Abstract
An internal tandem duplication (ITD) of the juxtamembrane (JM) domain of FLT3 (FLT3/ITD) has been found in 20% of patients with acute myeloid leukemia (AML) and is correlated with leukocytosis and a poor prognosis. Here, we compared the antiapoptotic effects of wild-type FLT3 (WtFLT3) and FLT3/ITD in terms of the regulation of Bcl-2 family members. In a murine myeloid cell line, 32D, interleukin-3 (IL-3) deprivation induced apoptosis following the down-regulation of Bcl-XL and the dephosphorylation of Bad. However, the expression levels of Bcl-2, Bax, Bak, and Mcl-1 were unchanged. In WtFLT3-transfected 32D (WtFLT3-32D) cells, FLT3 ligand (FL) stimulation did not restore the down-regulation of Bcl-XL but maintained the phosphorylation of Bad. Combined treatment with phosphatidylinositol 3-kinase (PI3K) inhibitor, LY294002, and mitogen-activated protein kinase kinase (MEK) inhibitor, PD98059, dephosphorylated Bad and induced apoptosis in WtFLT3-32D cells stimulated with FL. Induction of nonphosphorylated Bad induced remarkable apoptosis. These findings suggest that the FL stimulation is associated with antiapoptosis through Bad phosphorylation. On the other hand, FLT3/ITD-transfected 32D (FLT3/ITD-32D) cells survived in an IL-3–or FL-deprived state. Furthermore, the dephosphorylation of Bad using LY294002 and PD98059 was insufficient for apoptosis, and the down-regulation of Bcl-XL using antisense treatment was needed to induce apoptosis. FLT3 kinase inhibitor, AG1296, alone not only dephosphorylated Bad but also down-regulated Bcl-XL, leading FLT3/ITD-32D cells into apoptosis. These findings suggest that the antiapoptotic pathways from FLT3/ITD are more divergent than those from WtFLT3 and may represent targets for drug discovery with the potential of inducing selective cell death of human leukemia cells.
Introduction
FLT3 is a member of the class III receptor tyrosine kinase family and plays an important role in regulating the proliferation, differentiation, and survival of hematopoietic cells.1 Binding of FLT3 ligand (FL) to FLT3 induces receptor dimerization leading to activation of the tyrosine kinase domain and autophosphorylation of tyrosine residues.2 Subsequent signal transduction is mediated through the association and/or phosphorylation of phosphatidylinositol 3-kinase (PI3K), RAS, phospholipase C γ (PLCγ), VAV, SHC, growth factor receptor-bound protein 2 (GRB2), CBL, and signal transducers and activators of transcription 5a (STAT5a).3-5
Aberrant activation of receptor tyrosine kinases is a common event in human cancer, including leukemia.6 Of patients with acute myeloid leukemia (AML), 20% have an internal tandem duplication (ITD) of the juxtamembrane (JM) domain of FLT3 (FLT3/ITD),7,8 which is associated with leukocytosis and a poor prognosis.9,10 This mutation is also reported to be associated with disease progression or relapse of AML,11 and with a poor prognosis independently of leukocytosis.12 In terms of biologic activity, the ITD causes constitutive FL-independent dimerization and tyrosine phosphorylation,13,14 and activates RAS/mitogen-activated protein kinase (MAPK), Akt, and STAT5 in stably transfected cell lines.15,16 However, the antiapoptotic downstream pathways triggered by FLT3/ITD have not been fully elucidated.
In this study, we attempted to determine the antiapoptotic effects of wild-type FLT3 (WtFLT3) and FLT3/ITD mainly in terms of Bcl-2 family members, which participate in the regulation of cell survival in multiple hematopoietic cell lineages.17,18 It is reported that an antiapoptotic molecule, Bcl-2, is required for the survival of mature murine lymphocytes,19 and its homologue, Bcl-XL, is involved in the survival of immature murine lymphocytes, primitive human hematopoietic precursors, and interleukin-3 (IL-3)–dependent cell lines.20-22 A proapoptotic molecule, Bad, is involved in the survival function of many hematopoietic systems, including IL-3–dependent cell lines, and is regulated through the phosphorylation of 2 serine residues (Ser112 and Ser136).23,24 A proapoptotic molecule, Bim, is a principal regulator of murine hematopoietic homeostasis.25,26 Using 32D, a murine IL-3–dependent myeloid progenitor cell line, as a parent, we generated stable transfectants of WtFLT3 and FLT3/ITD. And we examined the effects of kinase inhibitors on the signaling pathways related to FLT3 kinase activity. It is reported that AG1296 inhibits both WtFLT3 and FLT3/ITD tyrosine kinase activity in vivo and induces apoptosis in FLT3-mediated transformed leukemia cells and primary AML cells.27,28 PI3K inhibitor, LY294002, and mitogenactivated protein kinase kinase (MEK) inhibitor, PD98059, impair cell growth and survival of AML cell lines and primary AML cells.29,30 Here, we show that the antiapoptotic pathways from FLT3/ITD are more divergent than those from WtFLT3. It may be useful not only in understanding the oncogenic properties of FLT3 in leukemia cells but also in providing new molecular targets for antileukemia drug discovery.
Materials and methods
Reagents and cells
Recombinant human FL was purchased from R&D Systems (Minneapolis, MN). Recombinant murine IL-3 was a generous gift from Kirin Brewery (Tokyo, Japan). PD98059 and LY294002 were obtained from Sigma-Aldrich (St Louis, MO). AG1296 was from Calbiochem (San Diego, CA). These reagents were dissolved in dimethyl sulfoxide (DMSO) at appropriate concentrations and stored at –20°C until use. A murine IL-3–dependent myeloid progenitor cell line, 32D, was obtained from RIKEN cell bank (Tsukuba, Japan) and maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS; Gibco-BRL, Gaithersburg, MD) and 1.0 ng/mL recombinant murine IL-3. MOLM-13, a cell line of AML-M5a with FLT3/ITD, was kindly provided by Dr Yoshinobu Matsuo (Fujisaki Cell Center, Okayama, Japan).8,31 Other leukemia cell lines have been maintained in our laboratory.32 MOLM-13, HL60, K562, and NB4 cell lines were cultured in RPMI 1640 medium supplemented with 10% FCS.
Antibodies, immunoprecipitation, and immunoblot analysis
Antiphospho Bad (Ser112 and Ser136), antiphospho MAPK, anti-MAPK, antiphospho Akt, and anti-Akt antibodies were obtained from New England Biolabs (Beverly, MA). Anti-FLT3, anti-Bax, anti-STAT5a, anti-Bad, and anti–Mcl-1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antiactin antibody was from Roche Diagnostics (Tokyo, Japan). Antimouse Bcl-2, antihuman Bcl-2, and anti–Bcl-XL antibodies were from Pharmingen (San Diego, CA). Antiphosphotyrosine antibody 4G10 and anti-Bak antibody were from Upstate Biotechnology (Lake Placid, NY). Anti-Bid and anti-Bim antibodies were kindly provided by Dr Stanley J. Korsmeyer (Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA) and Dr Toshiya Inaba (Hiroshima University, Hiroshima, Japan), respectively.26,33 Immunoprecipitation and immunoblotting analyses were described previously.34
Transfection of FLT3 and Bad into 32D cells
Transfection of WtFLT3 and different types of FLT3/ITD into 32D cells and the selection of each clone on the basis of flow cytometry and Western blotting analyses were described previously.13,14 Wild-type Bad (WtBad) and double-mutated Bad (DM-Bad) expression vectors (Ser112Ala and Ser136Ala), which were constructed by cloning Bad cDNA into the EcoRI site of pSSFV, were kindly provided by Dr Stanley J. Korsmeyer.23 Stable transfection of each Bad expression vector into WtFLT3-32D cells was performed with TransFast Transfection Reagent (Promega, Madison, WI), and the neomycin selection was carried out with a higher concentration than in the FLT3 transfection. Each clone expressing a comparable level of Bad was selected on the basis of the results of Western blotting analysis of whole-cell lysates and immunoprecipitation with anti-Bad antibody.
Growth inhibition assay and determination of apoptosis
Each stably transfected clone (1.0 × 105/mL) was seeded in 24-well culture plates. After overnight culture, tyrosine kinase inhibitors were added at various concentrations in triplicate. Viable cells were counted using the trypan blue dye–exclusion assay. The DNA histogram assay was described previously.34
Reverse-transcription polymerase chain reaction (PCR) assay
Total RNA from cells was extracted using the QIAamp RNA Blood Mini Kit (QIAGEN, Tokyo, Japan). Each cDNA was synthesized from each RNA using random primer and Molony murine leukemia virus reverse transcriptase (SUPERSCRIPT II; Invitrogen, Carlsbad, CA). The following primers of Bcl-XL and β-actin were synthesized by Nihon Gene Research Laboratories (Miyagi, Japan): 5′ TGG TCG ACT TTC TCT CCT AC 3′ (5′Bcl-XL), 5′ AGA GAT CCA CAA AGA TGT CC 3′ (3′Bcl-XL), 5′ GTG GGC CGC CCT AGG CAC CAG 3′ (5′β-actin), and 5′ CTC TTT GAT GTC ACG CAC GAT TTC 3′ (3′β-actin).35 The cDNA of Bcl-XL and β-actin was amplified by PCR using 30 cycles under the same conditions, except for the annealing temperatures, which were 54°C and 56°C, respectively. The PCR products were size-separated by electrophoresis on 2% agarose gels and detected by ultraviolet transillumination of ethidium bromide–stained DNA.
Treatment with antisense oligonucleotides
The antisense and sense morpholino oligonucleotides for Bcl-XL were designed and synthesized by Gene Tools (Philomath, OR) as follows: 5′ CCG GTT GCT CTG AGA CAT TTT TAT A 3′ (antisense) and 5′ TAT AAA AAT GTC TCA GAG CAA CCG G 3′ (sense). These sequences were determined by the following guidelines: (1) Select a target sequence in the postspliced mRNA in the region from the 5′ cap to about 25 bases 3′ to the AUG translational start site. (2) Make sure the selected sequence has little or no selfcomplementarity. (3) The oligonucleotides may show reduced water solubility if they contain more than about 36% guanines or more than 3 contiguous guanines. (4) For most applications, 25-mer is recommended. Antisense oligonucleotide assays were performed using EPEI Special Delivery solution (Gene Tools). Each cell line was preincubated with antisense or sense oligonucleotides using 24-well culture plates.
Results
WtFLT3 signaling pathways transduce antiapoptotic but not proliferative signals in WtFLT3-32D cells
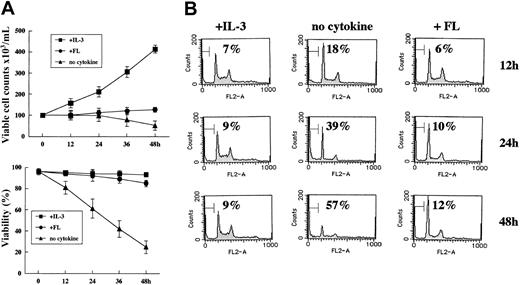
To evaluate the effect of WtFLT3 signaling on cell survival and proliferation, we used 32D cells stably transfected with WtFLT3 expression vector. In the presence of IL-3, WtFLT3-32D cell growth was highly comparable with that of parental 32D cells and resulted in apoptosis by the deprivation of IL-3 (Figure 1A). By adding FL, WtFLT3-32D cells were rescued from apoptosis, but the number of viable cells stayed at almost the same level. Analysis of the DNA contents using flow cytometry showed that the sub-G1 population increased by cytokine deprivation in WtFLT3/32D cells (Figure 1B). When FL was added to the culture medium without IL-3, the percentage of the sub-G1 population did not increase. These results indicated that signaling from WtFLT3 stimulated antiapoptotic pathways but not proliferative pathways.
WtFLT3 signaling pathways transduce antiapoptotic but not proliferative signals in WtFLT3-32D cells. (A) The growth and viability of WtFLT3-32D cells after IL-3 deprivation. Cells were washed 3 times with RPMI 1640 containing 10% FCS and resuspended with the same medium in the presence or absence of IL-3 (1.0 ng/mL) or FLT3 ligand (FL, 50 ng/mL). Viable cells were counted at the indicated times using the trypan blue dye–exclusion assay. The data shown are means and SDs of 3 independent experiments. (B) DNA histogram of WtFLT3-32D cells (cultured with IL-3, no cytokine, and FL). The percentage of the sub-G1 fraction is indicated.
WtFLT3 signaling pathways transduce antiapoptotic but not proliferative signals in WtFLT3-32D cells. (A) The growth and viability of WtFLT3-32D cells after IL-3 deprivation. Cells were washed 3 times with RPMI 1640 containing 10% FCS and resuspended with the same medium in the presence or absence of IL-3 (1.0 ng/mL) or FLT3 ligand (FL, 50 ng/mL). Viable cells were counted at the indicated times using the trypan blue dye–exclusion assay. The data shown are means and SDs of 3 independent experiments. (B) DNA histogram of WtFLT3-32D cells (cultured with IL-3, no cytokine, and FL). The percentage of the sub-G1 fraction is indicated.
Changes in the expression and phosphorylation of Bcl-2 family members after deprivation or stimulation of cytokines in WtFLT3-32D cells
The phosphorylation status of FLT3 and STAT5a was down-regulated by IL-3 deprivation (Figure 2A). FLT3 phosphorylation was rescued by FL stimulation but STAT5a phosphorylation was not. To explore the antiapoptotic signaling from WtFLT3, we examined the expression level of Bcl-2 family members. By cytokine deprivation, the expression levels of Bcl-2, Bax, Bak, and Mcl-1 were unchanged (Figure 2B). The expression level of Bcl-XL was down-regulated and not rescued by FL stimulation, suggesting that Bcl-XL expression was maintained by IL-3 signaling. In WtFLT3-32D cells, the expressions of Bim and Bid were undetectable in Western blotting analysis even after cytokine deprivation or FL stimulation (data not shown). The expression of endogenous Bad was undetectable in Western blotting analysis of whole cell lysates. Using immunoprecipitation by anti-Bad antibody, the phosphorylation status of Bad (Ser112 and Ser136) was down-regulated by IL-3 deprivation but rescued by FL stimulation (Figure 2C). These results suggested that the phosphorylation of Bad was associated with the survival of WtFLT3-32D cells by FL stimulation.
Changes in the expression and phosphorylation of Bcl-2 family members after deprivation or stimulation of cytokines in WtFLT3-32D cells. (A) Phosphorylation of FLT3 and STAT5a in WtFLT3-32D cells. Cells were washed 3 times with RPMI 1640 containing 10% FCS and resuspended with the same medium in the presence or absence of FLT3 ligand (FL, 50 ng/mL). After 0, 12, and 24 hours, each cell lysate was immunoprecipitated by anti-FLT3 antibody or anti-STAT5a antibody, and the immunocomplex was analyzed by immunoblotting with antiphosphotyrosine antibody 4G10. (B) The expression levels of Bcl-2 family members in WtFLT3-32D cells. After 0, 12, 24, and 36 hours, each sample was lysed and Western blotting analysis was performed with anti–Bcl-2, Bcl-XL, Bax, Bak, and Mcl-1 antibodies. (C) Immunoprecipitation by anti-Bad antibody at each time and immunoblotting by antiphospho Bad (Ser112) and antiphospho Bad (Ser136) specific antibodies were carried out.
Changes in the expression and phosphorylation of Bcl-2 family members after deprivation or stimulation of cytokines in WtFLT3-32D cells. (A) Phosphorylation of FLT3 and STAT5a in WtFLT3-32D cells. Cells were washed 3 times with RPMI 1640 containing 10% FCS and resuspended with the same medium in the presence or absence of FLT3 ligand (FL, 50 ng/mL). After 0, 12, and 24 hours, each cell lysate was immunoprecipitated by anti-FLT3 antibody or anti-STAT5a antibody, and the immunocomplex was analyzed by immunoblotting with antiphosphotyrosine antibody 4G10. (B) The expression levels of Bcl-2 family members in WtFLT3-32D cells. After 0, 12, 24, and 36 hours, each sample was lysed and Western blotting analysis was performed with anti–Bcl-2, Bcl-XL, Bax, Bak, and Mcl-1 antibodies. (C) Immunoprecipitation by anti-Bad antibody at each time and immunoblotting by antiphospho Bad (Ser112) and antiphospho Bad (Ser136) specific antibodies were carried out.
Survival function of WtFLT3 is mediated by Bad phosphorylation at Ser112 via MEK and Ser136 via Akt
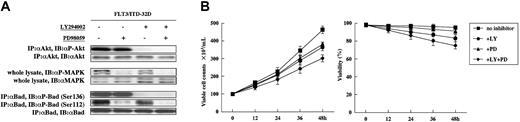
To elucidate the signaling molecules between WtFLT3 and Bad, we examined the effects of several kinase inhibitors. In Western blotting analysis, treatment with PI3K inhibitor, LY294002, dephosphorylated Akt and Bad at Ser136 (Figure 3A). On the other hand, treatment with MEK inhibitor, PD98059, dephosphorylated MAPK and Bad at Ser112. The single treatment with LY294002 or PD98059 had a marginal effect on the viability of WtFLT3-32D cells, and their combination had a synergistic effect (Figure 3B). These results suggested that both MEK/MAPK and PI3K/Akt pathways, which converge on the phosphorylation of Bad, mainly mediated the survival of WtFLT3-32D cells. To study whether the phosphorylation of Bad contributes to antiapoptosis in WtFLT3-32D cells, we generated stable transfectants overexpressing wild-type Bad (WtBad) and mutated Bad harboring mutations of both the phosphorylation sites (DM-Bad) (Figure 3C). In the presence of IL-3 stimulation, Bad expression between WtBad clones and DM-Bad clones was equivalent in Western blotting analysis of whole cell lysates. Although the Bad phosphorylation of WtBad clones was down-regulated by cytokine deprivation and fully rescued by FL stimulation in Western blotting analysis of whole cell lysates, that of DM-Bad clones was not detected by FL stimulation (data not shown). In the presence of FL stimulation, the viability of DM-Bad clones was lower than that of WtBad clones (Figure 3D). Taken together, the phosphorylation of Bad is implicated in the survival function of WtFLT3 in WtFLT3-32D cells.
Survival function of WtFLT3 is mediated by Bad phosphorylation at Ser112 via MEK and Ser136 via Akt. (A) After treatment with 10 μM LY294002 (LY) and 10 μM PD98059 (PD) for 4 hours, each sample was lysed and Western blotting analysis was performed with each antibody. (B) Inhibitory effects of kinase inhibitors on the viability of WtFLT3-32D cells. Treatment with 10 μM LY and 10 μM PD was used. The data shown are means and SDs of 3 independent experiments. (C) Establishment of 3 clones (vector control [▪], wild-type Bad [WtBad; ▧], and double-mutated Bad [DM-Bad; □]) in WtFLT3-32D cells. In the steady state, they were cultured with IL-3 stimulation. (D) IL-3 was washed out and each cell count was carried out at the indicated times after 50 ng/mL FL stimulation. The data shown are the means and SDs of 3 separate experiments.
Survival function of WtFLT3 is mediated by Bad phosphorylation at Ser112 via MEK and Ser136 via Akt. (A) After treatment with 10 μM LY294002 (LY) and 10 μM PD98059 (PD) for 4 hours, each sample was lysed and Western blotting analysis was performed with each antibody. (B) Inhibitory effects of kinase inhibitors on the viability of WtFLT3-32D cells. Treatment with 10 μM LY and 10 μM PD was used. The data shown are means and SDs of 3 independent experiments. (C) Establishment of 3 clones (vector control [▪], wild-type Bad [WtBad; ▧], and double-mutated Bad [DM-Bad; □]) in WtFLT3-32D cells. In the steady state, they were cultured with IL-3 stimulation. (D) IL-3 was washed out and each cell count was carried out at the indicated times after 50 ng/mL FL stimulation. The data shown are the means and SDs of 3 separate experiments.
Antiapoptotic effect of FLT3/ITD is different from that of WtFLT3
To compare FLT3/ITD's survival signaling with WtFLT3's, we evaluated the effect of FLT3/ITD in 32D cells. As observed in WtFLT3/32D cells, phospho-Bad (Ser136) and phospho-Akt, and phospho-Bad (Ser112) and phospho-MAPK were down-regulated by LY294002 and PD98059, respectively, in FLT3/ITD-32D cells (Figure 4A). The expression levels of Bcl-2 and Bcl-XL were unchanged by these treatments (data not shown). FLT3/ITD-32D cells proliferated independently of IL-3 stimulation (Figure 4B). By the combined treatment with LY294002 and PD98059 that induced apoptosis in WtFLT3-32D cells, the growth and viability of FLT3/ITD-32D cells were partially inhibited. These results suggested that antiapoptotic signaling of FLT3/ITD was different from that of WtFLT3.
Antiapoptotic effect of FLT3/ITD is different from that of WtFLT3. (A) After 4-hour treatment of inhibitors, each sample was lysed and subjected to immunoblotting. The experiment was the same as in Figure 3A. (B) Inhibitory effects of kinase inhibitors on the growth and viability of FLT3/ITD-32D cells. The data shown are means and SDs of 3 independent experiments.
Antiapoptotic effect of FLT3/ITD is different from that of WtFLT3. (A) After 4-hour treatment of inhibitors, each sample was lysed and subjected to immunoblotting. The experiment was the same as in Figure 3A. (B) Inhibitory effects of kinase inhibitors on the growth and viability of FLT3/ITD-32D cells. The data shown are means and SDs of 3 independent experiments.
Inhibition of FLT3/ITD kinase activity down-regulates the expression of Bcl-XL and Bad phosphorylation in FLT3/ITD-32D cells
A tyrosine kinase inhibitor, AG1296, has been reported to inhibit both WtFLT3 and FLT3/ITD tyrosine kinase activity and induce apoptosis in FLT3-mediated transformed leukemia cells.27,28 As expected, treatment with AG1296 induced apoptosis in both FL-cultured WtFLT3-32D cells and FLT3/ITD-32D cells, but not in parental 32D cells (Figure 5A). The AG1296 treatment completely inhibited the phosphorylation of FLT3 and STAT5a (Figure 5B). The growth-inhibitory effect of AG1296 on FLT3/ITD-32D cells was completely abrogated by the addition of IL-3 (Figure 5C). By the treatment of FLT3/ITD-32D cells with AG1296, not only phospho-Bad (Ser112 and Ser136) (Figure 6A), but also the expression level of Bcl-XL, was down-regulated (Figure 6B). In FLT3/ITD-32D cells, the expressions of Bim and Bid were undetectable in Western blotting analysis even after the treatment with AG1296 (data not shown). Reverse-transcription PCR assay showed that the mRNA level of Bcl-XL was down-regulated by AG1296 treatment in FLT3/ITD-32D cells (Figure 6C). These results showed that the expression level of Bcl-XL was involved in the antiapoptotic effect of FLT3/ITD and suggested that different antiapoptotic signaling pathways are activated by FLT3/ITD in comparison with WtFLT3.
Inhibition of FLT3/ITD kinase activity induces apoptosis in FLT3/ITD-32D cells. (A) Inhibitory effects of AG1296 on the viability of parental 32D, WtFLT3-32D, and FLT3/ITD-32D cells. Cells were cultured with medium in the presence or absence of IL-3 (1.0 ng/mL) or FL (50 ng/mL). AG1296 was added to the culture medium at the indicated concentrations, and viable cells were counted by the trypan blue dye–exclusion assay after 48-hour culture. (B) Dephosphorylation of FLT3 and STAT5a in FLT3/ITD-32D cells treated with AG1296. Cells were treated with AG1296 at 0.3, 1.0, and 3.0 μ for 4 hours. Each cell lysate was immunoprecipitated by anti-FLT3 antibody or anti-STAT5a antibody, and the immunocomplex was analyzed by immunoblotting with the indicated antibodies. (C) Effect of IL-3 on FLT3/ITD-32D treated with AG1296. IL-3 (1.0 ng/mL) and AG1296 (1.0 μM) were used. The data shown are means and SDs of 3 independent experiments.
Inhibition of FLT3/ITD kinase activity induces apoptosis in FLT3/ITD-32D cells. (A) Inhibitory effects of AG1296 on the viability of parental 32D, WtFLT3-32D, and FLT3/ITD-32D cells. Cells were cultured with medium in the presence or absence of IL-3 (1.0 ng/mL) or FL (50 ng/mL). AG1296 was added to the culture medium at the indicated concentrations, and viable cells were counted by the trypan blue dye–exclusion assay after 48-hour culture. (B) Dephosphorylation of FLT3 and STAT5a in FLT3/ITD-32D cells treated with AG1296. Cells were treated with AG1296 at 0.3, 1.0, and 3.0 μ for 4 hours. Each cell lysate was immunoprecipitated by anti-FLT3 antibody or anti-STAT5a antibody, and the immunocomplex was analyzed by immunoblotting with the indicated antibodies. (C) Effect of IL-3 on FLT3/ITD-32D treated with AG1296. IL-3 (1.0 ng/mL) and AG1296 (1.0 μM) were used. The data shown are means and SDs of 3 independent experiments.
Inhibition of FLT3/ITD kinase activity down-regulates the expression of Bcl-XL and Bad phosphorylation in FLT3/ITD-32D cells. (A) In treatment with AG1296 at increasing doses for 4 hours, each cell lysate was immunoprecipitated by anti-Bad antibody and immunoblotted by antiphospho Bad specific antibodies. (B) In parental 32D cells stimulated with IL-3 and FLT3/ITD-32D cells, each cell lysate at the indicated times was immunoblotted by anti–Bcl-2, Bcl-XL, Bax and Bak, and Mcl-1 antibodies. (C) The mRNA level of Bcl-XL was examined by reverse-transcription PCR assay using 30 amplification cycles. For the control of RNA recovery, β-actin expression was used.
Inhibition of FLT3/ITD kinase activity down-regulates the expression of Bcl-XL and Bad phosphorylation in FLT3/ITD-32D cells. (A) In treatment with AG1296 at increasing doses for 4 hours, each cell lysate was immunoprecipitated by anti-Bad antibody and immunoblotted by antiphospho Bad specific antibodies. (B) In parental 32D cells stimulated with IL-3 and FLT3/ITD-32D cells, each cell lysate at the indicated times was immunoblotted by anti–Bcl-2, Bcl-XL, Bax and Bak, and Mcl-1 antibodies. (C) The mRNA level of Bcl-XL was examined by reverse-transcription PCR assay using 30 amplification cycles. For the control of RNA recovery, β-actin expression was used.
The expression of Bcl-XL and Bad phosphorylation contribute to survival function of FLT3/ITD in FLT3/ITD-32D cells
In order to evaluate whether the expression of Bcl-XL contributes to the antiapoptosis effect of FLT3/ITD, we transfected Bcl-XL/antisense (Bcl-XL/AS) or Bcl-XL/sense (Bcl-XL/S) oligonucleotides into FLT3/ITD-32D cells. Bcl-XL/AS treatment suppressed the expression of Bcl-XL, while sense oligonucleotides did not in FLT3/ITD-32D cells (Figure 7A). By an additional 4-hour treatment with LY294002 and PD98059 after 24-hour treatment with each oligonucleotide, the phosphorylation of Bad was down-regulated and the expression of Bcl-XL was unchanged. The combined treatment with Bcl-XL/AS and the kinase inhibitors induced additional apoptosis in FLT3/ITD-32D cells (Figure 7B). These results showed that the expression of Bcl-XL and Bad phosphorylation contributed to the survival function of FLT3/ITD.
The expression of Bcl-XL and Bad phosphorylation contribute to survival function of FLT3/ITD in FLT3/ITD-32D cells. (A) In FLT3/ITD-32D cells, after 24-hour treatment with Bcl-XL/antisense (Bcl-XL/AS) or Bcl-XL/sense (Bcl-XL/S) oligonucleotides and an additional 4-hour treatment with or without kinase inhibitors (10 μM LY294002 [LY] and 10 μM PD98059 [PD]), each sample was lysed and immunoblotted by anti–Bcl-XL antibody. Each cell lysate was immunoprecipitated by anti-Bad antibody and immunoblotted by antiphospho Bad specific antibodies. Immunoblotting by antiactin antibody is for the control of total protein level. (B) Inhibitory effect of Bcl-XL/AS and kinase inhibitors on the viability of FLT3/ITD-32D cells. After 24-hour treatment with each oligonucleotide, LY (10 μM) and PD (10 μM) were or were not applied. The data shown are means and SDs of 3 independent experiments.
The expression of Bcl-XL and Bad phosphorylation contribute to survival function of FLT3/ITD in FLT3/ITD-32D cells. (A) In FLT3/ITD-32D cells, after 24-hour treatment with Bcl-XL/antisense (Bcl-XL/AS) or Bcl-XL/sense (Bcl-XL/S) oligonucleotides and an additional 4-hour treatment with or without kinase inhibitors (10 μM LY294002 [LY] and 10 μM PD98059 [PD]), each sample was lysed and immunoblotted by anti–Bcl-XL antibody. Each cell lysate was immunoprecipitated by anti-Bad antibody and immunoblotted by antiphospho Bad specific antibodies. Immunoblotting by antiactin antibody is for the control of total protein level. (B) Inhibitory effect of Bcl-XL/AS and kinase inhibitors on the viability of FLT3/ITD-32D cells. After 24-hour treatment with each oligonucleotide, LY (10 μM) and PD (10 μM) were or were not applied. The data shown are means and SDs of 3 independent experiments.
FLT3 kinase inhibitor induces apoptosis while suppressing Bcl-XL expression and Bad phosphorylation in MOLM-13 cells
To evaluate whether the results in FLT3/ITD transfectants are applicable to human leukemia cell lines, we used the following cell lines: FLT3/ITD-expressing MOLM-13, WtFLT3-expressing NB4, FLT3-negative HL60, and K562.8 Treatment with AG1296 induced apoptosis selectively in MOLM-13 cells (Figure 8A). By the treatment with AG1296, the expression levels of Bcl-XL and Bad phosphorylation (Ser112 and Ser136) were down-regulated in MOLM-13 cells (Figure 8B-C).
FLT3 kinase inhibitor induces apoptosis while suppressing Bcl-XL expression and Bad phosphorylation in MOLM-13 cells. (A) Inhibitory effect of AG1296 on the viability of human leukemia cell lines. AG1296 was added at the indicated concentrations, and viable cells were counted by the trypan blue dye–exclusion assay after 48-hour culture. The data shown are means and SDs of 3 independent experiments. (B) In MOLM-13 cells, each cell lysate at the indicated times was immunoblotted by antihuman Bcl-2 and Bcl-XL antibodies. Immunoblotting by antiactin antibody is for the control of total protein level. WL indicates whole lysate. (C) In treatment with AG1296 at increasing doses for 4 hours, each cell lysate was immunoprecipitated by anti-Bad antibody and immunoblotted by antiphospho Bad specific antibodies.
FLT3 kinase inhibitor induces apoptosis while suppressing Bcl-XL expression and Bad phosphorylation in MOLM-13 cells. (A) Inhibitory effect of AG1296 on the viability of human leukemia cell lines. AG1296 was added at the indicated concentrations, and viable cells were counted by the trypan blue dye–exclusion assay after 48-hour culture. The data shown are means and SDs of 3 independent experiments. (B) In MOLM-13 cells, each cell lysate at the indicated times was immunoblotted by antihuman Bcl-2 and Bcl-XL antibodies. Immunoblotting by antiactin antibody is for the control of total protein level. WL indicates whole lysate. (C) In treatment with AG1296 at increasing doses for 4 hours, each cell lysate was immunoprecipitated by anti-Bad antibody and immunoblotted by antiphospho Bad specific antibodies.
The expression of Bcl-XL and Bad phosphorylation contribute to survival in MOLM-13 cells
To evaluate whether the expression of Bcl-XL contributes to survival in MOLM-13 cells, we transfected Bcl-XL/AS or Bcl-XL/S oligonucleotides into MOLM-13 cells. The expression level of Bcl-XL was down-regulated by Bcl-XL/AS treatment (Figure 9A). By an additional 4-hour treatment with LY294002 and PD98059 after 24-hour treatment with each oligonucleotide, the phosphorylation of Bad was down-regulated and the expression of Bcl-XL was unchanged. The combined treatment with Bcl-XL/AS and the kinase inhibitors induced additional apoptosis in MOLM-13 cells (Figure 9B). These results showed that the expression of Bcl-XL and Bad phosphorylation contributed to survival in a FLT3/ITD-expressing human leukemia cell line, MOLM-13.
The expression of Bcl-XL and Bad phosphorylation contribute to survival in MOLM-13 cells. (A) In MOLM-13 cells, after 24-hour treatment with Bcl-XL/antisense (Bcl-XL/AS) or Bcl-XL/sense (Bcl-XL/S) oligonucleotides and an additional 4-hour treatment with or without kinase inhibitors (10 μM LY294002 [LY] and 10 μM PD98059 [PD]), each sample was lysed and immunoblotted by anti–Bcl-XL antibody. Each cell lysate was immunoprecipitated by anti-Bad antibody and immunoblotted by antiphospho Bad specific antibodies. Immunoblotting by antiactin antibody is for the control of total protein level. (B) Inhibitory effect of Bcl-XL/AS and kinase inhibitors on the viability of MOLM-13 cells. After 24-hour treatment with each oligonucleotide, LY (10 μM) and PD (10 μM) were or were not applied. The data shown are means and SDs of 3 independent experiments.
The expression of Bcl-XL and Bad phosphorylation contribute to survival in MOLM-13 cells. (A) In MOLM-13 cells, after 24-hour treatment with Bcl-XL/antisense (Bcl-XL/AS) or Bcl-XL/sense (Bcl-XL/S) oligonucleotides and an additional 4-hour treatment with or without kinase inhibitors (10 μM LY294002 [LY] and 10 μM PD98059 [PD]), each sample was lysed and immunoblotted by anti–Bcl-XL antibody. Each cell lysate was immunoprecipitated by anti-Bad antibody and immunoblotted by antiphospho Bad specific antibodies. Immunoblotting by antiactin antibody is for the control of total protein level. (B) Inhibitory effect of Bcl-XL/AS and kinase inhibitors on the viability of MOLM-13 cells. After 24-hour treatment with each oligonucleotide, LY (10 μM) and PD (10 μM) were or were not applied. The data shown are means and SDs of 3 independent experiments.
Discussion
In this study, we analyzed the antiapoptotic pathways from FLT3 to Bcl-2 family members regarding expression levels and phosphorylation status. In a murine myeloid cell line, 32D, IL-3 deprivation induced apoptosis following the down-regulation of Bcl-XL and the dephosphorylation of Bad. We showed that FLT3 signal did not restore the down-regulation of Bcl-XL but is associated with antiapoptosis through Bad phosphorylation.
The FLT3/ITD mutation, frequently observed in AML, is a poor prognostic factor.9,10,12 Identifying the biologic differences between wild-type and mutant FLT3 is important in understanding the oncogenic properties of FLT3 in leukemia cells and developing a molecular therapy targeted toward FLT3.36,37 In this study, we showed that the dephosphorylation of Bad was insufficient for apoptosis and the down-regulation of Bcl-XL was needed to induce apoptosis in FLT3/ITD-32D cells. However, the inhibitory effect of Bcl-XL/AS treatment on the viability was partial and Bcl-XL-targeted therapy might produce toxicity to normal tissues because Bcl-XL is critical for the survival of hematopoietic precursors and neural cells.20,21
We also showed that inhibition of FLT3/ITD kinase activity by FLT3 inhibitor, AG1296, down-regulated not only the phosphorylation of Bad but also the phosphorylation of STAT5a and the expression of Bcl-XL, and induced apoptosis in FLT3/ITD-32D cells. It is reported that STAT5 up-regulates transcription of the Bcl-XL gene in IL-3–dependent cell lines and may be involved in the regulation of apoptosis in hematopoietic cells.22,38,39 Bcr-Abl activates transcription of the Bcl-XL gene through STAT540 and blockade of the Bcr-Abl kinase activity induces apoptosis of chronic myelogenous leukemia cells by suppressing STAT5-dependent expression of Bcl-XL.41 Our results also imply the aberrant activation of the STAT5a/Bcl-XL pathway via FLT3/ITD, although we have not examined the direct regulation of Bcl-XL expression via STAT5a or ruled out the other pathways that regulate Bcl-XL expression. Several groups have shown the activation of STAT5 by FLT3/ITD,15,16 but the exact mechanism-involved remains unclear. It is reported that the platelet-derived growth factor (PDGF) receptor induces the activation of STAT5 directly.42 On the other hand, cytoplasmic tyrosine kinases belonging to the Src family (eg, Hck and Lyn) mediate the activation of STAT5 in order to transduce signals from receptors activated by IL-3, erythropoietin, and epidermal growth factor.43-45 Hck couples Bcr-Abl to STAT5 activation in myeloid leukemia cells.46 Development of mutant FLT3-specific inhibitors by cell-based drug screening and examination of DNA microarray assays will be useful in elucidating the molecules involved in the aberrant activation. Targeting such molecules will be useful for selective treatment. For example, we have reported that FLT3/ITD forms a complex with a molecular chaperone, heat shock protein 90 (Hsp90).34 Although further study is required, Hsp90 might be associated with the formation of large mobilized complexes of signal transduction molecules as a scaffolding protein and targeting Hsp90 might be promising for the treatment of AML.
We showed that AG1296 induced apoptosis in not only FLT3/ITD-32D cells but also a human leukemia cell line expressing FLT3/ITD, MOLM-13. AG1296 inhibits PDGF-induced cell proliferation, and does not interfere with PDGF binding or with PDGF receptor dimerization but acts by competitively inhibiting the binding of adenosine triphosphate (ATP).47 The FLT3 receptor is closely related to the PDGF receptor and the ATP-binding domains are highly conserved among the class III receptors. We have observed that AG1296 has little effect on 32D cells harboring an activating mutation within the activation loop of FLT3 (data not shown),48 which also demonstrates how AG1296 functions on FLT3. Targeting the activated FLT3, which is located at the most upstream end of the signals, is a more rational approach than targeting downstream cascades. Now several antagonists of FLT3 kinase activity have been identified,49-51 and some clinical phase studies are under way.37 It will be of considerable interest to determine whether FLT3 can be successfully inhibited in leukemic cells upon treatment of AML patients in vivo and whether this has a beneficial therapeutic effect.
In conclusion, we have described a diversity of FLT3/ITD-induced antiapoptotic pathways that might contribute to malignant progression by conferring a survival advantage through the suppression of apoptotic cell death. The diversity of pathways may represent targets for drug discovery with the potential of inducing selective cell death of human leukemia cells.
Prepublished online as Blood First Edition Paper, July 3, 2003; DOI 10.1182/blood-2002-12-3813.
Supported by Grants-in-Aid from the Japanese Ministry of Health, Labor and Welfare, the Ministry of Education, Culture, Sports, Science and Technology, Novartis Foundation for the Promotion of Science, and Uehara Memorial Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We gratefully acknowledge Dr Stanley J. Korsmeyer, Dr Oliver Rosnet, Dr Toshio Kitamura, Dr Toshiya Inaba, Dr Yoshinobu Matsuo, and Kirin Brewery for providing us with materials. We also thank M. Agr. Manabu Ninomiya, Ms Kyoko Aoyama, Ms Yoko Yokozawa, and Ms Manami Kira for their technical and secretarial assistance.


![Figure 3. Survival function of WtFLT3 is mediated by Bad phosphorylation at Ser112 via MEK and Ser136 via Akt. (A) After treatment with 10 μM LY294002 (LY) and 10 μM PD98059 (PD) for 4 hours, each sample was lysed and Western blotting analysis was performed with each antibody. (B) Inhibitory effects of kinase inhibitors on the viability of WtFLT3-32D cells. Treatment with 10 μM LY and 10 μM PD was used. The data shown are means and SDs of 3 independent experiments. (C) Establishment of 3 clones (vector control [▪], wild-type Bad [WtBad; ▧], and double-mutated Bad [DM-Bad; □]) in WtFLT3-32D cells. In the steady state, they were cultured with IL-3 stimulation. (D) IL-3 was washed out and each cell count was carried out at the indicated times after 50 ng/mL FL stimulation. The data shown are the means and SDs of 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/8/10.1182_blood-2002-12-3813/6/m_h82035076003.jpeg?Expires=1769209100&Signature=3wW53zKzGJOhkcnHAFpnJFA4mB6FWk3eftot1y6c6Qbmkj2pvOD8macF1XI7FVloosLova~tLyyQP3zXEe4Y03Rz231rIkWPlJWJl12CJ4RQqQeUBPNdniVI579M80U5Nw72pN5V4-jHARM2LjzqMXROw8S2onD4ly8LoqJgId8RozuxFOSeWIvcy~YL6umO0GKY2YbtqlOzjo5u9L3s9oZqg~G562XtEJZ5je9D066tshWYiZNj~d-ih0D8hDtMtjCRK92jwFeSYI5HJIM0HOOVfr41fDWrHULwE6w21xUA6M06uGuLtRilo41AIjQPkDLeZOZ8q2WlkTVaa4WOsw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 7. The expression of Bcl-XL and Bad phosphorylation contribute to survival function of FLT3/ITD in FLT3/ITD-32D cells. (A) In FLT3/ITD-32D cells, after 24-hour treatment with Bcl-XL/antisense (Bcl-XL/AS) or Bcl-XL/sense (Bcl-XL/S) oligonucleotides and an additional 4-hour treatment with or without kinase inhibitors (10 μM LY294002 [LY] and 10 μM PD98059 [PD]), each sample was lysed and immunoblotted by anti–Bcl-XL antibody. Each cell lysate was immunoprecipitated by anti-Bad antibody and immunoblotted by antiphospho Bad specific antibodies. Immunoblotting by antiactin antibody is for the control of total protein level. (B) Inhibitory effect of Bcl-XL/AS and kinase inhibitors on the viability of FLT3/ITD-32D cells. After 24-hour treatment with each oligonucleotide, LY (10 μM) and PD (10 μM) were or were not applied. The data shown are means and SDs of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/8/10.1182_blood-2002-12-3813/6/m_h82035076007.jpeg?Expires=1769209100&Signature=nw~JQeOYT3eby0KQP8U15IrsGnNE1~ze1NrFsYk40gBirh44tmoIhxUSzIiFIEbpQOh~nsyxaFvysKZWZVsTY3rIwOMyNyW0NifMMrmlCCAj3mj31xXRu64Wxzn3QQMXjRFB5FBKbVoFTVTBIp6B9yS7Pj5NdcGPY3tFwGVkv9kiA3HE5C1LsJk2D8JZrDqny5hE9MyKRCGKV8T025yRu5KfbXBRRdiCjjqOWRl6Hmni4BLStSvn8oEe~8rm7vdf464mSMzcjVwPfpmQGqrtC4fbueAyu9D9LIN5XxfyClOu4ywtT3oiQRcyha52~OGCZ0r8wX9ZZQGDti8nQ182FQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 9. The expression of Bcl-XL and Bad phosphorylation contribute to survival in MOLM-13 cells. (A) In MOLM-13 cells, after 24-hour treatment with Bcl-XL/antisense (Bcl-XL/AS) or Bcl-XL/sense (Bcl-XL/S) oligonucleotides and an additional 4-hour treatment with or without kinase inhibitors (10 μM LY294002 [LY] and 10 μM PD98059 [PD]), each sample was lysed and immunoblotted by anti–Bcl-XL antibody. Each cell lysate was immunoprecipitated by anti-Bad antibody and immunoblotted by antiphospho Bad specific antibodies. Immunoblotting by antiactin antibody is for the control of total protein level. (B) Inhibitory effect of Bcl-XL/AS and kinase inhibitors on the viability of MOLM-13 cells. After 24-hour treatment with each oligonucleotide, LY (10 μM) and PD (10 μM) were or were not applied. The data shown are means and SDs of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/8/10.1182_blood-2002-12-3813/6/m_h82035076009.jpeg?Expires=1769209100&Signature=JNhBb-A0zk6KvDnSnRZT0F7gWL2ogzD6CBCz9XHWQDQoAW8-TQuCUU~AyAbmeLI41p9heSQCpklqOIzQ5Vy3Bt9ccC0FWYtC1ZtRbmQpufR03usfItjduI3px1jUn3fYLNq0-~z6hr4ReB3N4YXJMqOdWLrpSOFJq8TG5yLl576fDyPGAPpbc~PmKAFY4C9SkE-czI4v9vP1P-pxU-2PcFLezY51azOCtMmLTflGLjSyW~rxiDdNDytqhugA0GjTjoMWQ03pPmV8dPoYjnVaTyxn8oFxQj6cgeRuingiHtZPvZ9QCBKj3r2FoCXGTogtwCQPt0H5vYDfVK94yGxP2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal