Abstract
Human-virus–specific CD8+ T cells that are found during primary infection have been studied almost exclusively in the peripheral blood, and it is unclear whether these cells are regulated in the same way as those in secondary lymphoid tissue. We investigated, therefore, the control of apoptosis and telomere erosion of Epstein-Barr virus (EBV)–specific CD8+ T cells found in the blood and tonsils of the same patients during acute infectious mononucleosis (AIM). Although the clonal composition of CD8+ T cells as determined by heteroduplex analysis was similar in both compartments, there was greater CD28 expression in the tonsil population, indicating that they were less differentiated. EBV-specific CD8+ T cells in both tissue types were extremely susceptible to apoptosis related to low Bcl-2 expression and were dependent on exogenous cytokines such as interleukin-2 (IL-2), IL-15, and interferon-α/β (IFN-α/β) for survival. In both compartments, however, these cells maintained their telomere lengths through telomerase induction. Thus, apoptosis-prone EBV-specific CD8+ T cells found during acute infection have to be rescued from death to persist as a memory population. However, signals that induce telomerase ensure that the rescued cells retain their replicative capacity. Significantly, these processes operate identically in cells found in blood and secondary lymphoid tissue.
Introduction
After primary infection in animals, virus-specific T cells found in different tissues are not identical.1,2 In addition, the extensive lymphadenopathy that occurs during Epstein-Barr virus (EBV) and other primary viral infections in humans suggests that the lymphoid tissue is a major site at which the virus-specific CD8+ T cells may be activated.3 However, most studies on EBV-specific CD8+ T cells during primary infection in humans have been performed on populations in peripheral blood only,4-8 and the relationship of these cells to those found in secondary lymphoid tissue is unknown. Important questions that remain unanswered are whether virus-specific CD8+ T cells of the same specificity are found in different anatomic sites, whether they are at the same stage of differentiation, and whether mechanisms that control their survival and persistence operate identically in different lymphoid compartments.
Apoptosis induced by cytokine deprivation9,10 and telomere erosion are 2 mechanisms that control the survival and replicative capacity of virus-specific CD8+ T cells after the primary response.9,11 Telomeres are repeating hexameric units of DNA and protein found at the ends of chromosomes.12 In the absence of compensatory mechanisms, repeated cell division results in critically short telomeres that signal growth arrest or replicative senescence.12 Previous studies have shown that peripheral blood populations of EBV-specific CD8+ T cells are susceptible to apoptosis.13,14 It is unknown, however, whether these cells are destined for clearance and those found in lymphoid tissues are preferentially selected for survival. In addition, it has been shown previously that there is considerable proliferation of the EBV-specific peripheral blood pool of CD8+ T cells during acute infection,8 and it has been proposed that the extent of this proliferation is sufficient to result in loss of replicative capacity caused by telomere erosion.9,15 Although EBV-specific CD8+ T-cell populations in peripheral blood may avoid this by inducing telomerase activity,16,17 it is unclear whether this is a general phenomenon that also applies to cells found in secondary lymphoid tissue during acute infectious mononucleosis (AIM).
For an efficient memory pool to persist after primary infection, not only must specific CD8+ T cells escape apoptosis, they must retain enough replicative capacity to allow subsequent proliferation in response to virus restimulation throughout life.18 In this study we demonstrate a close clonal relationship between EBV-specific CD8+ T cells in the blood and tonsils of the same patients during AIM. We also show that mechanisms that prevent apoptosis and telomere erosion operate equally in the CD8+ T cells from both compartments, and we suggest that both sets of signals have to be engaged during primary infection to enable the selection of specific cells that persist to form the memory pool.
Patients, materials, and methods
Patient and control tissues
Tonsil specimens were obtained from 26 patients in whom AIM was diagnosed. Fourteen patients with chronic tonsillitis with lymphofollicular hyperplasia were used as controls. Peripheral blood mononuclear cells (PBMCs) from 12 of the AIM patients and 6 of the control patients at the time of tonsillectomy were also studied. This study received ethical approval from the Royal Free Hampstead National Health Service (NHS) Trust (London) and the Ethics Committee of the Faculty of Clinical Medicine Mannheim, Ruprecht-Karls University of Heidelberg, where the tonsillectomies were performed.
Sample isolation, preparation, and storage.
Fresh tonsil specimens were cryopreserved in liquid nitrogen or were formalin-fixed and embedded in paraffin or were disaggregated with isolated mononuclear fractions cryopreserved in liquid nitrogen. PBMCs were prepared from heparinized peripheral blood at the time of tonsillectomy, and CD8+ T cells were isolated by negative selection using VARIO MACS (Miltenyi Biotech, Surrey, United Kingdom). EBV-specific CD8+ T cells were isolated by positive selection using phycoerythrin (PE)–conjugated major histocompatibility complex (MHC) class I tetrameric complexes followed by anti-PE magnetic bead isolation, as described.8,17
Flow cytometric analysis of cell phenotype.
FACScalibur (Becton Dickinson, Oxford, United Kingdom) 4-parameter analysis of T-cell phenotype was performed as described.8,17 EBV-specific tetramers coupled to streptavidin-PE or to streptavidin-Cy5 were used to identify different EBV-lytic, epitope-specific CD8+ T cells.8,17 Lytic epitopes used were the HLA-A2–restricted ligands GLCTLVAML and YVLDHLIVV and the HLA-B8–restricted peptide ligand RAKFKQLL.
Telomere length measurement by flow-FISH
Telomere length was measured using a modified version of the flow cytometric–fluorescent in situ hybridization (flow-FISH) protocol previously described.17 A cross-linking agent (BS3) was used for 2- or 3-color protocols, as described.19 In addition, further controls to standardize the technique were routinely used. These included the use of a no-probe control, fluorescence-labeled beads (DAKO, Ely, Cambrigeshire, United Kingdom), and cryopreserved PBMC samples with previously characterized telomere fluorescence.
Susceptibility to apoptosis and telomerase activity
PBMCs were cultured in complete medium in the presence or absence of exogenous growth factors, as described.8 Viable cell recovery was assessed by trypan blue dye exclusion, and cell survival in culture was calculated as previously described.8,17 The expression of Bcl-2, Bax, Bcl-x, and β-actin mRNA was determined using reverse transcription–polymerase chain reaction (RT-PCR).20 Telomerase activity was measured using the telomeric repeat amplification protocol (TRAP; TRAPeze Telomerase Detection Kit; Intergen Company, Oxford, United Kingdom).
Histologic analysis of tonsil samples
Paraffin-embedded tonsillectomy specimens of 14 AIM patients (age range, 14-41 years; mean age, 22 years; 8 male/6 female) and 8 controls (age range, 12-34 years; mean age, 18.5 years; 4 male/4 female) with chronic tonsillitis were analyzed using immunoperoxidase staining for expression of various molecules, as described previously.21 The extent of EBV infection was determined by probing for EBER (EBV early RNA; Novocastra, Newcastle upon Tyne, United Kingdom) using in situ hybridization. Apoptosis was investigated by the terminal dUTP nick-end labeling (TUNEL) technique. Semiquantitative assessment of histologic staining was performed by scoring the percentage of labeled cells within the interfollicular zones. Dual staining for EBER and TUNEL has been described previously.21
Assessment of clonality of AIM T cells by heteroduplex analysis
The clonal relationship between CD8+ T cells in blood and tonsils from AIM patients was determined by comparing the CDR3 region of different T-cell receptor (TCR) Vβ families in paired blood and tonsil CD8+ T-cell samples of patients. PCR was performed on 26 Vβ families using specificVβ and common Cβ primers. Each sample PCR was mixed with a Vβ-matched DNA carrier, denatured, and reannealed. The product was blotted and hybridized with a probe to the external Cβ region of the carrier. The probe was detected using anti–digoxigenin-AP Fab fragments and CDP-Star substrate (Roche Diagnostics, Mannheim, Germany). Identical clones within CD8+ T-cell populations from blood and tonsils were detected as heteroduplex bands with identical migration patterns.22 The presence of heteroduplexes has previously been shown to relate to the presence of an expanded clone, as determined by sequencing of the PCR product.22
Results
Relationship between EBV-specific T cells in blood and tonsils during primary infection
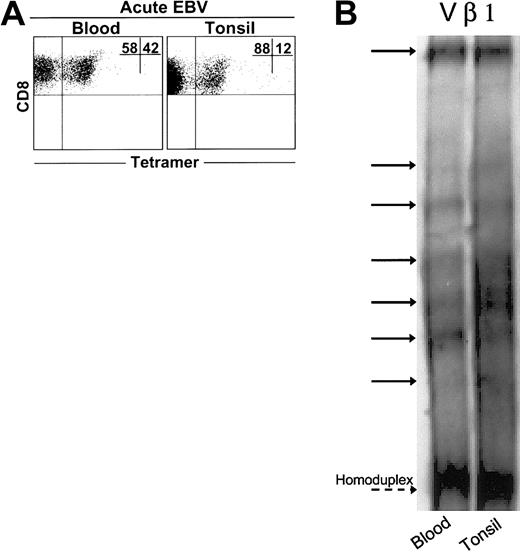
We found expanded populations of EBV-lytic, epitope-specific CD8+ T cells in blood and tonsil compartments during AIM (Figure 1A). However, these cells constituted a greater percentage of CD8+ T-cell populations in blood than in tonsils in 5 of 6 patients investigated (not shown). We next compared CD8+ T cells during AIM in both tissue types using the heteroduplex technique (Figure 1B). We examined 23 different TCR Vβ families in each of 3 patients. With rare exceptions, no clones were found in CD8+ T-cell populations from healthy age-matched controls.22 A total of 237 expanded clones were detected in CD8+ T cells from the blood of 3 patients, whereas 270 clones were detected in the tonsils of the same patients. Of these, 92% ± 8% of clones found in blood CD8+ T cells were also found in tonsil CD8+ populations. Conversely, 82% ± 10% of clones in tonsils were also found in blood. Collectively, these results indicate close identity between virus-specific CD8+ T cells in blood and tonsils during primary EBV infection.
Clonal analysis of expanded CD8+T cells from AIM blood and tonsil samples. (A) Representative dot plot of CD8 versus tetramer (RAK) staining in matched blood and tonsil samples. Heteroduplex analysis was used to assess the clonality of purified CD8+ T cells from matched blood and tonsil samples taken from 3 AIM patients. PCR reactions were performed for 23 different Vβ families in the blood and tonsils of each patient. (B) Data from a representative Vβ family showing heteroduplex bands shared between CD8+ T cell blood and tonsils, as indicated by solid arrows, are shown. Dashed arrow indicates the homoduplex band.
Clonal analysis of expanded CD8+T cells from AIM blood and tonsil samples. (A) Representative dot plot of CD8 versus tetramer (RAK) staining in matched blood and tonsil samples. Heteroduplex analysis was used to assess the clonality of purified CD8+ T cells from matched blood and tonsil samples taken from 3 AIM patients. PCR reactions were performed for 23 different Vβ families in the blood and tonsils of each patient. (B) Data from a representative Vβ family showing heteroduplex bands shared between CD8+ T cell blood and tonsils, as indicated by solid arrows, are shown. Dashed arrow indicates the homoduplex band.
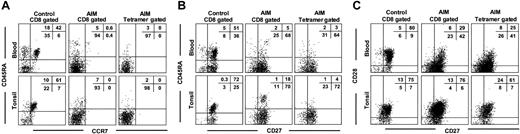
We found that the EBV-lytic, epitope-specific CD8+ T cells and total CD8+ T cells from blood and tonsils of AIM patients expressed low levels of CD45RA and the CCR7 chemokine receptor, indicating that they underwent considerable expansion (Figure 2A). In contrast, 40 ± 7 (mean ± SEM of 3 experiments) control CD8+ T cells in blood and 46 ± 12 of these cells in tonsils expressed both CD45RA and CCR7, indicating that they are less differentiated. Most EBV-specific and total CD8+ T-cell populations in the blood and tonsils of AIM patients that lose CD45RA expression retain CD27 expression (Figure 2B). Large numbers of CD8+ T cells in control blood and tonsil samples expressed both CD27 and CD45RA, again indicating that these populations are less differentiated than equivalent populations found during AIM.
Phenotypic analysis of blood and tonsil CD8+T cells from control and AIM patients. Expression of (A) CD45RA and CCR7, (B) CD45RA and CD27, and (C) CD27 and CD28 was investigated on CD8+ T cells from paired samples of control and AIM blood and tonsil samples. Paired blood and tonsil samples from AIM patients were investigated for the expression of the same markers in total CD8+ T cells (middle panels) or tetramer-gated, antigen-specific CD8+ T cells (right panels). Representative results from 1 of at least 3 separate paired blood and tonsil specimens are shown. Percentages of gated cells in each quadrant are shown as an inset in each panel.
Phenotypic analysis of blood and tonsil CD8+T cells from control and AIM patients. Expression of (A) CD45RA and CCR7, (B) CD45RA and CD27, and (C) CD27 and CD28 was investigated on CD8+ T cells from paired samples of control and AIM blood and tonsil samples. Paired blood and tonsil samples from AIM patients were investigated for the expression of the same markers in total CD8+ T cells (middle panels) or tetramer-gated, antigen-specific CD8+ T cells (right panels). Representative results from 1 of at least 3 separate paired blood and tonsil specimens are shown. Percentages of gated cells in each quadrant are shown as an inset in each panel.
The loss of the costimulatory molecule CD28 has been shown to precede that of CD27 during progressive differentiation of CD8+ T cells.4,7,23-25 We found that although the total CD8+ and EBV-specific T-cell populations from AIM tonsils expressed 70% ± 10% and 57% ± 16% of CD27 and CD28 T cells, respectively, only 28% ± 2% and 24% ± 1% of these cells in blood expressed both molecules (Figure 2C). This indicates that virus-specific CD8+ T cells from tonsils are less differentiated than those from blood. These differences appear to be intrinsic to the EBV-specific CD8+ T-cell populations because control blood and tonsils show similar proportions of cells expressing both molecules (Figure 2C).
The observation that 18% of hetroduplex-defined clones are found in tonsils but not in blood (Figure 1), together with the observation that EBV-specific CD8+ T cells from tonsils are less differentiated than their blood counterparts (Figure 2), suggests that the expansion of specific CD8+ cells occurs initially in the secondary lymphoid tissue during AIM and that more differentiated cells enter the bloodstream preferentially. We are investigating the relationship between different lytic and latent epitope-specific CD8+ populations in paired blood and tonsil samples. Our preliminary observations indicate that EBV latent epitope-specific CD8+ T cells were also less differentiated in the tonsils than in the blood of the same patients during AIM (not shown).
Extensive CD8+ T-cell proliferation in AIM is related to extent of B-cell infection
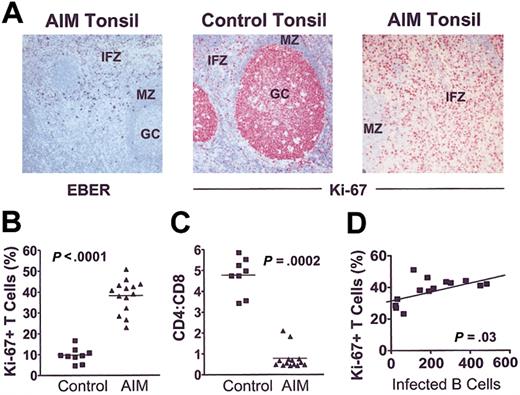
EBER-positive cells were observed mainly in the stroma underlying the crypt epithelium and in the superficial interfollicular zone (Figure 3A, left). Combined in situ hybridization for EBERs and immunostaining confirmed the EBER+ cells to be CD3–CD20+ B cells in all patients in whom this was investigated. Immunostaining for Ki-67 revealed high proliferative activity in germinal centers (GCs) but not in the interfollicular zone (IFZ) or the mantle zone (MZ) of control tonsils (Figure 3A, middle panel). In contrast, there were significantly higher numbers of Ki-67+ cells in the IFZ in tonsils from AIM patients (Figure 3A, right; 3B). More than 99% of these cells were CD3+CD20– cells. The mean CD4/CD8 ratio was 5-fold lower than in control tonsils, indicating a disproportionate expansion of CD8+ T cells (Figure 3C). The extent of interfollicular T-cell proliferation was significantly correlated with the number of EBV-infected B cells (Figure 3D). These results indicate that preferential CD8+ T-cell expansion during primary EBV infection is directly linked to the extent of B-cell infection by the virus.
CD8+T-cell proliferation is linked to extent of B-cell expansion in AIM. (A) (left panel) Acute EBV tonsil contains EBER+ cells in the IFZ (original magnification × 100). (middle and right panels) Ki-67 staining in control and AIM tonsil sections, respectively (original magnifications × 100). Proliferative activity is low in the interfollicular zones and the follicular MZ compared with GCs in control (middle panel), whereas AIM tonsils show high proliferative activity in the expanded interfollicular T-cell zones (right panel). (B) Proliferation index (percentage of Ki-67+) of interfollicular T cells is markedly increased in AIM patients compared with controls (Mann-Whitney U test; P < .0001). (C) Ratio of CD4- to CD8-expressing cells was counted exclusively in the interfollicular zones. (D) Linear regression analysis showed that the increase in proliferative activity correlated with the number of EBV-infected cells, expressed as number of EBER+ cells per 10 000 lymphocytes (r = 0.57).
CD8+T-cell proliferation is linked to extent of B-cell expansion in AIM. (A) (left panel) Acute EBV tonsil contains EBER+ cells in the IFZ (original magnification × 100). (middle and right panels) Ki-67 staining in control and AIM tonsil sections, respectively (original magnifications × 100). Proliferative activity is low in the interfollicular zones and the follicular MZ compared with GCs in control (middle panel), whereas AIM tonsils show high proliferative activity in the expanded interfollicular T-cell zones (right panel). (B) Proliferation index (percentage of Ki-67+) of interfollicular T cells is markedly increased in AIM patients compared with controls (Mann-Whitney U test; P < .0001). (C) Ratio of CD4- to CD8-expressing cells was counted exclusively in the interfollicular zones. (D) Linear regression analysis showed that the increase in proliferative activity correlated with the number of EBV-infected cells, expressed as number of EBER+ cells per 10 000 lymphocytes (r = 0.57).
CD8+ T cells from tonsils and blood are susceptible to apoptosis during AIM
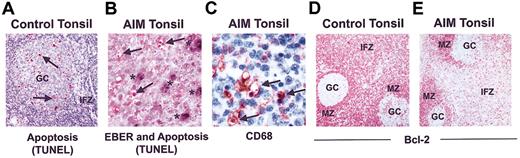
The relationship between apoptosis and extent of EBV infection during AIM is unknown. In control tonsils, apoptotic activity was predominantly observed in germinal centers (Figure 4A), and only 0.2% ± 0.07% of T cells in the IFZ were apoptotic. In contrast, a significantly higher proportion of T cells in the interfollicular T-cell zone of tonsils from AIM patients were apoptotic (1.7% ± 0.45%; P < .0001), and these cells were observed throughout this compartment (Figure 4B). This level of apoptotic activity was comparable to that observed in florid germinal centers of control tonsil tissue (1.8% ± 0.34%). EBER in situ hybridization coupled to TUNEL demonstrated that the apoptotic cells in the interfollicular T cell zone were not EBER-positive B cells (Figure 4B). In addition, many apoptotic bodies appeared to be localized together within vacuoles (Figure 4B) that were identified by CD68 staining to be the cytoplasm of macrophages (Figure 4C).
Apoptosis in control and AIM tonsil samples. (A) Apoptotic cells as identified by TUNEL in control tonsils are predominantly in the GCs and not in the IFZ (original magnification × 50). (B) Apoptotic cells in AIM tonsils, defined by TUNEL staining and indicated by arrows, do not costain with EBER, indicated by asterisks (original magnification × 400). (C) CD68 staining showing apoptotic cells with condensed nuclei being engulfed by macrophages (original magnification × 400). Bcl-2 expression in representative (D) control and (E) AIM tonsils is shown. Cells in the IFZ and MZ of control tonsils expressed high levels of Bcl-2, whereas GCs contained few positive cells (original magnification × 100). Results are representative of 14 AIM and 8 control tonsils investigated.
Apoptosis in control and AIM tonsil samples. (A) Apoptotic cells as identified by TUNEL in control tonsils are predominantly in the GCs and not in the IFZ (original magnification × 50). (B) Apoptotic cells in AIM tonsils, defined by TUNEL staining and indicated by arrows, do not costain with EBER, indicated by asterisks (original magnification × 400). (C) CD68 staining showing apoptotic cells with condensed nuclei being engulfed by macrophages (original magnification × 400). Bcl-2 expression in representative (D) control and (E) AIM tonsils is shown. Cells in the IFZ and MZ of control tonsils expressed high levels of Bcl-2, whereas GCs contained few positive cells (original magnification × 100). Results are representative of 14 AIM and 8 control tonsils investigated.
In control tonsils, the antiapoptotic molecule Bcl-2 was absent from germinal centers, whereas it was expressed at high levels by cells in the MZ and IFZ (54% ± 12%; Figure 4D). However, this protein was significantly decreased in T-cell areas but not in the MZ of AIM tonsils (33% ± 10%; P < .0012; Figure 4E). Levels of T-cell apoptosis (r = 0.65; P = .01) and Bcl-2 expression (r = 0.72; P = .003) in the in the IFZ were significantly correlated with the number of infected B cells present in 14 AIM tonsils investigated.
Cytokine-mediated rescue of CD8+ T cells from apoptosis in AIM patients
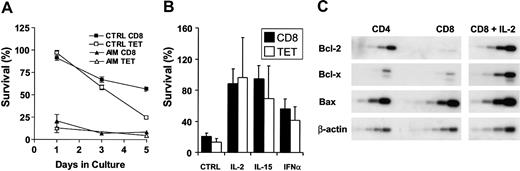
Peripheral blood populations of CD8+ and EBV-lytic, epitopespecific T-cell populations of AIM patients were susceptible to apoptosis when cultured without exogenous cytokines compared with the same cells taken from persons between 3 and 10 years after recovery from AIM (Figure 5A). We found that apoptosis was not inevitable and that cytokines such as IL-2, IL-15, and interferon-β (IFN-β) could promote the survival of total CD8+ or EBV-lytic, epitope-specific CD8+ T cells of AIM patients (Figure 5B) and of patients with chronic EBV infection (not shown). The susceptibility to apoptosis of CD8+ T cells from the peripheral blood of AIM patients was also related to low Bcl-2 expression, and rescue from apoptosis by IL-2 resulted in an increased level of Bcl-2 and a second antiapoptotic molecule, Bcl-XL, but not the proapoptotic molecule Bax (Figure 5C). This suggests that virus-specific CD8+ T cells found in blood and secondary lymphoid tissue during AIM are susceptible to apoptosis. However, some cells can be rescued from death by cytokines, and this may allow a memory pool to this virus to persist.
Total CD8+and antigen-specific T cells from AIM patients are susceptible to apoptosis in vitro but can be rescued by cytokines. (A) Freshly isolated PBMCs from AIM patients or from those with previous history of AIM were cultured in complete culture medium for 5 days. Survival of total CD8+ and tetramer-positive CD8+ T cells was calculated as a percentage of the initial number of CD8 or tetramer-positive T cells present before culture. Mean ± SEM of triplicate determinations from 1 of 3 separate experiments with similar results are shown. (B) Survival of CD8+ and EBV-lytic, epitope-specific CD8+ T cells cultured in the presence or absence of IL-2, IL-15, or type 1 IFN for 24 hours was investigated. Mean ± SEM percentage survival of 3 separate experiments is shown. (C) Expression of Bcl-2, Bcl-x, and Bax mRNA levels were determined by RT-PCR, performed over a range of cycles. Representative blot shows the level of expression in freshly isolated CD4+ and CD8+ T cells from an AIM patient and the same CD8+ T cells cultured in the presence of IL-2 for 24 hours.
Total CD8+and antigen-specific T cells from AIM patients are susceptible to apoptosis in vitro but can be rescued by cytokines. (A) Freshly isolated PBMCs from AIM patients or from those with previous history of AIM were cultured in complete culture medium for 5 days. Survival of total CD8+ and tetramer-positive CD8+ T cells was calculated as a percentage of the initial number of CD8 or tetramer-positive T cells present before culture. Mean ± SEM of triplicate determinations from 1 of 3 separate experiments with similar results are shown. (B) Survival of CD8+ and EBV-lytic, epitope-specific CD8+ T cells cultured in the presence or absence of IL-2, IL-15, or type 1 IFN for 24 hours was investigated. Mean ± SEM percentage survival of 3 separate experiments is shown. (C) Expression of Bcl-2, Bcl-x, and Bax mRNA levels were determined by RT-PCR, performed over a range of cycles. Representative blot shows the level of expression in freshly isolated CD4+ and CD8+ T cells from an AIM patient and the same CD8+ T cells cultured in the presence of IL-2 for 24 hours.
Extensive T-cell proliferation does not lead to telomere loss during AIM
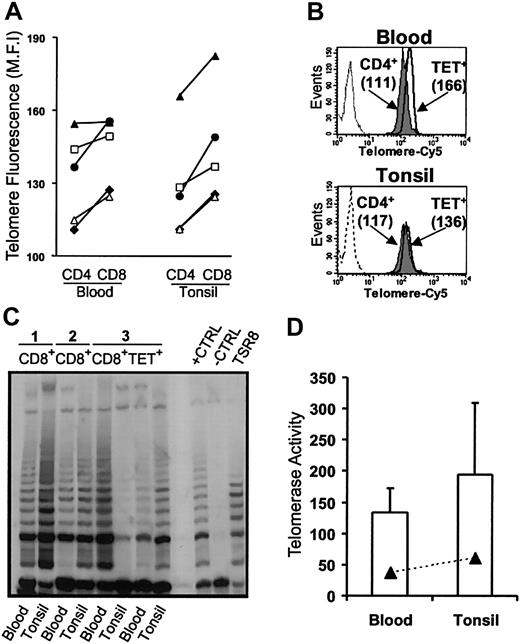
The calculated net expansion of EBV-specific CD8+ T cells during AIM is sufficient to lead to replicative senescence resulting from telomere erosion.15 We next investigated whether this was a constraint on the virus-specific CD8+ T cells in the blood and tonsils of patients during AIM. In healthy persons, CD4+ T cells have longer telomeres than does the CD8+ subset.16,17,26 However, despite preferential CD8+ T-cell expansion (Figure 3C), CD8+ T cells from blood and tonsils had consistently longer telomeres than the CD4 populations in the same persons (Figure 6A). This increase in telomere length relative to the CD4 population was also observed when isolated populations of EBV-lytic, epitope-specific CD8+ T cells from blood and tonsils were compared with CD4+ T cells (Figure 6B). CD8+ T cells from AIM patients that were not specific for the lytic epitopes investigated also had longer telomeres than the CD4+ T-cell population. This is probably because other EBV-specific CD8+ T cells with different specificities are also found within this population.19 A no-probe control (Figure 6B) that showed minimal fluorescence was also used in blood and tonsil samples to demonstrate the specificity of binding.
Relative telomere length and telomerase activity in blood and tonsils from AIM patients. (A) Telomere lengths were assessed using flow FISH in CD4+ and CD8+ T cells. Each symbol represents blood and tonsil samples taken from individual patients. (B) Telomere lengths were also investigated in isolated EBV-specific CD8+ T cells relative to the CD4+ population in the same patient. CD4+ (gray histogram) and tetramer-positive CD8+ T cells (open histogram) from blood (top panel) and tonsil (bottom panel) were isolated from AIM patients, and telomere lengths were determined by flow FISH. The no-probe control is also shown as a dashed profile. (C) Telomerase activity in blood and tonsils was measured in purified CD8+ T cells using the TRAP assay in 3 patients. In patient 3, telomerase activity was also measured in purified tetramer-positive CD8+ T cells. Positive and negative controls were run according to manufacturer's instructions. (D) Mean ± SEM telomerase activity for CD8+ and tetramer-positive T cells from the 3 separate samples were analyzed.
Relative telomere length and telomerase activity in blood and tonsils from AIM patients. (A) Telomere lengths were assessed using flow FISH in CD4+ and CD8+ T cells. Each symbol represents blood and tonsil samples taken from individual patients. (B) Telomere lengths were also investigated in isolated EBV-specific CD8+ T cells relative to the CD4+ population in the same patient. CD4+ (gray histogram) and tetramer-positive CD8+ T cells (open histogram) from blood (top panel) and tonsil (bottom panel) were isolated from AIM patients, and telomere lengths were determined by flow FISH. The no-probe control is also shown as a dashed profile. (C) Telomerase activity in blood and tonsils was measured in purified CD8+ T cells using the TRAP assay in 3 patients. In patient 3, telomerase activity was also measured in purified tetramer-positive CD8+ T cells. Positive and negative controls were run according to manufacturer's instructions. (D) Mean ± SEM telomerase activity for CD8+ and tetramer-positive T cells from the 3 separate samples were analyzed.
We found that the increase in telomere length was caused by an up-regulation of the enzyme telomerase in CD8+ T cells from blood and tonsils and in EBV-lytic, epitope-specific CD8+ T cells isolated from both tissue types (Figure 6C-D). The similar level of telomerase induction in CD8+ T cells specific for only 1 EBV protein and found in the CD8+ T-cell population as a whole in the same AIM patients suggests that most enzymatic activity observed in AIM is found within EBV-specific CD8+ T cells. Collectively these results demonstrate that preventing apoptosis and inducing telomerase are 2 mechanisms that have to be integrated when selecting the EBV-specific CD8+ T-cell pool after AIM.
Discussion
We have found that during primary infection, EBV-lytic, epitope-specific CD8+ T cells in blood and tonsils are closely related clonally and are regulated similarly by apoptosis and telomere erosion. Hence, EBV-specific CD8+ T cells in the circulation are representative of those in secondary lymphoid tissue during primary infection, which validates previous studies performed on blood populations only.4-8 An important observation was that both the proliferation and the susceptibility to apoptosis of the EBV-specific CD8+ T cells in tonsils was related to the extent of B-cell infection, indicating an antigen-driven component in the immune expansion and in the death of CD8+ T cells during AIM. We are performing a detailed analysis of the relationship between the extent of lytic and latent virus expression and the nature of the specific CD8 + T-cell response in these samples.
Although the expanded populations of virus-specific CD8+ T cells during AIM are programmed to die, they can be rescued by cytokines, and maintaining the telomere lengths of these cells ensures that those rescued from death will keep their replicative capacity. Nevertheless, the most highly expanded clones of virus-specific cells may be lost after primary infection, and this may be attributed to telomere erosion.15,17 This raises the possibility that telomerase induction may not compensate indefinitely for telomere loss in virus-specific CD8+ T cells,27 and is supported by the development of shorter telomeres in EBV-specific CD8+ T cells studied in the same patients during and 14 years after AIM.17
Important questions that remain to be addressed are how telomere-related replicative senescence and apoptosis are regulated in EBV-specific CD8+ T cells during chronic infection. Previous studies have shown that Bcl-2 is up-regulated on virus-specific memory CD8+ T cells, indicating that they are less susceptible to apoptosis than those found during acute infection.8,28 However, it is unclear whether the increase in Bcl-2 is caused by the influence of cytokines such as IL-2 and IL-15, which induce the survival and up-regulation of this molecule,20,29 or whether it is caused by a reprogramming of apoptosis in the virus-specific CD8+ T-cell population.8 Nevertheless, the apoptosis-resistant phenotype has also been observed in T cells that have been aged in vitro,30 suggesting that although apoptosis plays an important role during acute infection, this process may not be the major constraint on the persistence of virus-specific CD8+ T cells during the chronic phase of the disease.
The extent of apoptosis observed in the tonsils of AIM patients is dependent on the availability of antiapoptotic cytokines, and we are investigating the expression of cytokines that regulate death in tonsils from patients with AIM. However, the rate of clearance of apoptotic CD8+ T cells by macrophages determines the level of apoptosis observed at any given time.31 We have found that numerous apoptotic cells in AIM tonsils are found within macrophages, suggesting that there is a high incidence of death coupled to intensive clearance of these cells in situ.
Telomere levels in virus-specific CD8+ T cells decrease during chronic infection,17 but it is unknown whether this is sufficient to impose a limit on the persistence or replication of virus-specific memory CD8+ T cells in elderly persons. The loss of telomeres is related to the decreased ability of T cells that are repeatedly activated to up-regulate telomerase.11,32 Studies in most murine systems may not provide an accurate portrayal of the impact of telomere erosion on immune memory in humans because of the 10-fold longer telomeres found in mice33 and because of their much shorter lifespans. Nevertheless, the observation that sixth-generation telomerase-deficient mice show signs of premature aging34 and of dramatic reductions in the numbers of germinal centers formed after immunization35 does point to a role for telomere erosion in regulating immune responses.
A role for telomerase in regulating the replicative capacity of lymphocytes has been demonstrated in studies in which transfection of the catalytic unit of the enzyme prolongs replicative potential36-38 and restores cytotoxic function39 in human T lymphocytes.
The development of new murine models that have short telomeres may provide representative data on whether telomere erosion constrains memory T-cell persistence in humans.18 Given that the human life expectancy worldwide is increasing,40 the memory T-cell pool to infectious agents will have to be maintained for increasing periods in the future. An important question is whether this will ultimately lead to an increased incidence of infection caused by the development of replicative senescence in antigen-specific T-cell pools that have to be maintained for more than 8 decades in vivo.41
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-06-1791.
Supported by the British Biotechnology and Biological Research Council, The Henry Smith's Charity, Dermatrust, and Research into Aging.
M.V.D.S, F.J.P., and C.S.V. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank all the blood and tonsil donors for agreeing to take part in this study. We also thank Professor A. B. Rickinson and Dr A. Hislop for discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal