Abstract
Platelet adhesion and activation at the vascular wall are the initial steps leading to arterial thrombosis and vascular occlusion. Prostacyclin and nitric oxide inhibit platelet adhesion, acting via cyclic adenosine monophosphate (cAMP)– and cyclic guanosine monophosphate (cGMP)–dependent protein kinases. A major downstream target for both cAMP- and cGMP-dependent protein kinases is the vasodilator-stimulated phosphoprotein (VASP). To test the significance of VASP for the regulation of platelet adhesion in vivo, we studied platelet–vessel wall interactions using VASP-deficient (VASP–/–) mice. Under physiologic conditions, platelet adhesion to endothelial cells was significantly enhanced in VASP null mutants when compared with wild-type mice (P < .05). Platelet recruitment in VASP null mice involved P-selectin and the fibrinogen receptor glycoprotein IIb-IIIa (GPIIb-IIIa). Under pathophysiologic conditions, the loss of VASP increased platelet adhesion to the postischemic intestinal microvasculature, to the atherosclerotic endothelium of ApoE-deficient mice, and to the subendothelial matrix following endothelial denudation (P < .05 vs wild type). Importantly, platelet adhesion in VASP null mutants was unresponsive to nitric oxide. These data show for the first time in vivo that VASP is involved in down-regulation of platelet adhesion to the vascular wall under both physiologic and pathophysiologic conditions.
Introduction
The adhesion of platelets to the vascular wall is central to the pathogenesis of atherogenesis and arterial thrombosis.1,2 Nitric oxide (NO) and prostacyclin are of major importance for the regulation of platelet–vessel wall interactions. They activate soluble guanylyl cyclase and adenylate cyclase, respectively, initiating a subsequent rise in platelet cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP).3-5 Cyclic GMPdependent protein kinase I (cGKI) and cAMP-dependent protein kinase (cAK) are thought to be the major downstream targets of the NO/cGMP and prostacyclin/cAMP signaling cascades in platelets.3,6,7
A common substrate of both cAK and cGK is the vasodilator-stimulated phosphoprotein (VASP).8-10 VASP was isolated initially from human platelets but it is also expressed in a wide variety of other cells and tissues.11 VASP is the founding member of a family of proline-rich proteins designated the Ena/VASP protein family, which comprises VASP, Drosophila Enabled (Ena), a substrate of the Abelson tyrosine kinase (Abl), the mammalian Ena homolog Mena, and the Ena-VASP–like protein Evl.12-14 These proteins share highly homologous N-terminal and C-terminal domains (Ena-VASP homology domains 1 and 2, designated EVH1 and EVH2) and proline-rich central domains.12-14 VASP has been found to be associated with focal adhesions, stress fibers, cell-cell contacts, and highly dynamic membrane regions in platelets, smooth muscle cells, endothelial cells, and fibroblasts.11,15 VASP directly binds to profilin, zyxin, and to the focal adhesion and cell-cell contact protein vinculin12-20 and is thought to play an important role in the regulation of cytoskeletal (re)organization and cell motility.15,21-26
In platelets, VASP is strategically located at the intersection of 2 major inhibitory pathways. In response to the cyclic nucleotide-regulating platelet antagonists NO and prostacyclin, VASP is phosphorylated at serine-157, serine-239, and threonine-278 by both cAK and cGK.27-29 VASP phosphorylation closely correlates with platelet inhibition and is paralleled by the inhibition of platelet fibrinogen receptor glycoprotein IIb-IIIa (GPIIb-IIIa) activation.27,30 Platelets deficient in VASP exhibit enhanced agonist-induced activation of P-selectin expression and fibrinogen binding to GPIIb-IIIa integrin.31,32 Antagonism of the Gi-coupled platelet adenosine diphosphate (ADP) receptor P2Y12 by thienopyridines abolishes the inhibitory effects of ADP on prostaglandin E1–stimulated, cAMP-dependent VASP phosphorylation and prevents platelet aggregation.33 VASP, therefore, appears to be involved in the regulation of platelet activation in vitro. However, an important but unanswered question is whether or not VASP is essential for the regulation of platelet function and platelet–vessel wall interactions in vivo.
Materials and methods
Animals
VASP–/– mice were generated as described.31 For experiments, 8- to 12-week-old male VASP–/– and wild-type (VASP+/+) mice on C57BL/6/129sv background were used. To assess the adhesion of VASP–/– or wild-type platelets to atherosclerotic endothelium, ApoE–/– (C57BL/6J-ApoEtm1Unc) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Four-week-old ApoE–/– consumed a 0.25% cholesterol diet (Harlan Research diets, 0% cholate; Harlan Teklad, Madison, WI) for 6 weeks. All experimental procedures performed on animals met the requirements of the German legislation on protection of animals.
In vivo fluorescence microscopy
Platelets, isolated from VASP–/– or wild-type mice as described,34 were labeled in vitro with 5-carboxyfluorescein diacetat succinimidyl ester (DCF). Fluorescent platelets (0.2 × 109) were infused intravenously via polyethylene catheters (Portex, Hythe, England) implanted into the right jugular vein of VASP–/– or wild-type recipient mice and were visualized in situ by in vivo video microscopy of the right common carotid artery. The genotypes (wild-type [WT], VASP–/–, or ApoE–/–) of platelet donors and recipients are indicated in the figures. We monitored platelet–endothelial cell interactions using a Zeiss Axiotech microscope (× 20 water immersion objective, W 20 × /0.5; Zeiss, Munich, Germany) with a 100 W HBO mercury lamp for epi-illumination (Osram GmbH, Eichstätt, Germany). All videotaped images were evaluated using a computer-assisted image analysis program (Cap Image 7.1, Dr Zeintl, Heidelberg, Germany34,35 ). The number of adherent platelets was assessed by counting the cells that did not move or detach from the endothelial surface within 10 seconds.
In 2 separate sets of experiments, fluorescent VASP–/– platelets were injected together with 50 μg function-blocking anti–GPIIb-IIIa (JON/A-F(ab′)2)36 or anti–P-selectin (Pharmingen, Hamburg, Germany) monoclonal antibody (mAb), respectively, into VASP null recipients. Subsequently, platelets were visualized in the common carotid artery (n = 6-7 per group) by in vivo video microscopy as described in this section.
Assessment of platelet adhesion following ischemia-reperfusion
Platelet–vessel wall interactions were assessed prior to and following intestinal ischemia-reperfusion (I/R) as described.6 Briefly, C57BL/6/129sv mice of either genotype (n = 8-10 litter- or age-matched animals per group) were laparotomized, and syngeneic-labeled platelets (200 × 106, wild-type or VASP–/–) were infused as bolus via a venous catheter inserted into the right jugular vein. Segmental jejunal ischemia (60 minutes) was induced with subsequent reperfusion. Platelet–endothelial cell interactions in intestinal arterioles and venules (15 μm < vessel diameter < 85 μm) were analyzed by intravital videofluorescence microscopy prior to (baseline) and following I/R as described.6,34,35
Platelet adhesion in ApoE–/– mice
To define the role of VASP in the regulation of platelet adhesion to the atherosclerotic vascular wall, DCF-labeled platelets of either genotype (wild-type or VASP–/–, respectively) were infused intravenously into VASP+/+ApoE–/– mice, which had consumed a cholesterol diet for 6 weeks. Platelet adhesion was visualized in the right common carotid artery in situ by in vivo video microscopy as described above.
Assessment of platelet adhesion following vascular injury: inhibition of platelet adhesion by nitric oxide
Wild-type mice were anesthetized by intraperitoneal injection of a solution of midazolame (5 mg/kg body weight; Ratiopharm, Ulm, Germany), medetomidine (0.5 mg/kg body weight; Pfizer, Karlsruhe, Germany), and fentanyl (0.05 mg/kg body weight; CuraMed Pharma GmbH, Munich, Germany). Polyethylene catheters (Portex) were implanted into the right jugular vein. The right common carotid artery was dissected free and ligated vigorously near the carotid bifurcation for 5 minutes to induce vascular injury. Fluorescent wild-type or VASP–/– platelets (200 × 106/250 μL) were preincubated at room temperature for 5 minutes with either phosphate-buffered saline (PBS) or the NO-donor spermine-NO (Alexis, Grünberg, Germany; 100 nM final concentration). After preincubation, the samples were stimulated for 5 minutes with 0.2 U/mL mouse thrombin (Sigma-Aldrich, Deisenhofen, Germany), a concentration that does not induce full platelet aggregation in vitro. Thrombin activation was employed since VASP has been implicated previously in thrombin-induced affinity modulation of platelet integrins,31,32 a process that facilitates platelet adhesion at sites of vascular injury. In addition, nitric oxide, which triggers VASP phosphorylation, has been demonstrated to attenuate thrombin-induced platelet integrin activation.37
Following thrombin activation, the fluorescent platelets were infused intravenously and platelet adhesion to the injured carotid artery was monitored in situ by in vivo video microscopy. Tethered platelets were defined as all platelets establishing initial contact with the injured vessel wall, followed by slow surface translocation (at a velocity significantly lower than the centerline velocity) or by firm adhesion (their numbers are given as cells/mm2 endothelial surface). The number of adherent platelets was assessed by counting the cells that did not move or detach from the endothelial surface within 10 seconds.
In a separate set of experiments, we assessed the role of the platelet fibrinogen receptor for adhesion of VASP–/– platelets following vascular injury. Fluorescent VASP–/– platelets were preincubated with 50 μg/mL anti–GPIIb-IIIa (JON/A-F(ab′)2)36 or PBS for 10 minutes. Wild-type mice were subjected to carotid injury as described above. Subsequently, fluorescent VASP null platelets preincubated with anti–GPIIb-IIIa or PBS were infused and visualized at the site of carotid injury (n = 4-5) using intravital videomicroscopy as described above.
Statistical analysis
Comparisons between group means were performed using Mann-Whitney U test. Data represent mean ± SEM. A P value of .05 was regarded as significant.
Results
VASPregulates platelet adhesion under physiologic conditions in vivo
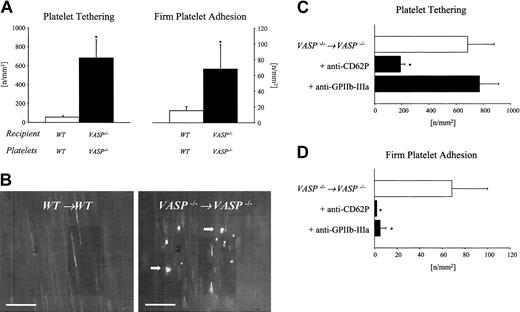
To address the significance of VASP for the homeostasis of platelet-endothelium interactions in vivo, we assessed platelet adhesion in the carotid artery. Fluorescent VASP–/– platelets were injected into VASP–/– mice and were visualized by intravital videofluorescence microscopy. Wild-type platelets transfused into wild-type recipients served as controls. While wild-type platelets rarely interacted with wild-type endothelium under physiologic conditions, the loss of VASP significantly enhanced platelet–endothelial cell interactions in vivo (Figure 1A-B). Platelet tethering to the endothelial surface was increased approximately 11-fold, while the number of platelets firmly attached to the vascular wall increased more than 4-fold in VASP–/– mutants compared with wild-type mice (P < .05). To clarify whether VASP expressed by platelets or present in the vascular wall is necessary to inhibit platelet–endothelial cell interactions under physiologic conditions, VASP–/– platelets were infused into wild-type mice. Interestingly, under physiologic conditions the isolated loss of platelet VASP was not associated with enhanced firm platelet adhesion to the vascular wall of wild-type animals (not shown).
Role of VASP in the regulation of platelet adhesion in the common carotid artery in vivo. (A) Platelet–endothelial cell interactions were investigated in VASP–/– mice by in vivo fluorescence microscopy of the common carotid artery in situ. Wild-type animals served as controls. The panels summarize platelet tethering (left) and firm platelet adhesion (right). Tethered and adherent platelets were classified according to their interaction with the endothelial cell lining as described in “Materials and methods” and are given per mm2 of vessel surface. Mean ± SEM, n = 8-10 each group; * indicates significant difference compared with wild-type mice; P < .05. (B) The microphotographs show representative in vivo fluorescence microscopy images. White arrows indicate adherent platelets. Bars represent 50 μm. Role of P-selectin and GPIIb-IIIa for platelet tethering (C) and firm platelet adhesion (D) in VASP null mice. VASP–/– mice were injected with 50 μg function-blocking anti–P-selectin or anti–GPIIb-IIIa mAb, respectively, prior to in vivo videofluorescence microscopy. Untreated VASP null mice served as controls. Tethered and adherent platelets were classified according to their interaction with the endothelial cell lining as described in “Materials and methods” and are given per mm2 of vessel surface. Mean ± SEM; n = 6-7 carotid arteries; * indicates significant difference compared with untreated VASP null mice; P < .05.
Role of VASP in the regulation of platelet adhesion in the common carotid artery in vivo. (A) Platelet–endothelial cell interactions were investigated in VASP–/– mice by in vivo fluorescence microscopy of the common carotid artery in situ. Wild-type animals served as controls. The panels summarize platelet tethering (left) and firm platelet adhesion (right). Tethered and adherent platelets were classified according to their interaction with the endothelial cell lining as described in “Materials and methods” and are given per mm2 of vessel surface. Mean ± SEM, n = 8-10 each group; * indicates significant difference compared with wild-type mice; P < .05. (B) The microphotographs show representative in vivo fluorescence microscopy images. White arrows indicate adherent platelets. Bars represent 50 μm. Role of P-selectin and GPIIb-IIIa for platelet tethering (C) and firm platelet adhesion (D) in VASP null mice. VASP–/– mice were injected with 50 μg function-blocking anti–P-selectin or anti–GPIIb-IIIa mAb, respectively, prior to in vivo videofluorescence microscopy. Untreated VASP null mice served as controls. Tethered and adherent platelets were classified according to their interaction with the endothelial cell lining as described in “Materials and methods” and are given per mm2 of vessel surface. Mean ± SEM; n = 6-7 carotid arteries; * indicates significant difference compared with untreated VASP null mice; P < .05.
Since VASP has been implicated in the negative regulation of P-selectin surface expression and in the inhibition of agonist-induced GPIIb-IIIa affinity modulation,31 we next addressed the role of P-selectin and GPIIb-IIIa for enhanced platelet adhesion under physiologic conditions in VASP null mutants. VASP–/– mice were injected with 50 μg function-blocking anti–P-selectin or anti–GPIIb-IIIa mAb prior to in vivo videofluorescence microscopy. Infusion of anti–P-selectin mAb reduced tethering by approximately 70% and virtually abolished firm platelet adhesion (P < .05) in VASP null mice (Figure 1C-D). In contrast, anti–GPIIb-IIIa did not affect loose platelet tethering but strongly reduced firm platelet adhesion (P < .05). Together, this indicates that P-selectin mediates platelet tethering in VASP null mice and thereby facilitates subsequent firm platelet adhesion via platelet GPIIb-IIIa.
VASPattenuates platelet adhesion to the postischemic microvascular endothelium
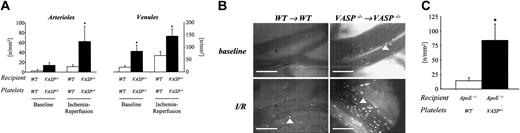
Interactions between circulating platelets and the vascular wall are required for maintenance of vascular integrity and hemostasis. However, in certain pathophysiologic processes, in particular I/R, the adhesion and aggregation of platelets may also contribute to vascular injury.38,39 To evaluate the relevance of VASP for the regulation of platelet-vessel interactions under pathophysiologic conditions in vivo, we determined platelet adhesion within the microcirculation of an ischemic-reperfused segment of the jejunum using intravital video microscopy. Wild-type or VASP–/– mice were subjected to intestinal ischemia (60 minutes). Platelet–vessel wall interactions were visualized within intestinal arterioles and venules by intravital fluorescence microscopy prior to and after I/R. Under control conditions without I/R, wild-type platelets did not interact with wild-type endothelium (6 ± 4 and 26 ± 8 firmly adherent platelets/mm2 in arterioles and venules, respectively; Figure 2A-B). In contrast, as reported above for the macrovasculature (carotid artery), numerous platelets were found attached to the vascular endothelium of intestinal microvessels in VASP–/– mice (14 ± 6 and 85 ± 25 firmly adherent platelets/mm2 in arterioles and venules, respectively; P < .05 vs wild type). In response to I/R, 11 ± 3 and 67 ± 16 platelets/mm2 were found firmly attached in arterioles and venules of wild-type mice, respectively (Figure 2A-B). The loss of VASP strongly enhanced postischemic platelet–endothelial cell interactions. Within both arterioles and venules the number of adherent platelets was increased 2- to 5-fold when compared with wild-type animals (62 ± 30 and 146 ± 29 firmly adherent platelets/mm2 in arterioles and venules, respectively; Figure 2A-B). Hence, VASP participates in the regulation of platelet–vessel wall interactions in the macrovasculature and microvasculature both under physiologic conditions and following postischemic reperfusion.
Role of VASP in the regulation of platelet adhesion under pathophysiologic conditions. (A) Platelet–vessel wall interactions were assessed prior to and following intestinal ischemia-reperfusion (I/R) as described in “Materials and methods.” Fluorescent wild-type or VASP–/– platelets were transfused into recipient mice of either genotype. Segmental jejunal ischemia (60 minutes) was induced with subsequent reperfusion. Platelet–endothelial cell interactions in intestinal arterioles and venules were analyzed by intravital videofluorescence microscopy prior to (baseline) and following I/R in arterioles (left) and venules (right).6,35 Mean ± SEM; * indicates significant difference compared with wild type; P < .05. (B) The microphotographs show representative in vivo fluorescence microscopy images of platelet adhesion prior to (top) and following intestinal I/R (bottom) in control animals (left) or VASP–/– mice (right). Arrowheads indicate adherent platelets. Bars represent 50 μm. (C) To evaluate the role of VASP in the regulation of platelet adhesion to the atherosclerotic vascular wall, wild-type or VASP–/– platelets were infused intravenously into ApoE–/– mice. Platelet adhesion was visualized in the right common carotid artery in situ by in vivo video microscopy as described above. Mean ± SEM; * indicates significant difference compared with wild-type platelets; P < .05.
Role of VASP in the regulation of platelet adhesion under pathophysiologic conditions. (A) Platelet–vessel wall interactions were assessed prior to and following intestinal ischemia-reperfusion (I/R) as described in “Materials and methods.” Fluorescent wild-type or VASP–/– platelets were transfused into recipient mice of either genotype. Segmental jejunal ischemia (60 minutes) was induced with subsequent reperfusion. Platelet–endothelial cell interactions in intestinal arterioles and venules were analyzed by intravital videofluorescence microscopy prior to (baseline) and following I/R in arterioles (left) and venules (right).6,35 Mean ± SEM; * indicates significant difference compared with wild type; P < .05. (B) The microphotographs show representative in vivo fluorescence microscopy images of platelet adhesion prior to (top) and following intestinal I/R (bottom) in control animals (left) or VASP–/– mice (right). Arrowheads indicate adherent platelets. Bars represent 50 μm. (C) To evaluate the role of VASP in the regulation of platelet adhesion to the atherosclerotic vascular wall, wild-type or VASP–/– platelets were infused intravenously into ApoE–/– mice. Platelet adhesion was visualized in the right common carotid artery in situ by in vivo video microscopy as described above. Mean ± SEM; * indicates significant difference compared with wild-type platelets; P < .05.
VASPmodulates platelet–endothelial cell adhesion in early atherosclerosis of the carotid artery
The adhesion of platelets to the vascular endothelium is of central importance in the initiation of the atherogenetic process. Platelet adhesion occurs early in the process of atherosclerosis and strictly precedes leukocyte infiltration and plaque formation.2 To evaluate the participation of VASP in the regulation of platelet adhesion in developing atherosclerosis, fluorescent wild-type or VASP null platelets were transfused into ApoE-deficient recipient mice, which had consumed a cholesterol-enriched diet. Platelet adhesion was monitored in the carotid arterial bifurcation by in vivo fluorescence microscopy.2 The adhesion of wild-type platelets (ApoE+/+, VASP+/+) to ApoE null endothelium was increased 2-fold when compared with wild-type recipients (Figure 2C). The loss of VASP in platelets further enhanced platelet adhesion in atherosclerosisprone ApoE–/– mice. In fact, the number of VASP null platelets firmly adherent to the vascular wall of ApoE–/– mice increased approximately 4-fold compared with VASP+/+ platelets. Although both platelets and endothelial cells express VASP, the isolated loss of platelet VASP was associated with enhanced platelet–vessel wall interactions in atherosclerotic mice. This indicates that under the pathophysiologic conditions of atherosclerosis, VASP expressed in the vascular wall is not sufficient to prevent platelet adhesion.
VASPnull platelets show enhanced adhesion following endothelial denudation
In advanced atherosclerosis, fissuring or rupture of the atherosclerotic lesion leads to endothelial denudation and exposure of the thrombogenic subendothelial matrix to circulating platelets, initiating platelet recruitment to the injured vessel wall. In order to address whether, apart from modulating platelet–endothelial cell interactions, VASP might be involved in the regulation of platelet adhesion to the subendothelial matrix, we assessed platelet–vessel wall interactions following vascular injury of the mouse carotid artery. Carotid vascular injury was induced in wild-type animals by vigorous ligation for 5 minutes,40 which consistently causes complete loss of the endothelial cell layer and initiates platelet adhesion at the site of injury, as demonstrated by scanning electron microscopy (Figure 3A). Subsequently, the adhesion of thrombin-activated (0.2 U/mL) wild-type or VASP null platelets to the injured carotid artery was determined by in vivo fluorescence microscopy.
Platelet adhesion following endothelial denudation. (A) Scanning electron micrographs of carotid arteries prior to (left) and after (right) vascular injury. Endothelial denudation induces platelet adhesion. Bars represent 5 μm. (B-C) Fluorescent wild-type or VASP–/– platelets were preincubated with either PBS (Control) or the NO-donor spermine-NO. After preincubation, the samples were stimulated with 0.2 U/mL mouse thrombin or with PBS. Platelet tethering (B) and firm platelet adhesion (C) to the carotid artery were assessed by intravital videofluorescence microscopy. Mean ± SEM; n = 5 each group; * indicates significant difference compared with control; ns indicates no significant difference; P < .05. (D) The microphotographs show representative in vivo fluorescence microscopy images in illustrating adhesion of wild-type or VASP–/– platelets following endothelial denudation in the absence or presence of spermine-NO. Bars represent 50 μm. (E) Role of GPIIb-IIIa for tethering and adhesion of VASP null platelets following endothelial denudation. Wild-type mice were injected with VASP–/– platelets preincubated with 50 μg function-blocking anti–GPIIb-IIIa mAb. Platelet recruitment was visualized at the site of injury by videofluorescence microscopy. The graph shows percent inhibition of platelet tethering and adhesion by anti–GPIIb-IIIa mAb (n = 5) compared with PBS-treated mice (n = 4). Mean ± SEM.
Platelet adhesion following endothelial denudation. (A) Scanning electron micrographs of carotid arteries prior to (left) and after (right) vascular injury. Endothelial denudation induces platelet adhesion. Bars represent 5 μm. (B-C) Fluorescent wild-type or VASP–/– platelets were preincubated with either PBS (Control) or the NO-donor spermine-NO. After preincubation, the samples were stimulated with 0.2 U/mL mouse thrombin or with PBS. Platelet tethering (B) and firm platelet adhesion (C) to the carotid artery were assessed by intravital videofluorescence microscopy. Mean ± SEM; n = 5 each group; * indicates significant difference compared with control; ns indicates no significant difference; P < .05. (D) The microphotographs show representative in vivo fluorescence microscopy images in illustrating adhesion of wild-type or VASP–/– platelets following endothelial denudation in the absence or presence of spermine-NO. Bars represent 50 μm. (E) Role of GPIIb-IIIa for tethering and adhesion of VASP null platelets following endothelial denudation. Wild-type mice were injected with VASP–/– platelets preincubated with 50 μg function-blocking anti–GPIIb-IIIa mAb. Platelet recruitment was visualized at the site of injury by videofluorescence microscopy. The graph shows percent inhibition of platelet tethering and adhesion by anti–GPIIb-IIIa mAb (n = 5) compared with PBS-treated mice (n = 4). Mean ± SEM.
Within the first minutes after endothelial denudation, numerous wild-type platelets were tethered to the vascular wall (2530 ± 82 platelets/mm2; Figure 3B-D). Virtually all wild-type platelets establishing contact with the subendothelium showed ensuing irreversible stable arrest (2089 ± 58 platelets/mm2; Figure 3B-D). The isolated loss of VASP in platelets significantly enhanced platelet tethering (3191 ± 144 platelets/mm2; P < .05 vs wild-type platelets) and firm platelet adhesion after vascular injury (2753 ± 128 platelets/mm2; P < .05 vs wild-type platelets). GPIIb-IIIa, which mediates platelet adhesion to the intact endothelium in VASP null mice, is also involved in recruitment of VASP null platelets to the site of vascular injury. Correspondingly, preincubation of VASP null platelets with function-blocking anti–GPIIb-IIIa mAb strongly reduced adhesion to the area of endothelial denudation (by 62% compared with PBS-pretreated platelets), while platelet tethering was not significantly altered (Figure 3E). Together, this supports the concept that platelet VASP is involved in the regulation of both platelet–endothelial cell and platelet-subendothelium interactions, and loss of VASP-dependent GPIIb-IIIa inhibition might at least in part account for the increase in adhesion of VASP null platelets to both the intact endothelium and to sites of vascular injury.
VASPnull platelets are unresponsive to nitric oxide
NO is known to be a very important endogenous platelet antagonist,41,42 signaling platelet inhibition through cGKI.6 To determine whether VASP mediates NO/cGMP-dependent regulation of platelet adhesion, we next investigated the effects of NO on adhesion of wild-type and VASP–/– platelets to the injured vascular wall. Fluorescent wild-type or VASP–/– platelets were preincubated with the NO-donor spermine-NO followed by stimulation with thrombin. Adhesion of thrombin-activated platelets to the carotid artery of wild-type mice was monitored by intravital videofluorescence microscopy. In wild-type platelets, pretreatment with NO decreased platelet tethering and adhesion to the injured vascular wall by approximately 56% and 57% (Figure 3B-D). In contrast, in platelets lacking VASP the response to NO was nearly absent. While platelet tethering was reduced by 8%, firm platelet arrest was attenuated by 9% (P < .05 vs wild-type platelets).
Discussion
Under both physiologic and pathophysiologic conditions, platelet adhesion is strongly inhibited by cAMP- and cGMP-elevating agents,5,34 acting via cGK and cAK.3,6,7 However, the downstream signaling pathway involved in cAMP- and cGMP-dependent inhibition of platelet–vessel wall interactions remains controversial. A major substrate of both cAK and cGK is VASP, a 46/50 kDa vasodilator-stimulated protein, expressed in high concentrations in platelets, endothelial cells, and smooth muscle cells.8-10 However, the in vivo significance of VASP in platelet function is poorly understood.
In the present study we used intravital fluorescence microscopy to directly examine the role of VASP for the regulation of platelet–vessel wall interactions in vivo. The data show that VASP is associated with the inhibition of platelet–endothelial cell interactions under physiologic conditions in the common carotid artery and in the intestinal microcirculation. Moreover, the loss of VASP strongly enhanced platelet–vessel wall interactions (i) following I/R, (ii) in atherosclerotic mice, or (iii) in response to endothelial denudation. P-selectin and GPIIb-IIIa were involved in platelet–endothelial cell interactions in VASP null mice under physiologic conditions, while GPIIb-IIIa contributed to adhesion of VASP null platelets following endothelial denudation. Together, these findings support the concept that under both physiologic and pathophysiologic conditions (I/R, atherogenesis, and endothelial denudation), cGMP- and cAMP-elevating agents, such as NO and prostacyclin, act via VASP to attenuate platelet adhesion in vivo. Interestingly, the isolated loss of VASP in platelets resulted in increased platelet adhesion following vascular injury or in atherosclerosis-prone mice. In contrast, in the absence of endothelial cell dysfunction or endothelial denudation, the isolated loss of platelet VASP was not associated with enhanced platelet adhesion to the vascular wall. This implicates that the presence of VASP in the vascular wall (endothelial cells, smooth muscle cells) is essential to control platelet–endothelial cell interactions under physiologic conditions but is not sufficient to prevent platelet adhesion in the presence of endothelial dysfunction or following endothelial denudation.
Importantly, a previous investigation by Aszodi and coworkers32 clearly demonstrated that VASP null platelets show a more rapid shape change and aggregation in response to collagen compared with wild-type platelets. Likewise, agonist-induced fibrinogen binding to the platelet surface was significantly enhanced in the absence of VASP. However, the alterations in platelet function observed in VASP null mice did not translate into changes of tail-bleeding time. Here we show that platelet–endothelial cell interactions are enhanced in VASP null mice even under physiologic conditions. This apparent discrepancy may suggest that VASP deficiency induces subtle changes in platelet–vessel wall interactions, which are overcome by other inhibitory pathways during hemostasis. However, it appears important to note that the value of tail bleeding assays or platelet counts in predicting the events that underlie normal hemostasis is not established.43 In particular, there is no clear correlation between reduced bleeding time and thrombotic risk.43
Unlike VASP–/– mutants, mice deficient in cGKI showed enhanced platelet adhesion following I/R but not under resting conditions.6 This suggests that under physiologic conditions, the action of either cGMP or cAMP is sufficient to maintain the antiadhesive properties of the endothelial surface. Hence, in the physiologic state a defect in the NO/cGKI/VASP cascade is compensated by endothelium-derived prostacyclin signaling through the unperturbed cAK/cAMP/VASP pathway.3,6 In VASP null mice, platelet adhesion was significantly increased even in the absence of vascular injury. This further supports the concept that VASP is located at the intersection of both antiadhesive signaling cascades, and loss of VASP cannot be compensated for by either cGKI- or cAMP-dependent pathways.
While prostacyclin has been postulated to act as the basal controller of platelet reactivity in the physiologic state,3 the NO/cGMP/cGKI signaling cascade appears to be particularly relevant for inhibition of platelet–vessel wall interactions under pathophysiologic conditions.6,41 Therefore, to delineate further the significance of VASP in NO-dependent inhibition of platelet adhesion under pathophysiologic conditions, we determined the effects of NO on the adhesion of wild-type or VASP null platelets following vascular injury. In wild-type mice, NO strongly attenuated platelet tethering and adhesion to the injured vessel segment. By contrast, in platelets lacking VASP, NO was not effective in preventing platelet recruitment to the vessel wall following endothelial denudation. Hence, VASP is essential for NO-dependent inhibition of platelet adhesion following vascular injury in vivo, and the loss of VASP cannot be compensated by other cGKI pathways, such as phosphorylation of the thromboxane receptor,44 inositol 1,4,5-trisphophate receptor–associated cGMP kinase substrate (IRAG),45,46 or other potential substrates.
Recent evidence supports a role of VASP in the regulation of adhesion receptors, which are essentially involved in platelet–vessel wall interactions, including P-selectin and the platelet fibrinogen receptor GPIIb-IIIa. Hauser and colleagues31 reported earlier that VASP is involved in the inhibition of agonist-induced P-selectin expression on platelets. In addition, there is increasing evidence implicating a role of VASP for the modulation of GPIIb-IIIa ligand binding affinity: (i) VASP and integrins colocalize along highly dynamic filamentous membrane structures11,15 ; (ii) VASP phosphorylation correlates with reduced fibrinogen binding affinity of GPIIb-IIIa30 ; (iii) VASP null mutants show increased activation of platelet GPIIb-IIIa and enhanced aggregation in response to collagen31,32 ; and (iv) inhibition of platelet aggregation by low doses of cyclic nucleotides is impaired in VASP-deficient mice.31,32 In the present manuscript we demonstrate for the first time in vivo that both P-selectin and GPIIb-IIIa are essentially involved in platelet recruitment in VASP null mice under physiologic conditions. In addition, GPIIb-IIIa also contributed to firm adhesion of VASP-deficient platelets following arterial denudation. This supports the notion that loss of VASP is associated with enhanced P-selectin and GPIIb-IIIa exposure/activation and further substantiates the biologic significance of VASP as a negative regulator in the platelet adhesion cascade.
Importantly, nitric oxide has been demonstrated to inhibit agonist-induced GPIIb-IIIa activation on platelets and to attenuate P-selectin expression on both endothelial cells and platelets. Interestingly, platelets per se release nitric oxide in substantial amounts upon activation and, therefore, appear to have the ability to self-regulate their adhesion and aggregation by a NO-dependent autocrine/paracrine mechanism. Based on our present findings that platelet adhesion to the endothelium is enhanced in the absence of VASP and that VASP null platelets are unresponsive to nitric oxide, it can be speculated that loss of VASP disrupts NO-dependent platelet autocrine or paracrine regulation and is associated with enhanced activation of platelet and/or endothelial adhesion receptors, resulting in increased P-selectin surface expression and fibrinogen binding capacity of platelets and/or endothelial cells under both physiologic and pathophysiologic conditions. Apart from P-selectin and GPIIb-IIIa, additional receptor-ligand interactions, such as GPVI- or GPIa-IIa–collagen interactions, might be controlled by a NO/VASP pathway. In fact, our observation that platelet tethering, a process which appears to be more or less GPIIb-IIIa independent, is increased in VASP null platelets supports this notion. However, further studies will be required to address this important issue.
The exact mechanisms of VASP-dependent regulation of P-selectin expression and GPIIb-IIIa function have not been identified thus far. However, since VASP participates in the regulation of actin assembly22,47 and phosphorylation of VASP negatively regulates its interaction with actin filaments48 and its localization at focal adhesion sites in intact cells,49 it can be envisaged that VASP-dependent regulation of platelet integrins involves either an unidentified modulation of integrin-cytoskeletal linkage or VASP-regulated stress fiber bundling, resulting in a corresponding change in integrin clustering and ligand binding avidity. Similar mechanisms might account for VASP-dependent regulation of α-granule release and thus P-selectin expression.
In conclusion, we have demonstrated in vivo that VASP is involved in the negative regulation of platelet adhesion to the vascular wall under physiologic conditions. Moreover, the loss of VASP in platelets is associated with an increase in platelet accumulation in the postischemic intestinal microvasculature and with a significant enhancement of platelet adhesion to the atherosclerotic vascular wall. The latter appears particularly important since reduced phosphorylation levels of VASP have been reported in the atherosclerotic vasculature. Inhibition of agonist-induced platelet adhesion through the NO/cGMP pathway is defective in VASP null platelets. These data extend our knowledge on how platelet adhesion is regulated in vivo and point to the importance of VASP in this process.
Prepublished online as Blood First Edition Paper, August 21, 2003; DOI 10.1182/blood-2002-11-3417.
Supported by the Deutsche Forschungsgemeinschaft (SFB 355) and by the Graduate program 438 “Vascular Biology in Medicine” (Deutsche Forschungsgemeinschaft).
S.M., S.G., and I.K. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank K. Langenbrink, S. Kerstan, A. Wallmuth, and H. Wehnes for excellent technical assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal