been described.6 The anti-ELMO1 antibody was developed by immunizing rabbits with amino terminal peptide of ELMO1. Cell lysates were incubated with anti-HA affinity matrix (Roche Diagnostics, Indianapolis, IN), and the precipitates were subject to immunoblotting with the use of anti-V5 antibody (Invitrogen), anti-HA antibody (Roche Diagnostics), anti-ELMO1 antibody, or anti-CrkL antibody (Santa Cruz Biotechnology, Santa Cruz, CA). The pull-down assay to detect activated Rac was done as previously described.6
Immunofluorescence microscope
Cells were stained with Alexa Fluor 568–labeled phalloidin (Molecular Probes, Eugene, OR) as previously described.6
Results and discussion
We first assessed the expression of DOCK2 and ELMO1 in T-, B- and PC-cell lines by Northern blot analysis. As we previously reported,6 DOCK2-expression was detected in all cell lines tested, with the exception of BW5147α–β– and its derivative BEα16-3 (Figure 1B). On the other hand, ELMO1 was expressed in all T- and B-cell lines, but its expression was not detected in 2 PC cell lines, NS1 and J558 (Figure 1B).
The mouse DOCK2 encodes 1828 amino acid residues, including an SH3 domain located at the amino-terminus.6 When ELMO1 or CrkL was expressed in 293T cells with full-length DOCK2, the association of DOCK2 with ELMO1 was readily detected (Figure 1C). However, we were unable to detect DOCK2-CrkL interaction (Figure 1C). The association with ELMO1 was also found when DOCK2delC or DOCK2N was coexpressed with ELMO1 (Figure 1D). In contrast, such association was not detected in the case of DOCK2delN (Figure 1D). Since the amino-terminal region of DOCK2 required for ELMO1-binding includes SH3 domain, we examined whether this domain would be involved in the interaction with ELMO1 by analyzing the DOCK2 mutants where one amino acid substitution was introduced in the consensus sequence of the SH3. When DOCK2L27E (a mutant in which leucine at position 27 is replaced with glutamic acid) was expressed with ELMO1 in 293T cells, the association with ELMO1 was still observed (Figure 1E). However, no such association was detected with DOCK2G32E, DOCK2P60E, or DOCK2F63E where glycine, proline, or phenylalanine at position 32, 60, or 63 was replaced with glutamic acid (Figure 1E). These results indicate that DOCK2 interacts with ELMO1 through its SH3 domain.
ELMO1 encodes 727 amino acid residues, including a pleckstrin homology (PH)–like domain located at the 555 to 676 region.8,9 To identify the DOCK2-binding region of ELMO1, we expressed several ELMO1 deletion mutants with full-length DOCK2 in 293T cells. Similar to the case of wild-type ELMO1, ELMO1del1 and ELMO1del8 were found to be associated with DOCK2 (Figure 1F). However, such association could not be detected when ELMO1del10 was coexpressed with DOCK2 (Figure 1F). These results indicate that carboxyl-terminal region of ELMO1 is required for DOCK2-binding.
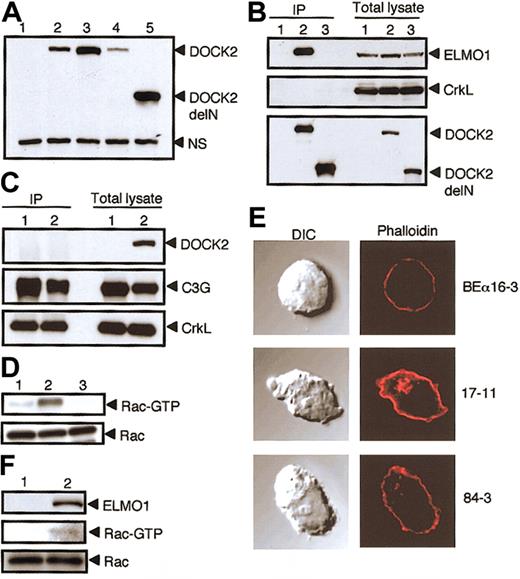
To examine the association of DOCK2 with ELMO1 under nearly physiologic condition, we used BEα16-3 transfectants, 25-7 and 17-11 expressing full-length DOCK2 with or without HA tag, respectively, and 84-3 expressing DOCK2delN with HA tag (Figure 2A). When cell extracts were immunoprecipitated with anti-HA antibody, the association of DOCK2 with the endogenous ELMO1 was detected with 25-7, but not 84-3, and BEα16-3 (Figure 2B). These results clearly indicate that DOCK2 associates with ELMO1 in T cells. On the other hand, we were unable to detect the association of DOCK2 with CrkL in 25-7 (Figure 2B). When cell extracts of 17-11 were immunoprecipitated with anti-CrkL antibody, C3G was clearly coimmunoprecipitated with CrkL (Figure 2C). However, the association between DOCK2 and CrkL was again not detected in 17-11 (Figure 2C). CrkL is implicated to play an important role in integrin signaling through the interaction with C3G that functions as guanosine diphosphate/guanosine triphosphate (GDP/GTP) exchange factor for Rap1.11,12 Although DOCK2-CrkL interaction might be induced upon stimulation, we have found that DOCK2 deficiency has no effect on chemokineinduced integrin activation in T cells (J.V.S. et al, manuscript in preparation). Therefore, the question of whether CrkL really functions in the DOCK2 signaling pathway needs to be investigated in the future studies.
Effect of the association of DOCK2 with ELMO1. The association of DOCK2 with ELMO1 is critical for DOCK2-mediated Rac activation and cytoskeletal reorganization. (A) The expression of DOCK2 or DOCK2delN in BEα16-3 (lane 1), N3-5 (lane 2), 17-11 (lane 3), 25-7 (lane 4), and 84-3 (lane 5) was analyzed with the use of anti-DOCK2 antibody. A nonspecific band (NS) is included as a loading control. (B) Cell extracts were prepared from BEα16-3 (lane 1), 25-7 (lane 2), and 84-3 (lane 3); immunoprecipitated with anti-HA affinity matrix (to precipitate DOCK2 or DOCK2delN); and analyzed by immunoblotting with anti-ELMO1 antibody (top panel), anti-CrkL antibody (middle panel), or anti-HA antibody (to detect DOCK2 or DOCK2delN) (bottom panel). The expression of ELMO1, CrkL, DOCK2, or DOCK2delN in each cell line is shown in the 3 right lanes of each subpanel. (C) Cell extracts were prepared from BEα16-3 (lane 1) and 17-11 (lane 2), immunoprecipitated with anti-CrkL antibody, and analyzed by immunoblotting with anti-DOCK2 antibody (top panel), anti-C3G antibody (middle panel), or anti-CrkL antibody (bottom panel). The expression of DOCK2, C3G, and CrkL in each cell line is shown in the 2 right lanes. (D) Cell extracts were prepared from 84-3 (lane 1), 17-11 (lane 2), and BEα16-3 (lane 3), and analyzed for Rac or for the GTP-bound, activated Rac by means of glutathione S-transferase (GST)–fusion, Rac-binding domain of p21-activated kinase 1 (PAK1). (E) BEα16-3, 17-11, and 84-3 were analyzed for actin polymerization by means of staining with phalloidin. The differential interference contrast (DIC) images are shown in the left panels. Original magnification, × 600. (F) NS1 (lane 1) and NS1 transfectant expressing ELMO1 (lane 2) were analyzed for Rac (bottom panel) or the GTP-bound, activated Rac (middle panel) as described in panel D. The expression of ELMO1 is shown in the top panel.
Effect of the association of DOCK2 with ELMO1. The association of DOCK2 with ELMO1 is critical for DOCK2-mediated Rac activation and cytoskeletal reorganization. (A) The expression of DOCK2 or DOCK2delN in BEα16-3 (lane 1), N3-5 (lane 2), 17-11 (lane 3), 25-7 (lane 4), and 84-3 (lane 5) was analyzed with the use of anti-DOCK2 antibody. A nonspecific band (NS) is included as a loading control. (B) Cell extracts were prepared from BEα16-3 (lane 1), 25-7 (lane 2), and 84-3 (lane 3); immunoprecipitated with anti-HA affinity matrix (to precipitate DOCK2 or DOCK2delN); and analyzed by immunoblotting with anti-ELMO1 antibody (top panel), anti-CrkL antibody (middle panel), or anti-HA antibody (to detect DOCK2 or DOCK2delN) (bottom panel). The expression of ELMO1, CrkL, DOCK2, or DOCK2delN in each cell line is shown in the 3 right lanes of each subpanel. (C) Cell extracts were prepared from BEα16-3 (lane 1) and 17-11 (lane 2), immunoprecipitated with anti-CrkL antibody, and analyzed by immunoblotting with anti-DOCK2 antibody (top panel), anti-C3G antibody (middle panel), or anti-CrkL antibody (bottom panel). The expression of DOCK2, C3G, and CrkL in each cell line is shown in the 2 right lanes. (D) Cell extracts were prepared from 84-3 (lane 1), 17-11 (lane 2), and BEα16-3 (lane 3), and analyzed for Rac or for the GTP-bound, activated Rac by means of glutathione S-transferase (GST)–fusion, Rac-binding domain of p21-activated kinase 1 (PAK1). (E) BEα16-3, 17-11, and 84-3 were analyzed for actin polymerization by means of staining with phalloidin. The differential interference contrast (DIC) images are shown in the left panels. Original magnification, × 600. (F) NS1 (lane 1) and NS1 transfectant expressing ELMO1 (lane 2) were analyzed for Rac (bottom panel) or the GTP-bound, activated Rac (middle panel) as described in panel D. The expression of ELMO1 is shown in the top panel.
Having found that the association of DOCK2 with ELMO1 was abrogated in 84-3, we compared Rac activation and actin polymerization in 17-11 and 84-3 where similar amounts of DOCK2 or DOCK2delN were expressed (Figure 2A). While the GTP-bound, activated Rac was detected in 17-11, DOCK2-mediated Rac activation was severely impaired in 84-3 expressing DOCK2delN (Figure 2D). When cells were stained with phalloidin, F-actin assembly was observed in 17-11, but not in 84-3 and 16-3 (Figure 2E). These results suggest that the interaction of DOCK2 with ELMO1 is required for Rac activation and subsequent remodeling of actin cytoskeleton. This was further confirmed by the results that the expression of ELMO1 induced Rac activation in NS1, which expresses DOCK2 but lacks ELMO1 expression (Figure 2F).
Our results indicate that DOCK2 activates Rac and regulates actin cytoskeleton through the interaction with ELMO1. Therefore, the DOCK2-ELMO1 interaction might be a therapeutic target for immunologic disorders, such as autoimmune diseases and graft rejection, caused by tissue infiltration of lymphocytes.
Prepublished online as Blood First Edition Paper, June 26, 2003; DOI 10.1182/blood-2003-01-0173.
Supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by Japan Science and Technology Corporation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal