Abstract
The CD11a/CD18 (leukocyte function-associated antigen 1 [LFA-1]) integrin mediates critical leukocyte adhesive interactions during immune and inflammatory responses. The CD11a promoter directs CD11a/CD18 integrin expression, and its activity in lymphoid cells depends on a functional RUNX1/AML-1–binding site (AML-110) within the MS7 sequence. We now report that MS7 contains a C/EBP-binding site (C/EBP-100), which overlaps with AML-110 and is bound by C/EBP factors in myeloid cells. C/EBP and RUNX/AML factors compete for binding to their respective cognate elements and bind to the CD11a promoter MS7 sequence in a cell lineage- and differentiation-dependent manner. In myeloid cells MS7 is primarily recognized by C/EBP factors in proliferating cells whereas RUNX/AML factors (especially RUNX3/AML-2) bind to MS7 in differentiated cells. RUNX3/AML-2 binding to the CD11a promoter correlates with increased RUNX3/AML-2 protein levels and enhanced CD11a/CD18 cell surface expression. The relevance of the AML-110 element is underscored by the ability of AML-1/ETO to inhibit CD11a promoter activity, thus explaining the low CD11a/CD18 expression in t(8;21)–containing myeloid leukemia cells. Therefore, the expression of the CD11a/CD18 integrin in myeloid cells is determined through the differential occupancy of the CD11a proximal promoter by transcription factors implicated in the pathogenesis of myeloid leukemia.
Introduction
CD11a/CD18 (leukocyte function-associated antigen 1 [LFA-1], αL/β2) is a member of the β2 integrin subfamily that mediates leukocyte interactions required for immune and inflammatory responses through the recognition of its counterreceptors CD50, CD54, and CD102.1,2 The functional relevance of CD11a/CD18 for leukocyte extravasation is underscored by the existence of the leukocyte adhesion deficiency syndrome, in which leukocytes exhibit a deficient expression of the leukocyte integrins and whose clinical symptoms are secondary to the lack of phagocyte migration into inflammatory sites.1,2 CD11a/CD18 also participates in T-cell and lymphoma metastasis,3 and ischemia-reperfusion syndromes, myocardial infarction, and allograft rejection have their origin in uncontrolled CD11a/CD18-dependent phagocyte extravasation into the tissues.4,5 CD11a/CD18 is exclusively expressed on cells of the hematopoietic lineage, and its expression is regulated during hematopoietic differentiation (reviewed by Corbí1 ). The proximal regulatory region of the CD11a gene confers leukocyte-restricted expression to reporter genes both in vitro and in vivo.6-10 The RUNX1/AML-1 transcription factor contributes to the tissue-specific activity of the CD11a promoter in lymphoid cells through recognition of the AML-110 element,11 and methylation and chromatin structure have also been implicated in the tissue-specific expression of CD11a.12 However, the involvement of RUNX factors in the basal and regulated expression of CD11a in myeloid cells has not been investigated.
The polyomavirus enhancer binding protein 2/core binding factor/acute myeloid leukemia (PEBP2/CBF/AML) family of heterodimeric (α/β) mammalian transcription factors includes 3 distinct α subunits (RUNX1/AML-1; RUNX3/AML-2; RUNX2/AML-3) and 1 β subunit (CBFβ, PEBP2β).13,14 The α subunits exhibit sequence-specific DNA-binding ability while the β subunit interacts with the α subunits and increases their DNA-binding affinity.15 RUNX1 and CBFβ are indispensable for the development of definitive hematopoiesis.16-20 The genes encoding the RUNX1/CBFβ transcription factor complex are frequent targets of chromosomal translocations in acute leukemias,14,15,21 as a high percentage of acute myeloid leukemia and B-lineage acute lymphoblastic leukemias have altered RUNX1 or CBFβ alleles. RUNX1 proteins are widely expressed in cells of the hematopoietic lineage and activate transcription of myeloid- and lymphoid-specific genes.13,14,22 Although RUNX factors are relatively weak transcriptional activators14,22 and may function as transcriptional repressors and in epigenetic silencing,23-26 RUNX1 potently enhances transcription rates through physical and functional cooperation with Myb,27,28 members of the C/EBP29,30 and Ets31,32 transcription factor families, and several transcriptional coactivators.33-35 RUNX3, which is also expressed in lymphoid and myeloid cells,13,14,22 critically contributes to development and survival of dorsal root ganglia neurons36,37 and functions as a tumor-suppressor gene in gastric cancers.38
The CCAAT/enhancer binding protein (C/EBP) family comprises 6 transcription factors (α, β, γ, δ, ϵ, and CHOP), which bind DNA as homodimers and heterodimers and whose expression within the hematopoietic system is largely restricted to the myeloid lineage.39,40 C/EBPα plays a critical role in neutrophilic differentiation41,42 while C/EBPβ and C/EBPϵ are mainly involved in regulating specialized functions and terminal differentiation of macrophages and granulocytes, respectively.43,44 C/EBPα is barely detectable on peripheral blood monocytes and highly expressed in neutrophils, while C/EBPϵ is preferentially expressed on CD34+ cells and during granulocytic differentiation.40,45 In fact, conditional expression of C/EBPα in bipotential myeloid progenitors is sufficient to trigger neutrophilic differentiation.46 Myeloid cells express 2 alternative isoforms of C/EBPα, C/EBPα42 and C/EBPα30, which differ in transcriptional activity and are generated by a ribosome-scanning mechanism.40 C/EBPα is highly expressed in proliferating myelomonocytic cell lines, and its expression is down-regulated during monocytic and erythroid, but not neutrophilic, differentiation.40,45 Conversely, C/EBPβ and C/EBPδ expression is up-regulated during monocytic differentiation.45 C/EBP proteins (α, β, and ϵ) regulate the promoters of several myeloid genes, including those of the granulocyte-macrophage colony-stimulating factor (GM-CSF), G-CSF, and M-CSF receptors.39,40,45
In the present paper we report that RUNX and C/EBP factors bind in a mutually exclusive manner to overlapping binding sites within the CD11a promoter MS7 element, whose integrity is essential for CD11a promoter activity in lymphoid and myeloid cells. Occupancy of the MS7 element is regulated during myeloid differentiation, with RUNX3 binding correlating with increased CD11a integrin cell surface expression. Therefore, CD11a expression in lymphoid and myeloid cells is controlled by RUNX1 and RUNX3, respectively, whose access to the CD11a promoter would be regulated by members of the C/EBP transcription factor family. Furthermore, AML-1/Eight-Twenty-One (ETO) was found to repress the CD11a promoter activity through MS7, which might explain the low expression of the CD11a/CD18 integrin in t(8;21)–containing acute myeloid leukemia M2 cells.
Materials and methods
Cell culture
The human cell lines K562 (chronic myelogenous leukemia); HeLa (epithelial carcinoma); Jurkat (T-cell lymphoma); JY, Ramos, and Raji (B lymphoblastoid); Kasumi-1 (acute myeloid leukemia M2 t(8;21)+); U937 (histiocytic lymphoma); HL-60 (acute promyelocytic leukemia); and THP-1 (monocytic leukemia) were cultured in RPMI supplemented with 10% fetal calf serum (FCS) at 37°C in a humidified atmosphere with 5% CO2. COS-7 cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% FCS. Induction of differentiation of U937, THP-1, and HL-60 cells was accomplished in the presence of phorbol myristate acetate (PMA) at 10 (U937 and THP-1) and 2 ng/mL (HL-60). Tumor-infiltrating lymphocytes (TILs) were provided by Dr P. Sánchez-Mateos (Hospital Gregorio Marañón, Madrid, Spain).
Transfections, plasmids, and site-directed mutagenesis
HeLa, COS-7, and K562 cells were transfected with Superfect (Qiagen, Hilden, Germany), according to the manufacturer's instructions. Transfections were done in 24-well plates using 1 μg of reporter plasmid on 4 × 104 (COS-7, HeLa) or 8 × 105 to 15 × 105 (K562) cells. THP-1 cells were transfected using O-diethylaminoethyl (DEAE)–dextran following standard procedures. The amount of DNA in each transfection was normalized by using the corresponding insertless expression vector CMV-0 as carrier. Each transfection experiment was performed at least 3 times with different DNA preparations. Transfection efficiencies were normalized by cotransfection with the pCMV-βgal plasmid and β-galactosidase levels determined using the Galacto-Light kit (Tropix, Bedford, MA).
The CD11a-based reporter gene constructs pCD11A170-Luc and pCD11A170(–110mut)-Luc, based on the pXP2 plasmid, have been previously described.7,8 pCD11A170-Luc contains the –170/+83 fragment of the CD11a promoter driving the expression of the firefly luciferase cDNA, while pCD11A170(–110mut)-Luc contains a mutated AML-110 RUNX1-binding site. The expression plasmids CMV-AML1B, CDM8-CBFβ1, CMV–AML-1/ETO, and CMV-Myb were generously provided by Drs Scott Hiebert (Vanderbilt Cancer Center, Vanderbilt University, Nashville, TN), and M. Krangel (Duke University Medical Center, Durham, NC), respectively. The expression plasmids for C/EBPα42, C/EBPα30, and C/EBPβ were kindly provided by Dr G. J. Darrington (Texas Children's Hospital, Houston). The RUNX3 expression plasmid pCGN–AML-2 has been previously reported.47
Site-directed mutagenesis was performed on the CD11a promoter construct pCD11A170-Luc using the QuikChange System (Stratagene, La Jolla, CA). For mutation of the CEBP-100 site, the oligonucleotides CEBPmutS (5′-CTCCCTGAACCCCTGCGGTTTCGGTCCTCCTGC-3′) and CEBPmutAS 5′-GCAGGAGGACCGAAACCGCAGGGGTTCAGGGAG-3′) were used, and the resulting plasmid was termed pCD11A170(–100mut)-Luc. Simultaneous mutation of AML-110 and CEBP-100 was accomplished with oligonucleotides RUNX/CEBPmutS (5′-CTCCCTGAACCCGAATTCTTTCGGTCCTCCTGC-3′) and RUNX/CEBPmutAS (5′-GCAGGAGGACCGAAAGAATTCGGGTTCAGGGAG-3′) to yield the plasmid pCD11A170 (–100/–110mut)-Luc. DNA constructs and mutations were confirmed by DNA sequencing.
Generation of stable Runx3 transfectants in U937 cells
The RUNX3 coding region was obtained by reverse transcriptase–polymerase chain reaction (RT-PCR) using oligonucleotides 5′-GGATCCCCTGACGGCCGCTGTTATG-3′ and 5′-GGAGCGCAGGTCCCATTC-3′, inserted into BamHI/EcoRI-digested pCDNA3.1+, and the resulting plasmid (20 μg, pCDNA3.1+-Runx3) transfected into U937 cells by electroporation (300 V, 960 microfarads [[mu]F]). Transfected cells were selected with G418 (1 mg/mL), cloned by limiting dilution, and analyzed for Runx3 expression by Western blot.
Electrophoretic mobility shift assays (EMSAs)
Electrophoretic mobility shift assays (EMSAs) were performed as described.7 Unlabeled competitor oligonucleotides were added to the nuclear extracts at a 100-fold molar excess and incubated at 4°C for 15 minutes before the addition of the radioactive probe. For antibody inhibition/supershift experiments, 0.5 μL sc-61X (polyclonal antiserum against C/EBPα; Santa Cruz Biotechnology, Santa Cruz, CA), R-3034 (polyclonal antiserum against RUNX1; generously provided by Dr N. A. Speck), or an anti-RUNX3 polyclonal antiserum47 was incubated with the nuclear extracts at 4°C for 30 minutes before the addition of the probe. Nuclear extracts were prepared according to Schreiber et al48 but including aprotinin, antipain, leupeptin, pepstatin, and Pefabloc (4-(2-aminoethyl)-benzenesulfonyl-fluoride, hydrochloride) as additional protease inhibitors. When indicated, EMSA was performed using a limiting amount of probe (approximately 50 pg). This amount of probe yielded readily detectable specific complexes and a weak/absent “free probe” band when incubated with the amount of extract normally used in EMSA (5 μg). The CD11a promoter-based oligonucleotides used for EMSA and their relative positions are shown in Figure 1A. The sequence of an MS7-based oligonucleotide where RUNX- and C/EBP-binding sites were separated by 10 base pair (bp) was 5′-CCCTGCGGTTTGGATCCGTTTTCACAACTCCTGC-3′ (MS7 + 10). Additional oligonucleotides used as competitors and/or probes included RUNXCONS, which contains a consensus RUNX/AML-binding site, CEBPCONS, including a C/EBP-binding site, and CEBPCONSmut, where the C/EBP-binding site is disrupted.
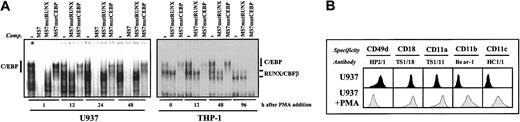
Sequence of the proximal CD11a promoter region around the MS7 element and recognition of the MS7 probe by nuclear extracts from lymphoid and myeloid cells. (A) CD11a proximal promoter sequence (–130 to –81) flanking the MS7 element. The oligonucleotides used throughout this paper are shown below the sequence, with mutated nucleotides in lowercase. The positions of the overlapping RUNX-binding site (AML-110) and C/EBP-binding sites (CEBP-100) are indicated. (B) EMSA was performed on the MS7 oligonucleotide probe using nuclear extracts from the indicated lymphoid and myeloid cells. (C) EMSA was carried out on the MS7 oligonucleotide probes using nuclear extracts from U937 cells and in the absence or presence of the indicated unlabeled competitor oligonucleotides (at 100-fold molar excess) or C/EBPα- or C/EBPβ-specific polyclonal antisera. The position of RUNX- or C/EBP-containing complexes is indicated.
Sequence of the proximal CD11a promoter region around the MS7 element and recognition of the MS7 probe by nuclear extracts from lymphoid and myeloid cells. (A) CD11a proximal promoter sequence (–130 to –81) flanking the MS7 element. The oligonucleotides used throughout this paper are shown below the sequence, with mutated nucleotides in lowercase. The positions of the overlapping RUNX-binding site (AML-110) and C/EBP-binding sites (CEBP-100) are indicated. (B) EMSA was performed on the MS7 oligonucleotide probe using nuclear extracts from the indicated lymphoid and myeloid cells. (C) EMSA was carried out on the MS7 oligonucleotide probes using nuclear extracts from U937 cells and in the absence or presence of the indicated unlabeled competitor oligonucleotides (at 100-fold molar excess) or C/EBPα- or C/EBPβ-specific polyclonal antisera. The position of RUNX- or C/EBP-containing complexes is indicated.
Western blot
Total cell lysates were obtained in 50 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (pH 7.5), 250 mM NaCl, 1 mM EDTA (ethylenediaminetetraacetic acid), 0.5% Triton X-100, 0.5 mM dithiothreitol (DTT), 10 mM NaF, 1 mM Na3VO4, 20 mM Pefabloc (4-(2-aminoethyl)-benzenesulfonyl-fluoride, hydrochloride), and 2 μg/mL aprotinin, antipain, leupeptin, and pepstatin. A total of 10 μg of each lysate was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and transferred onto an Immobilon polyvinylidene difluoride membrane (Millipore, Bedford, MA). After blocking with 5% nonfat dry milk in 50 mM Tris (tris(hydroxymethyl)aminomethane)–HCl (pH 7.6), 150 mM NaCl, and 0.1% Tween 20, protein detection was performed using the Supersignal West Pico Chemiluminescent system (Pierce, Rockford, IL). Detection of C/EBPα, C/EBPβ, RUNX1, and CBFβ was carried out using specific polyclonal antibodies sc-61X (C/EBPα) and sc-150 (C/EBPβ) from Santa Cruz Biotechnology and using Ab-2 (RUNX1) and PC288 (CBFβ) from Oncogene Research (Cambridge, MA). RUNX3 protein was detected using a previously described polyclonal antiserum.47
Flow cytometry and antibodies
Phenotypic analysis of proliferating, PMA-differentiated, and transfected U937 cells was carried out by indirect immunofluorescence. Monoclonal antibodies used for cell surface staining included T3b (anti-CD3) as a control, HP2/1 (anti-CD49d) (kindly provided by Dr F. Sánchez-Madrid, Hospital Universitario de La Princesa, Madrid, Spain), TS1/18 (anti-CD18), TS1/11 (anti-CD11a), Bear-1 (anti-CD11b), and HC1/1 (anti-CD11c). All incubations were done in the presence of 50 μg/mL human immunoglobulin G (IgG) to prevent binding through the Fc portion of the antibodies. Flow cytometry analysis was performed with an EPICS-CS (Coulter Científica, Madrid, Spain) using log amplifiers.
DNA precipitation
Detection of oligonucleotide-bound factors was carried out as previously described49 using biotinylated MS7 oligonucleotide and nuclear extracts from RUNX1- or AML1/ETO-transfected COS-7 cells. MS7-bound proteins were recovered using streptavidin-agarose, separated by SDS-PAGE, and subjected to Western blot with polyclonal antibodies against RUNX1 or ETO.
Chromatin immunoprecipitation (ChIP) assays
Chromatin immunoprecipitation (ChIP) assays were performed using the ChIP assay kit (Upstate Biotechnology, Lake Placid, NY) according to the manufacturer's recommendations. Briefly, untreated and PMA-differentiated (96 hours) THP-1 cells were cross-linked with 1% formaldehyde for 30 minutes at 37°C. After washing with ice-cold phosphate-buffered saline (PBS), cells were lysed in 200 μL of a solution containing 1% SDS, 10 mM EDTA, 50 mM Tris-HCl (pH 8.1) and including 1 μg/mL aprotinin, leupeptin, and pepstatin and 1 mM phenylmethylsulfonyl fluoride (PMSF). Chromatin samples were sonicated with 3 sets of 10-second pulses at 50% maximum power in a Soniprep 150 MSE to reduce DNA length to approximately 200 to 500 bp. Sonicated lysates were then diluted to 2 mL with 0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 167 mM NaCl, and 16.7 mM Tris-HCl (pH 8.1); and 20 μL of this solution was removed for later PCR analysis (input). After preclearing with salmon sperm DNA/protein A agarose for 1 hour at 4°C, antibodies (1 μg) were added and the sonicated lysates were incubated overnight at 4°C in a rocking platform. Immune complexes were collected with 60 μL of salmon sperm DNA/protein A agarose (1 hour at 4°C) and agarose beads washed with solutions of increasing ionic strength. After a final wash in 1 mM EDTA and 10 mM Tris-HCl (pH 8.0), bound immune complexes were eluted in a freshly prepared solution of 1% SDS and 0.1 M NaHCO3, and cross-links were reversed in the samples (and the input from the sonicated lysates) by heating at 65°C for 4 hours. Samples were then treated with proteinase K, and DNA was phenol chloroform–extracted and precipitated. DNA was resuspended (10 μL) and 2 μL used for detection of the CD11a promoter by PCR using the oligonucleotides 5′TGCCCTTTGGCCTCTTACAGTGGTACTTT-3′ and 5′ACCATGACAGCAGTGGAGACTGTCATCTCC-3′, which together amplify a 221-bp region between positions –227 and –6.8 DNA from the input was resuspended in 20 μL and 1 μL was used for PCR. Immunoprecipitating antibodies included rabbit polyclonal antisera against human RUNX3,47 C/EBPα (sc-61X), and CD40 (sc-9096) (Santa Cruz Biotechnology) as a control.
Statistical analysis
Data from multiple experiments were represented as mean group values ± SD. Statistical comparisons were performed using a paired, 2-tailed Student t test, with the probability of P less than .05 considered to be significant.
Results
Differential recognition of the CD11a promoter MS7 element in lymphoid and myeloid cells
RUNX1 regulates the CD11a integrin promoter activity in lymphoid cells through recognition of the AML-110 element within the MS7 sequence (Figure 1A).11 To evaluate whether RUNX factors also bind to the CD11a promoter in other hematopoietic lineages, EMSA was initially performed. RUNX factors were the only MS7-binding factors in both T (Jurkat and tumor-infiltrating lymphocytes) and B lymphoid (JY, Ramos, Raji) lineages (Figure 1B), whereas a different pattern of EMSA-retarded complexes was observed in myeloid cell lines (U937, HL-60, THP-1) (Figure 1B). Moreover, a distinct profile of retarded species was observed in proliferating and PMA-differentiated myeloid cells (Figure 1B), thus suggesting that factors other than RUNX interact with the MS7 sequence in myeloid extracts and that recognition of MS7 shifts during myeloid differentiation.
C/EBP factors recognize the MS7 element in myeloid cells
The MS7 oligonucleotide includes the sequence TTTCACAA, which matches the consensus C/EBP-binding sequence (TTGCGCAA) and overlaps with the previously reported AML-110 element (TGCGGT)11 (Figure 1A). To determine whether this particular sequence was involved in formation of the myeloid retarded complexes, MS7, MS7mutRUNX, and MS7mutCEBP were used as cold competitors in EMSA experiments (Figure 1A). Competition with MS7mutCEBP oligonucleotide left the pattern of complexes almost unaffected whereas MS7mutRUNX eliminated the more intense low-mobility complexes (Figure 1C). Similar results were obtained using THP-1 nuclear extracts (Figure 5), indicating that the sequence ACAA is specifically recognized by myeloid nuclear factors distinct from RUNX1. The first clue to the identity of the myeloid factor interacting with MS7 was obtained using a C/EBP consensus sequence (CEBPCONS) as a competitor, because it produced a pattern of recognition identical to that yielded by MS7mutRUNX, whereas CEBPCONSmut was without effect (Figure 1C). The specific interaction of C/EBP factors with MS7 was finally evidenced by the capacity of polyclonal antisera against C/EBPα, and to a lower extent C/EBPβ, to inhibit and supershift the more relevant complexes observed in U937 cells (Figure 1C).
Changes in MS7 recognition during myeloid cell differentiation: correlation with CD11a/CD18 cell surface expression. (A) EMSA was performed on the MS7 oligonucleotide probe using nuclear extracts obtained from U937 cells (left panel) or THP-1 cells (right panel) at different time points during PMA-induced differentiation. The position of RUNX1/CBFβ- or C/EBP-containing complexes is indicated. Where indicated, unlabeled competitor oligonucleotides (MS7, MS7mutRUNX, or MS7mutCEBP) were added at 100-fold molar excess. (B) Cell surface expression of CD49d, CD11a, CD11b, CD11c, and CD18 integrin subunits on proliferating (U937) and PMA-differentiated cells (U937+PMA). Flow cytometry was performed using the indicated monoclonal antibodies.
Changes in MS7 recognition during myeloid cell differentiation: correlation with CD11a/CD18 cell surface expression. (A) EMSA was performed on the MS7 oligonucleotide probe using nuclear extracts obtained from U937 cells (left panel) or THP-1 cells (right panel) at different time points during PMA-induced differentiation. The position of RUNX1/CBFβ- or C/EBP-containing complexes is indicated. Where indicated, unlabeled competitor oligonucleotides (MS7, MS7mutRUNX, or MS7mutCEBP) were added at 100-fold molar excess. (B) Cell surface expression of CD49d, CD11a, CD11b, CD11c, and CD18 integrin subunits on proliferating (U937) and PMA-differentiated cells (U937+PMA). Flow cytometry was performed using the indicated monoclonal antibodies.
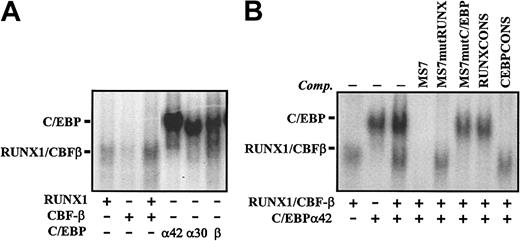
C/EBP binding to MS7 was directly demonstrated with nuclear extracts from COS-7 cells transfected with either C/EBPα42, C/EBPα30, or C/EBPβ. The 3 C/EBP factors bound MS7, and the C/EBP-dependent complexes exhibited lower mobility than those produced by RUNX1/CBFβ (Figure 2A), in agreement with the results obtained with U937 extracts. Therefore, RUNX1 and C/EBP factors bind specifically and independently to the MS7 element. However, while RUNX1 is the only factor interacting with MS7 in lymphoid cells, C/EBP factors are the major constituents of MS7-bound complexes in proliferating myeloid cells. To clarify the relationship between RUNX1 and C/EBP binding to the MS7 sequence, binding assays were performed using extracts from COS-7 cells transfected with either RUNX1, CBFβ, or C/EBPα. As shown in Figure 2B, recognition of MS7 by C/EBPα was completely abolished in the presence of cold MS7, CEBPCONS, or MS7mutRUNX but not by the MS7mutCEBP or RUNXCONS (Figure 2B). Conversely, recognition of MS7 by RUNX1/CBFβ was prevented by MS7, RUNXCONS, or MS7mutCEBP oligonucleotides but was not affected by CEBPCONS or MS7mutRUNX (Figure 2B). Therefore, RUNX1 binding to MS7 relies on the integrity of the AML-110 element, while recognition of the MS7 sequence by C/EBP factors, because it takes place in myeloid extracts, is completely dependent on the –100 element TTTCACAA (hereafter termed CEBP-100).
Nuclear factors interacting with the MS7 probe and identification of nucleotides involved in recognition of CEBP-100. (A) EMSA was performed on the MS7 oligonucleotide using nuclear extracts from COS-7 cells transfected with expression vectors for the indicated transcription factors. (B) EMSA was performed on the MS7 oligonucleotide using nuclear extracts from COS-7 cells transfected with either C/EBPα42 or RUNX1 plus CBFβ and in the absence or in the presence of the indicated competitor oligonucleotides. In all cases, the position of RUNX1- and C/EBP-containing complexes is indicated.
Nuclear factors interacting with the MS7 probe and identification of nucleotides involved in recognition of CEBP-100. (A) EMSA was performed on the MS7 oligonucleotide using nuclear extracts from COS-7 cells transfected with expression vectors for the indicated transcription factors. (B) EMSA was performed on the MS7 oligonucleotide using nuclear extracts from COS-7 cells transfected with either C/EBPα42 or RUNX1 plus CBFβ and in the absence or in the presence of the indicated competitor oligonucleotides. In all cases, the position of RUNX1- and C/EBP-containing complexes is indicated.
C/EBP factors positively contribute to CD11a promoter activity through recognition of CEBP-100
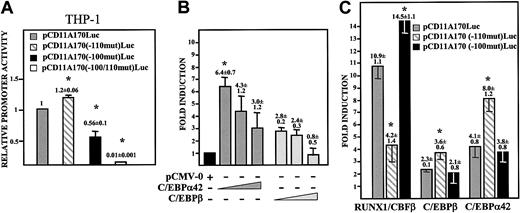
To evaluate the functional relevance of C/EBP binding to the CD11a promoter, the CEBP-100 site was mutated. As shown in Figure 3A, disruption of CEBP-100 significantly decreased the activity of the CD11a promoter in THP-1 cells, yielding an average of 60% of the activity of the wild-type construct. Conversely, mutation of AML-110 slightly increased the activity of the CD11a promoter (average of 120%) (Figure 3A), suggesting that both factors have opposite effects on the activity of the CD11a promoter in myeloid cells. The simultaneous mutation of AML-110 and CEBP-100 completely abolished promoter activity in THP-1 myeloid cells (Figure 3A). Altogether, these results indicate that C/EBP factors contribute to the CD11a promoter activity in proliferating myeloid cells.
Transactivation of the CD11a integrin gene promoter by C/EBP factors is dependent on both CEBP-100 and AML-110 elements. (A) THP-1 cells were transfected with the indicated reporter plasmids and luciferase activity determined after 24 hours. Promoter activity is expressed relative to the activity produced by the wild-type pCD11A170-Luc reporter plasmid in each transfected cell type after normalization for transfection efficiency. Data represent mean ± SD of triplicate determinations (*P = .03 for pCD11A170(–100mut)-Luc, P = .02 for pCD11A170(–110mut)-Luc, and P = 10–5 for pCD11A170(–100/–110mut)-Luc when compared with the activity of the wild-type construct). (B) K562 cells were transfected with 1 μg pCD11A170-Luc together with increasing amounts (30, 100, or 300 ng) of CMV-0, CMV-C/EBPα42, or CMV-C/EBPβ expression plasmids. In all cases, total DNA was kept constant (1.5 μg) by adding CMV-0 plasmid DNA. Fold induction represents the luciferase activity produced by each expression vector relative to the activity produced by a similar amount of empty CMV-0 vector. Data represent mean ± SD of 3 independent experiments (*P = .05 compared with the activity of pCMV-0–transfected cells). (C) K562 cells were transfected with 1 μg of the indicated reporter plasmids and in the absence or presence of RUNX1/CBFβ, C/EBPα42, or C/EBPβ expression plasmids. For each individual reporter construct, fold induction represents the luciferase activity yielded by an expression vector relative to the activity produced by a similar amount of empty CMV-0 plasmid. Data represent mean ± SD of 3 independent experiments using distinct DNA preparations (*P < .05 compared with the activity of pCD11A170-Luc in the presence of RUNX1/CBFβ, C/EBPβ, or C/EBPα, respectively).
Transactivation of the CD11a integrin gene promoter by C/EBP factors is dependent on both CEBP-100 and AML-110 elements. (A) THP-1 cells were transfected with the indicated reporter plasmids and luciferase activity determined after 24 hours. Promoter activity is expressed relative to the activity produced by the wild-type pCD11A170-Luc reporter plasmid in each transfected cell type after normalization for transfection efficiency. Data represent mean ± SD of triplicate determinations (*P = .03 for pCD11A170(–100mut)-Luc, P = .02 for pCD11A170(–110mut)-Luc, and P = 10–5 for pCD11A170(–100/–110mut)-Luc when compared with the activity of the wild-type construct). (B) K562 cells were transfected with 1 μg pCD11A170-Luc together with increasing amounts (30, 100, or 300 ng) of CMV-0, CMV-C/EBPα42, or CMV-C/EBPβ expression plasmids. In all cases, total DNA was kept constant (1.5 μg) by adding CMV-0 plasmid DNA. Fold induction represents the luciferase activity produced by each expression vector relative to the activity produced by a similar amount of empty CMV-0 vector. Data represent mean ± SD of 3 independent experiments (*P = .05 compared with the activity of pCMV-0–transfected cells). (C) K562 cells were transfected with 1 μg of the indicated reporter plasmids and in the absence or presence of RUNX1/CBFβ, C/EBPα42, or C/EBPβ expression plasmids. For each individual reporter construct, fold induction represents the luciferase activity yielded by an expression vector relative to the activity produced by a similar amount of empty CMV-0 plasmid. Data represent mean ± SD of 3 independent experiments using distinct DNA preparations (*P < .05 compared with the activity of pCD11A170-Luc in the presence of RUNX1/CBFβ, C/EBPβ, or C/EBPα, respectively).
RUNX and C/EBP factors compete for binding to the MS7 element
The contribution of CEBP-100 to the CD11a promoter activity was also analyzed by cotransfection experiments in K562 cells, where RUNX1/CBFβ transactivation depends on the AML-110 motif.11 C/EBPα42 transactivated the CD11a promoter, yielding higher values than C/EBPβ (Figure 3B), and CD11a promoter activity decreased upon transfection of higher amounts of both C/EBP factors, a result compatible with a squelching mechanism. Disruption of CEBP-100 did not significantly affect the transactivation ability of C/EBPα42 and C/EBPβ (Figure 3C). By contrast, C/EBPα42 and C/EBPβ transactivation was significantly higher on pCD11A170(–110mut)-Luc, where the AML-110 site had been mutated (P = .03 and .04, respectively) (Figure 3C). A similar phenomenon was found in RUNX1 cotransfection experiments: RUNX1 transactivation was reduced to 40% upon AML-110 mutation (P = .02), while it was increased by 30% in constructs exhibiting a mutated C/EBP-binding site (P = .008) (Figure 3C). These results indicate that the positive regulatory effects of RUNX1 (or C/EBPα) are more obvious under conditions in which the binding of C/EBPα (or RUNX1) is impaired, suggesting that both factors might bind the MS7 sequence in a competitive manner.
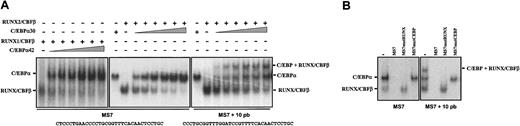
To experimentally address this hypothesis, and because their respective binding sequences appeared to overlap, nuclear extracts from COS-7 cells transfected with RUNX factors were tested for their ability to interact with MS7 in the presence of increasing amounts of C/EBP-containing extracts. When using MS7 probe excess, RUNX1 and C/EBP factors bound to MS7 regardless of the amount of C/EBP-transfected cell extract added to the binding reaction, and no ternary complex was detected at any of the RUNX1-C/EBP ratios tested (Figure 2B). However, when limiting amounts of probe were used, C/EBPα42 displaced RUNX1/CBFβ in a dose-dependent manner without formation of ternary complexes (Figure 4A). A similar experiment was performed using extracts from RUNX2-transfected and C/EBPα30-transfected cells. Again, the C/EBP factor displaced RUNX2/CBFβ from the MS7 probe in a dose-dependent manner, and no ternary complexes were detected (Figure 4A), suggesting that RUNX and C/EBP factors compete for MS7 binding. To rule out the possibility that ternary complexes were comigrating with C/EBPα30-containing complexes, a similar competition assay was performed on the MS7 + 10 pb probe, where AML-110 and CEBP-100 are separated by a 10-bp spacer. While single factor–containing complexes exhibited identical mobility with both probes, ternary complexes were observed even with the lowest amount of extract from C/EBPα30-transfected cells, and RUNX2-containing complexes were detected throughout the whole titration experiment (Figure 4A). Ternary complexes contained both RUNX2/CBFβ and C/EBPα30, as demonstrated by the ability of cold MS7mutRUNX or MS7mutCEBP to prevent its formation (Figure 4B). Therefore, RUNX and C/EBP factors appear to be unable to interact simultaneously with their respective cognate sequences on MS7 unless the AML-110 and CEBP-100 elements are separated, because it occurs in the MS7 + 10 pb probe. This result, together with the transactivation experiments shown in Figure 3C, suggests that RUNX and C/EBP factors compete for occupancy of MS7 and that C/EBP binding to CEBP-100 prevents RUNX binding to, or displaces RUNX from, the AML-110 site.
RUNX1/CBFβ and C/EBP factors compete for binding to the MS7 element. (A) EMSA was performed on a limiting amount of MS7 (left and center panels) or MS7 + 10 (right panel) oligonucleotide probes using nuclear extracts from COS-7 cells transfected with either RUNX1/CBFβ and C/EBPα42 (left panel) or with RUNX2/CBFβ and C/EBPα30 (center and right panels). Binding reactions were carried out using a constant amount of RUNX/CBFβ-transfected cells and increasing amounts of extracts from C/EBPα-transfected cells. The sequence of MS7 and MS7 + 10 is indicated. (B) Identification of factors in ternary complexes. EMSA was performed on a limiting amount of MS7 (left panel) or MS7 + 10 (right panel) oligonucleotide probes using equal amounts of nuclear extracts from COS-7 cells transfected with either RUNX2/CBFβ or C/EBPα30. Where indicated, unlabeled competitor oligonucleotides (MS7, MS7mutRUNX, or MS7mutCEBP) were added at 100-fold molar excess. In all cases, the position of RUNX/CBFβ- and C/EBP-containing complexes is indicated.
RUNX1/CBFβ and C/EBP factors compete for binding to the MS7 element. (A) EMSA was performed on a limiting amount of MS7 (left and center panels) or MS7 + 10 (right panel) oligonucleotide probes using nuclear extracts from COS-7 cells transfected with either RUNX1/CBFβ and C/EBPα42 (left panel) or with RUNX2/CBFβ and C/EBPα30 (center and right panels). Binding reactions were carried out using a constant amount of RUNX/CBFβ-transfected cells and increasing amounts of extracts from C/EBPα-transfected cells. The sequence of MS7 and MS7 + 10 is indicated. (B) Identification of factors in ternary complexes. EMSA was performed on a limiting amount of MS7 (left panel) or MS7 + 10 (right panel) oligonucleotide probes using equal amounts of nuclear extracts from COS-7 cells transfected with either RUNX2/CBFβ or C/EBPα30. Where indicated, unlabeled competitor oligonucleotides (MS7, MS7mutRUNX, or MS7mutCEBP) were added at 100-fold molar excess. In all cases, the position of RUNX/CBFβ- and C/EBP-containing complexes is indicated.
Differential occupancy of MS7 by RUNX and C/EBP during myeloid differentiation
Given the above results and the lineage-specific recognition of the MS7 sequence, we analyzed the occupancy of the AML-110 and CEBP-100 elements during myeloid differentiation by using nuclear extracts from THP-1 and U937 cells at distinct time points along PMA-triggered differentiation. MS7 was almost exclusively recognized by C/EBPα-containing complexes at early differentiation time points, because low levels of RUNX binding to AML-110 could be detected (Figure 5A). As differentiation progressed, RUNX binding to MS7 became apparent whereas C/EBP-containing complexes decreased (Figure 5A). At the end of the differentiation process (48 hours in U937 cells) RUNX-containing complexes were much more abundant than the C/EBP-containing species and exhibited a different mobility than in nondifferentiated cells (Figure 5A). The same shift in the occupancy of MS7 was observed during monocytic differentiation of THP-1 cells (Figure 5A). Therefore, a switch takes place on the CD11a proximal promoter during myeloid differentiation: C/EBPα is the main factor interacting with MS7 in undifferentiated cells through recognition of CEBP-100, while occupancy of AML-110 by RUNX factors only takes place at the final stages of myeloid differentiation and is concomitant with a dramatic reduction in the occupancy of CEBP-100. The differentiation-dependent change in the occupancy of CEBP-100 and AML-110 correlates with an increase in CD11a cell surface expression (Figure 5B), underscoring the relevance that the C/EBP-RUNX switch has on the CD11a/CD18 integrin expression during myeloid cell differentiation.
RUNX3 occupies MS7 in differentiated myeloid cells and influences CD11a cell surface expression
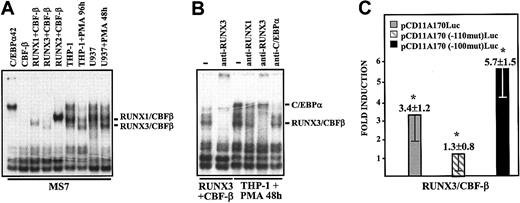
To identify the AML-110–binding factors in differentiated myeloid cells, EMSA complexes from undifferentiated and PMA-differentiated cells were compared with those yielded by RUNX-overexpressing COS-7 extracts. AML-110–dependent complexes in differentiated cells exhibited a similar mobility to that of RUNX3-containing extracts (Figure 6A). Moreover, an anti-RUNX3 polyclonal antiserum completely prevented formation of AML-110–dependent complexes in nuclear extracts from differentiated THP-1 cells (Figure 6B), thus indicating that occupancy of MS7 by RUNX3 correlates with the increase in CD11a expression at the final stages of myeloid differentiation. The functional consequence of RUNX3 binding to AML-110 was subsequently analyzed in transient transfection experiments. RUNX3 significantly transactivated the CD11a promoter (3.4-fold), and this transactivation was abrogated upon disruption of the AML-110 element (1.3-fold) (Figure 6C). Moreover, and like in the case of RUNX1, RUNX3 transactivation was stronger in the context of a mutated CEBP-100 element (5.7-fold) (Figure 6C).
RUNX3 binds to and transactivates the CD11a promoter through recognition of the AML-110 element. (A) EMSA was performed on the MS7 oligonucleotide probe using nuclear extracts from COS-7 cells transfected with the indicated factors (C/EBPα42, CBFβ, RUNX1+CBFβ, RUNX3+CBFβ, or RUNX2+CBFβ) or from undifferentiated or differentiated myeloid (U937 or THP-1) cells. The position of RUNX1- and RUNX3-containing complexes is indicated. (B) EMSA was performed on the MS7 oligonucleotide probe using nuclear extracts from COS-7 cells transfected with RUNX3+CBFβ or differentiated THP-1 cells and in the absence or in the presence of polyclonal antisera against C/EBPα, RUNX1, or RUNX3. The position of C/EBPα- and RUNX3-containing complexes is indicated. (C) K562 cells were transfected with 1 μg of the indicated reporter vector together with 400 ng pCGN–AML-2 (RUNX3) plus CDM8-CBFβ. “Fold induction” represents the luciferase activity produced by each expression vector combination relative to the activity produced by 400 ng CMV-0 plus empty pCGN. Data represent mean ± SD of 3 experiments using distinct DNA preparations (*P = .01 for pCD11A170-Luc activity in the presence of the RUNX3 expression vector versus pCMV-0; *P = .01 for RUNX3 transactivation on mutant constructs compared with transactivation on pCD11A170-Luc).
RUNX3 binds to and transactivates the CD11a promoter through recognition of the AML-110 element. (A) EMSA was performed on the MS7 oligonucleotide probe using nuclear extracts from COS-7 cells transfected with the indicated factors (C/EBPα42, CBFβ, RUNX1+CBFβ, RUNX3+CBFβ, or RUNX2+CBFβ) or from undifferentiated or differentiated myeloid (U937 or THP-1) cells. The position of RUNX1- and RUNX3-containing complexes is indicated. (B) EMSA was performed on the MS7 oligonucleotide probe using nuclear extracts from COS-7 cells transfected with RUNX3+CBFβ or differentiated THP-1 cells and in the absence or in the presence of polyclonal antisera against C/EBPα, RUNX1, or RUNX3. The position of C/EBPα- and RUNX3-containing complexes is indicated. (C) K562 cells were transfected with 1 μg of the indicated reporter vector together with 400 ng pCGN–AML-2 (RUNX3) plus CDM8-CBFβ. “Fold induction” represents the luciferase activity produced by each expression vector combination relative to the activity produced by 400 ng CMV-0 plus empty pCGN. Data represent mean ± SD of 3 experiments using distinct DNA preparations (*P = .01 for pCD11A170-Luc activity in the presence of the RUNX3 expression vector versus pCMV-0; *P = .01 for RUNX3 transactivation on mutant constructs compared with transactivation on pCD11A170-Luc).
Because of the ability of RUNX3 to bind and transactivate the CD11a promoter, U937 cells were transfected with RUNX3 (U937-RUNX3) to determine its influence on the expression of the CD11a/CD18 integrin. Western blot analysis demonstrated that RUNX3-transfected cells expressed considerably higher levels of RUNX3 than untransfected U937 cells (Figure 7A). In addition, independent clones of RUNX3-overexpressing U937 cells exhibited significantly higher levels of CD11a/CD18 cell surface expression than parental cells (Figure 7B). Therefore, RUNX3 expression not only correlates but has a direct influence on the cell surface expression of CD11a in myeloid cells.
In vivo occupancy of MS7 and influence of RUNX3 on CD11a cell surface expression. (A) Nuclear extracts were obtained from U937 and 2 independent clones (nos. 6 and 8) of U937-Runx3 cells; 10 μg from each extract was subjected to Western blot using polyclonal antisera-specific Runx3.47 The position of the Runx3 protein is indicated. (B) CD11a/CD18 and CD11c/CD18 cell surface expression on untransfected U937 and 2 independent U937-Runx3 cell clones, as determined by flow cytometry using the monoclonal antibodies TS1/11 (anti-CD11a) and HC1/1 (anti-CD11c). Negative control fluorescence was determined using the supernatant from the T3b hybridoma (anti-CD3). Data represent mean ± SD of the mean fluorescence intensity values obtained in 4 independent experiments (*P < .002 and P < .000 05 for CD11a expression in clone nos. 6 and 8, respectively, compared with the CD11a expression in untransfected U937 cells). (C) Chromatin immunoprecipitations on uninduced (THP-1) or PMA-differentiated (96 hours) THP-1 cells (THP-1+PMA), using antibodies specific for C/EBPα, RUNX3, CD40 (negative control), or no antibody. Precipitated chromatin was analyzed by PCR using a pair of CD11a promoter-specific primers that flank the MS7 element and amplify a 221 bp DNA fragment. Input lane represents the PCR analysis performed on the DNA precipitated from a 1:20 dilution of the starting sonicated lysate. Each experiment was performed twice with similar results, and 1 of the experiments is shown.
In vivo occupancy of MS7 and influence of RUNX3 on CD11a cell surface expression. (A) Nuclear extracts were obtained from U937 and 2 independent clones (nos. 6 and 8) of U937-Runx3 cells; 10 μg from each extract was subjected to Western blot using polyclonal antisera-specific Runx3.47 The position of the Runx3 protein is indicated. (B) CD11a/CD18 and CD11c/CD18 cell surface expression on untransfected U937 and 2 independent U937-Runx3 cell clones, as determined by flow cytometry using the monoclonal antibodies TS1/11 (anti-CD11a) and HC1/1 (anti-CD11c). Negative control fluorescence was determined using the supernatant from the T3b hybridoma (anti-CD3). Data represent mean ± SD of the mean fluorescence intensity values obtained in 4 independent experiments (*P < .002 and P < .000 05 for CD11a expression in clone nos. 6 and 8, respectively, compared with the CD11a expression in untransfected U937 cells). (C) Chromatin immunoprecipitations on uninduced (THP-1) or PMA-differentiated (96 hours) THP-1 cells (THP-1+PMA), using antibodies specific for C/EBPα, RUNX3, CD40 (negative control), or no antibody. Precipitated chromatin was analyzed by PCR using a pair of CD11a promoter-specific primers that flank the MS7 element and amplify a 221 bp DNA fragment. Input lane represents the PCR analysis performed on the DNA precipitated from a 1:20 dilution of the starting sonicated lysate. Each experiment was performed twice with similar results, and 1 of the experiments is shown.
RUNX3 binds to the CD11a promoter in vivo
Finally, to assess the in vivo occupancy of the CD11a promoter during myeloid cell differentiation, chromatin immunoprecipitation assays were performed on THP-1 cells using antibodies specific for C/EBPα and RUNX3. Both factors bound the CD11a promoter in proliferating THP-1 cells, whereas only RUNX3-specific antibodies immunoprecipitated the CD11a promoter above background levels in differentiated THP-1 cells (Figure 7C). The failure of CEBPα-specific antibodies to immunoprecipitate the CD11a promoter in PMA-treated THP-1 cells is in agreement with the absence of C/EBPα-dependent EMSA complexes in differentiated myeloid cells (Figure 5A) and correlates with the diminished expression of C/EBPα at the final stages of myeloid differentiation. Altogether, these results indicate that the CD11a promoter is differentially occupied by C/EBP and RUNX factors during myeloid cell line differentiation and that RUNX3 directly participates in the increased cell surface expression of CD11a during myeloid differentiation.
Regulated expression of RUNX3 during myeloid cell differentiation
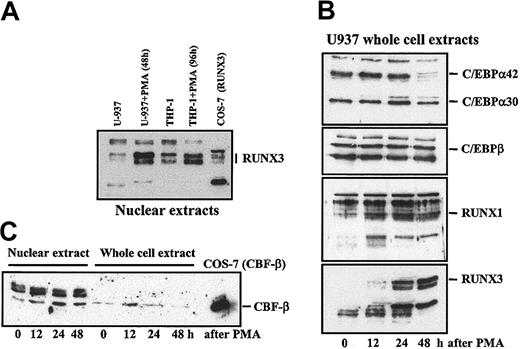
RUNX α subunits are synthesized and proteolytically degraded in the cytoplasm unless associated to CBF-β.50 To analyze the mechanism responsible for the differentiation-dependent occupancy of MS7, RUNX3 protein levels were determined along myeloid cell differentiation. Proliferating myeloid U937 and THP-1 cells exhibited a low level of nuclear RUNX3, whereas PMA differentiation led to a considerable increase in RUNX3 protein (Figure 8A). Kinetics analysis revealed that the increase in total RUNX3 levels (Figure 8B) correlated with an increase in CBF-β, which was observed in total cell lysates 12 hours after PMA treatment and in nuclear extracts after 24 hours (Figure 8C). As described,40,45 C/EBPα42 disappeared at the final stages of myeloid differentiation (Figure 8B). Therefore, occupancy of the MS7 element by RUNX3 at later stages of myeloid differentiation correlates with increased levels of RUNX3 and CBF-β, the latter probably playing a role in the stabilization of the heterodimer, and the shift in the occupancy of MS7 along myeloid differentiation might be explained in terms of loss of C/EBPα expression and up-regulated levels of RUNX3.
Expression levels of C/EBPα, C/EBPβ, RUNX1, RUNX3, and CBFβ in differentiating U937 and THP-1 myeloid cells. Nuclear extracts (A,C) and whole cell extracts (B,C) were obtained from U937 (A-C) and THP-1 (A) cells at the indicated time points along PMA-induced differentiation; 10 μg from each extract was subjected to Western blot using polyclonal antisera specific for C/EBPα, C/EBPβ (Santa Cruz Biotechnology), RUNX1, CBFβ (Oncogene Research), and RUNX3.47 The position of each protein is indicated.
Expression levels of C/EBPα, C/EBPβ, RUNX1, RUNX3, and CBFβ in differentiating U937 and THP-1 myeloid cells. Nuclear extracts (A,C) and whole cell extracts (B,C) were obtained from U937 (A-C) and THP-1 (A) cells at the indicated time points along PMA-induced differentiation; 10 μg from each extract was subjected to Western blot using polyclonal antisera specific for C/EBPα, C/EBPβ (Santa Cruz Biotechnology), RUNX1, CBFβ (Oncogene Research), and RUNX3.47 The position of each protein is indicated.
AML-1/ETO represses CD11a promoter activity and might prevent CD11a/CD18 integrin expression
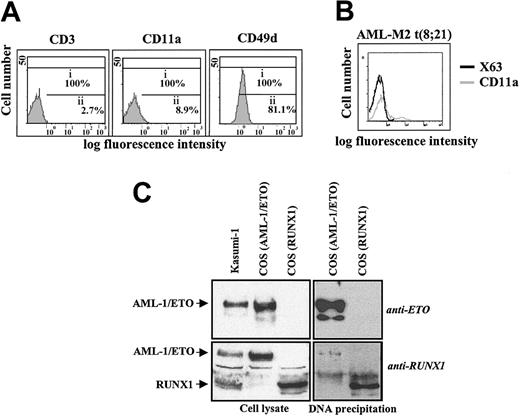
The chimeric protein AML-1(RUNX1)/ETO, which results from the t(8;21) chromosomal translocation found in 25% of AML-M2 leukemias,14,15,17 blocks RUNX1- and RUNX3-dependent transcriptional activation51-53 and abolishes C/EBPα expression.54 Because RUNX3 and C/EBPα bind the critical MS7 element in the CD11a promoter and CD11a/CD18 expression is weak/absent in t(8;21)+ cells,55 we reasoned that AML-1/ETO might directly affect CD11a promoter activity. Flow cytometry analysis of Kasumi-1 cells derived from an AML-M2 patient56 and bone marrow cells from an untreated AML-M2 patient confirmed an extremely weak CD11a expression (Figure 9A-B). Interestingly, DNA affinity precipitation experiments revealed that AML-1/ETO was capable of recognizing the RUNX-binding site within the MS7 element. MS7 oligonucleotide specifically retained RUNX1 from RUNX1-transfected cells and AML-1/ETO from AML-1/ETO-overexpressing COS-7 cells (Figure 9C), indicating that AML-1/ETO can bind to the critical AML-110 element within the CD11a proximal promoter.
Interactions on the MS7 element and CD11a/CD18 expression in t(8;21)–containing cells. (A) Determination of the cell surface expression of CD11a and CD49d integrins on AML1/ETO-expressing Kasumi-1 cells, as determined by flow cytometry using the monoclonal antibodies TS1/11 (anti-CD11a), ALC 1/6.3 (anti-CD49d), and T3b (anti-CD3) as control. i, whole population; ii, percentage of marker-positive cells. (B) CD11a/CD18 cell surface expression on cells from the bone marrow aspirate of a t(8;21)+ AML-M2 patient. CD11a expression was determined using the TS1/11 monoclonal antibody. Negative control fluorescence was determined using the supernatant from the myeloma P3X63 (X63). (C) DNA affinity precipitation on the MS7 element. COS cells were transfected with expression vectors for either RUNX1 or AML-1/ETO, and cells extracts were incubated with biotinylated MS7 oligonucleotide. DNA-protein complexes were isolated by centrifugation with streptavidin-agarose, and bound proteins separated by SDS-PAGE and subjected to Western blot using RUNX1 (lower panels) or ETO-specific (upper panels) polyclonal antibodies. As a control, cell lysates from transfected and Kasumi-1 cells (left panels) were analyzed in parallel.
Interactions on the MS7 element and CD11a/CD18 expression in t(8;21)–containing cells. (A) Determination of the cell surface expression of CD11a and CD49d integrins on AML1/ETO-expressing Kasumi-1 cells, as determined by flow cytometry using the monoclonal antibodies TS1/11 (anti-CD11a), ALC 1/6.3 (anti-CD49d), and T3b (anti-CD3) as control. i, whole population; ii, percentage of marker-positive cells. (B) CD11a/CD18 cell surface expression on cells from the bone marrow aspirate of a t(8;21)+ AML-M2 patient. CD11a expression was determined using the TS1/11 monoclonal antibody. Negative control fluorescence was determined using the supernatant from the myeloma P3X63 (X63). (C) DNA affinity precipitation on the MS7 element. COS cells were transfected with expression vectors for either RUNX1 or AML-1/ETO, and cells extracts were incubated with biotinylated MS7 oligonucleotide. DNA-protein complexes were isolated by centrifugation with streptavidin-agarose, and bound proteins separated by SDS-PAGE and subjected to Western blot using RUNX1 (lower panels) or ETO-specific (upper panels) polyclonal antibodies. As a control, cell lysates from transfected and Kasumi-1 cells (left panels) were analyzed in parallel.
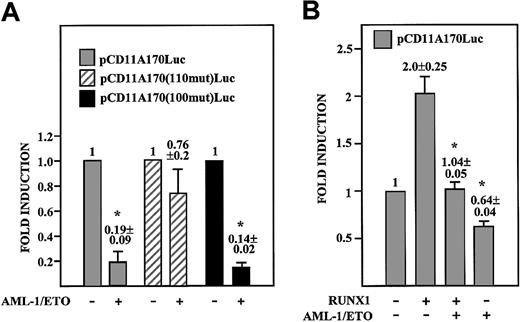
The functional relevance of AML-1/ETO binding to MS7 was analyzed by coexpressing AML-1/ETO with distinct CD11a promoter-based reporter constructs. AML-1/ETO reduced CD11a promoter activity to 10%, and the same repressive effect was observed in the presence of a disrupted C/EBP-binding site (14% of activity) (Figure 10A). By contrast, the repressive effect of AML-1/ETO was almost abolished when the AML-110 RUNX1-binding site was mutated (76% of wild-type activity) (Figure 10A), demonstrating that the AML-1/ETO repressive effect requires an intact RUNX-binding site. Moreover, RUNX1-mediated transactivation of the CD11a promoter was abolished in the presence of AML-1/ETO (Figure 10B). Because AML-1/ETO is capable of binding to MS7, these results indicate that AML-1/ETO represses CD11a promoter activity primarily through the AML-110 element and might consequently decrease CD11a integrin expression in AML-M2 leukemia cells.
AML-1/ETO represses CD11a integrin promoter activity: dependency on the AML-110 element. (A) K562 cells were transfected with 1 μg of the indicated reporter plasmids and in the absence or in the presence of a CMV–AML-1/ETO expression plasmid. In all cases, total DNA was kept constant by the addition of empty CMV-0 plasmid DNA. For each individual reporter construct, “fold induction” represents the luciferase activity produced by each individual expression plasmid relative to the activity in the presence of empty CMV-0 plasmid. The experiment was performed 4 times using distinct DNA preparations. Data represent mean ± SD of the 4 experiments (*P = .004 and P = .0001 when comparing the activity of pCD11A170-Luc and pCD11A170(–100mut)-Luc in the presence or absence of the CMV–AML-1/ETO expression plasmid). (B) HeLa cells were transfected with 1 μg of pCD11A170Luc reporter plasmid and in the absence or in the presence of either CMV–AML-1 (RUNX1) and/or CMV–AML-1/ETO expression plasmids. In all cases, total DNA was kept constant by the addition of empty CMV-0 plasmid DNA. For each individual reporter construct, “fold induction” represents the luciferase activity produced by each individual expression plasmid combination relative to the activity produced by a similar amount of empty CMV-0 plasmid. Data represent mean ± SD of 3 experiments using distinct DNA preparations (*P = .03 compared with the activity of the wild-type pCD11A170-Luc construct in the presence of RUNX1).
AML-1/ETO represses CD11a integrin promoter activity: dependency on the AML-110 element. (A) K562 cells were transfected with 1 μg of the indicated reporter plasmids and in the absence or in the presence of a CMV–AML-1/ETO expression plasmid. In all cases, total DNA was kept constant by the addition of empty CMV-0 plasmid DNA. For each individual reporter construct, “fold induction” represents the luciferase activity produced by each individual expression plasmid relative to the activity in the presence of empty CMV-0 plasmid. The experiment was performed 4 times using distinct DNA preparations. Data represent mean ± SD of the 4 experiments (*P = .004 and P = .0001 when comparing the activity of pCD11A170-Luc and pCD11A170(–100mut)-Luc in the presence or absence of the CMV–AML-1/ETO expression plasmid). (B) HeLa cells were transfected with 1 μg of pCD11A170Luc reporter plasmid and in the absence or in the presence of either CMV–AML-1 (RUNX1) and/or CMV–AML-1/ETO expression plasmids. In all cases, total DNA was kept constant by the addition of empty CMV-0 plasmid DNA. For each individual reporter construct, “fold induction” represents the luciferase activity produced by each individual expression plasmid combination relative to the activity produced by a similar amount of empty CMV-0 plasmid. Data represent mean ± SD of 3 experiments using distinct DNA preparations (*P = .03 compared with the activity of the wild-type pCD11A170-Luc construct in the presence of RUNX1).
Discussion
The CD11a/CD18 integrin is exclusively found on hematopoietic lineage cells, and its lineage-specific and differentiation-regulated expression is mainly controlled through the tissue-restricted activity of the CD11a gene promoter. We report that the activity of the CD11a proximal regulatory region is critically dependent on the integrity of the AML-110 and CEBP-100 elements within the MS7 sequence, which is recognized by RUNX and C/EBP factors in a competitive manner. Recognition of MS7 differs in lymphoid versus myeloid cells, and its occupancy is regulated in a differentiation-dependent manner in myeloid cells. MS7 is preferentially occupied by C/EBP factors in proliferating myeloid cells while RUNX factors, and especially RUNX-3, are the major MS7-bound factors in differentiated cells. Our results indicate that the increased CD11a/CD18 membrane expression in PMA-differentiated leukemia myeloid cell lines correlates with down-regulation of C/EBPα expression and increased RUNX3 expression. Moreover, RUNX3 overexpression enhances CD11a/CD18 cell surface expression, and C/EBPα is lost from the CD11a promoter at the later stages of myeloid differentiation. Altogether, these data indicate that RUNX and C/EBP factors control the lineage- and differentiation-dependent expression of the CD11a/CD18 integrin through the differential occupancy of MS7, reveal the positive effect of RUNX3 on CD11a/CD18 expression, and suggest the existence of an inverse correlation between CD11a transcription and C/EBPα levels. Analysis of the changes in gene expression during differentiation and maturation of monocyte-derived dendritic cells further supports such an inverse correlation between C/EBPα levels and CD11a expression, because the level of CD11a mRNA and cell surface expression drops during GM-CSF/interleukin-4 (GM-CSF/IL-4)–induced dendritic cell differentiation (Le Naour et al57 ; and A.P.-K. and A.L.C., unpublished data, November 2001), while C/EBPα mRNA levels increase during the process.57
RUNX factors regulate the lymphoid- and myeloid-restricted expression of several genes.13-15 In the lymphoid lineage, RUNX factors, in concert with c-Myb, increase the transcription of distinct T-cell receptor genes.28 The myeloid-specific expression of the neutrophil elastase,58 M-CSF receptor,29,30 and myeloperoxidase27 is mainly controlled through the concerted and synergistic action of RUNX1 with factors such as Myb, PU.1, and C/EBP, which bind closely located elements on their respective promoter regions. However, dissection of the MS7 element has revealed that RUNX and C/EBP factors do not bind simultaneously to MS7 but, on the contrary, compete for binding to their overlapping binding sites. As a consequence, it is the interplay between RUNX and C/EBP factors that dictates the level of CD11a promoter activity and, consequently, the level of expression of CD11a/CD18. To our knowledge, the CD11a promoter MS7 sequence represents the first example of an element involved in both lymphoid and myeloid expression through differential and competitive recognition by RUNX and C/EBP transcription factors.
Stable transfection experiments indicate that RUNX3 overexpression has a positive effect on the CD11a/CD18 integrin cell surface expression. However, disruption of the RUNX-binding site within MS7 results in an increased CD11a promoter activity. These 2 apparently contradictory results could be reconciled when considering the existence of RUNX and C/EBP isoforms, which differ in transcriptional potency and even act as repressors (AML1δN, C/EBPα30).40,59 Therefore, disruption of the AML-110 site within MS7 might affect not only the involvement of functionally distinct RUNX proteins in CD11a promoter activity but also the occupancy of the CEBP-100 site by C/EBP factors with either transactivating (C/EBPα42) or repressive (C/EBPα30) capabilities. We are currently examining these and alternative explanations and have obtained evidence that myeloid cells express alternative RUNX3 isoforms that are devoid of transactivation ability but retain their DNA-binding capacity (A.P.-K. and A.L.C., unpublished data, April 2003). Regardless of the precise mechanism, the relative levels of C/EBP and RUNX factors, through the differential occupancy of the MS7 element, appear to underlie the changes in CD11a/CD18 expression that occur during myeloid differentiation. Therefore, the MS7 element constitutes a valuable tool to systematically analyze the specific factors responsible for the basal and regulated expression of the CD11a integrin subunit at distinct stages during lymphoid and myeloid differentiation.
Finally, the molecular interactions that we have observed within the CD11a gene regulatory region have led to the finding that AML-1/ETO expression down-regulates its activity through the AML-110 element and, consequently, might explain the diminished or absent expression of CD11a/CD18 in leukemia cells containing the AML-1/ETO–encoding t(8;21) chromosomal translocation.56 In these cells, RUNX1-dependent CD11a transcription would be inhibited by AML-1/ETO, leaving C/EBP factors, and especially C/EBPα, as the only alternative for occupancy of the MS7 element and promotion of CD11a transcription. However, because AML-1/ETO down-regulates C/EBPα expression,55 the MS7-dependent transcription of the CD11a gene would be greatly impaired in AML-1/ETO–expressing cells, thus leading to a weak/absent CD11a/CD18 cell surface expression. It is also tempting to speculate that CD11a/CD18 expression might be altered in acute myeloid leukemias where dominant-negative mutations of C/EBPα have been identified.60 Whether the diminished expression of CD11a/CD18 contributes to leukemogenesis in AML-1/ETO–expressing cells deserves further investigation, especially considering that engagement of other adhesion receptors reverses the myeloid differentiation blockage observed in acute myeloid leukemia.61
Prepublished online as Blood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2003-02-0618.
Supported by grants 08.3/0026/2000.1 from Comunidad Autónoma de Madrid, 01/0063-01 from Fondo de Investigaciones Sanitarias, and SAF2002-04615-C02-01 from Ministerio de Ciencia y Tecnología (A.L.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We gratefully acknowledge Drs Ana Aranda and Aurora SánchezPacheco for their very generous help with ChIP assays.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal