Abstract
Constitutive activation of aberrant fibroblast growth factor receptor 1 (FGFR1) kinase as a consequence of gene fusion such as FOP-FGFR1 associated with t(6; 8)(q27;p11-12) translocation, is the hallmark of an atypical aggressive stem cell myeloproliferative disorder (MPD) in humans. In this study, we show that expression of FOP-FGFR1 in primary bone marrow cells induced by retroviral transduction generates a MPD in mice. Constitutive FOP-FGFR1 kinase activity was both essential and sufficient to cause a chronic myeloproliferative syndrome in the murine bone marrow transplantation model. In contrast to the human disorder, lymphoproliferation and progression to acute phase were not observed. Lymphoid symptoms, however, appeared when onset of the disease was delayed as the result of mutation of FOP-FGFR1 at tyrosine 511, the phospholipase C γ (PLCγ) binding site.
Introduction
Chromosomal translocations that rearrange the fibroblast growth factor receptor 1 (FGFR1) gene at 8p11-12 region1 are associated with an aggressive stem cell myeloproliferative disorder (MPD) that frequently presents with B- or T-cell lymphoma and transforms into acute myeloid leukemia.2,3 The resulting fusion proteins contain oligomerization domains from different partners, including FGFR1 oncogene partner (FOP),4 fused to the tyrosine kinase domain of FGFR1, which is constitutively activated,5-8 and induce cell survival.6 We have evaluated here the leukemogenic potential of FOP-FGFR1 through retroviral transduction and transplantation of bone marrow cells. FOP-FGFR1 kinase induces a rapid and fatal chronic MPD in mice. The binding site of phospholipase C γ (PLCγ) of FOP-FGFR16 is required to trigger the disease.
Study design
DNA constructs
Bone marrow infection and transplantation
Generation and titering of retroviral stocks were done as previously described.10-13 Bone marrow from male C57BL/6J mice (CER-Janvier, Le Genest-St-Isle, France) donors, treated with 150 mg/kg 5-fluorouracil (5-FU; TEVA Pharma, Paris, France) during 5 days, was harvested and enriched by using the Sca-1+ MultiSort kit (Miltenyi-Biotec, Paris, France). Sca-1+ cells14 were seeded (106 cells/mL) in RPMI complete medium supplemented with recombinant murine cytokines (murine interleukin 3 [mIL-3], mIL-6, 10 ng/mL; murine stem cell factor [mSCF], 100 ng/mL; R&D Systems, Minneapolis, MN). After 48 hours in culture, 106 stem cells were infected twice in Retronectin (BioWittaker, Walkersville, MD)–coated Petri dishes15 with 2 mL of each viral supernatant supplemented with cytokines. Cells (105-106) were transplanted into lethally irradiated female syngeneic mice. Blood and bone marrow smears and cytospin preparations were stained with May-Grünwald-Giemsa stain. Histopathologic analyses were done on tissues fixed for 24 hours in 10% neutral-buffered formalin and were paraffin-embedded.
In vitro myeloid clonogenic progenitor assays
Sca-1+–transduced cells (104) were plated in methylcellulose medium as described.16 Colonies consisting of more than 50 cells were scored after 7 days. Cells from myeloid colony-forming cells were harvested and used for subsequent replating.
DNA, RNA, and protein analyses
To assess the integrity of the provirus and the clonality of samples, KpnI- or BamHI-digested genomic DNAs were hybridized with radioactive neomycin probes.
Results and discussion
To determine whether FOP-FGFR1 could induce leukemic transformation of primitive hematopoietic stem cells in vivo, Sca-1+ cells20 were transduced with MSCVneo vectors with/without FOP-FGFR1 or one of its mutants (kinase defective, FOP-FGFR1K259/A; or PLCγ-binding defective, FOP-FGFR1Y511/F). FOP-FGFR1 protein expression and tyrosine-phosphorylation status were verified (not shown). In clonogenic assays for myeloid progenitors,16 the primary colonies derived from progenitors infected with either FOP-FGFR1 vectors were heterogeneous in size and were similar to those obtained for control vector (not shown). To determine if FOP-FGFR1–infected progenitors might display perturbation in their self-renewal or differentiation potential, methylcellulose cultures were harvested, and cells were used for subsequent replating (Figure 1A). The majority of the colonies derived from murine stem cell virus (MSCV)–infected progenitors were small and diffuse. Only the FOP-FGFR1 colonies were compact and resembled colonies generated by primitive hematopoietic cells even in the third generation (32 ± 10 versus 3 ± 1 for control). Thus, FOP-FGFR1 markedly enhances the self-renewal capacity and proliferative potential of clonogenic hematopoietic progenitors in vitro. Moreover, as previously shown for Ba/F3 cells,6 FOP-FGFR1 did not induce cytokine-independent growth of primary hematopoietic cells but did sustain cell survival (1 month in absence of growth factors).
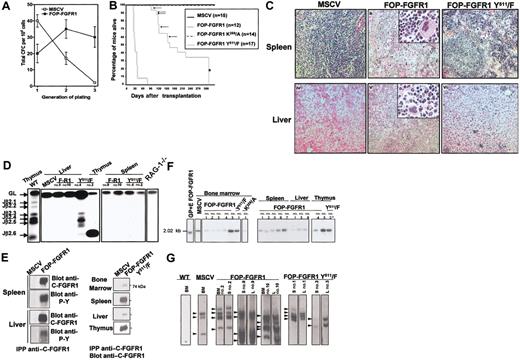
In vitro and in vivo characterization of the transforming properties of FOP-FGFR1 kinase. (A) Proliferative effect of FOP-FGFR1 on murine Sca-1+ hematopoietic progenitors. Each point represents the mean number (SEM) of total colony-forming cells (CFCs) per 104 cells generated after sequential plating in methylcellulose from three independent transduction experiments. (B) Kaplan-Meier plots of recipient mice survival after transplantation of bone marrow Sca-1+ stem cells infected with FOP-FGFR1 and its mutants as indicated (n=number of mice). Arrows and asterisk indicate the FOP-FGFR1 Y511/F mice that developed a thymic lymphoma and that were killed for characterization (n=10), respectively. (C) Histopathology of mice transplanted with FOP-FGFR1 and derived mutants. Views of spleen (ii, iii), and liver (v, vi) of transplanted mice. (i, iv) Normal tissues in a negative control mouse transplanted with Sca-1+ bone marrow cells retrovirally transduced with MSCV. In the FOP-FGFR1 animals, spleen (ii) and liver (v) showed effacement of normal architecture due to marked extramedullary hematopoiesis with maturing myeloid and erythroid precursors, and numerous megakaryocytes (original magnification × 100, inserts × 400). A slight extramedullary hematopoiesis was also found in the FOP-FGFR1 Y511/F spleens (iii). The liver from FOP-FGFR1 Y511/F mouse (vi) was almost normal, with only a few foci of inflammation. The 5-μm deparaffinized sections were hematoxylin-eosinstained. (D) TCR gene rearrangement in FOP-FGFR1 (F-R1) and FOP-FGFR1Y511/F (Y511/F) transplanted mice. Representative results of blot hybridization with J2 probes of gels containing PCR products from indicated tissues and mice using D2 5′ and J2 3′ PCR primers19 . Purified thymocytes from wild-type (WT) and RAG-1-/- mice were used as positive and negative controls, respectively. Autoradiographs were exposed for 4 hours so that the faint bands corresponding to TCR rearrangements of some mature T cells in spleen samples are not visible. (E) FOP-FGFR1 and FOP-FGFR1 PLC-binding–defective (Y511) are expressed in tissues involved in the myeloproliferative disease. To evaluate expression of the introduced FOP-FGFR1 (left panel) and FOP-FGFR1 Y511/F (right panel) proteins, cell lysates prepared from different tissues as indicated were subjected to immunoprecipitation with anti–C-FGFR1 and analyzed by Western blot with anti–C-FGFR1 and/or antiphosphotyrosine antibodies. Note the strong expression of the mutant in spleen, and thymus (in the animals with thymus hypertrophy) and the faint one in bone marrow and liver, in agreement with the histopathological findings. (F-G) Southern blot detection of provirus integration in tissues of FOP-FGFR1 mice. Genomic DNAs from bone marrow (BM), spleen (S), liver (L), and thymus from several FOP-FGFR1 and FOP-FGFR1 Y511/F transplanted and diseased mice, were digested with either KpnI (F) or BamHI (G) and hybridized with a radioactive probe from the neomycin resistance gene. (F) KpnI cuts in both retroviral LTRs; the single band of expected size demonstrates the presence of provirus. GP+E86 packaging cell line FOP-FGFR1 stably transfected (GP+E FOP-FGFR1), and bone marrow cells from one of the control vector mice (MSCV) were used as controls. Asterisk indicates the thymus sample from a FOP-FGFR1Y511/F mouse that developed a malignant lymphoma 126 days after transplantation. (G) BamHI cuts only once in the provirus; this results in bands of different sizes corresponding to 2 to 4 genomic integration sites (arrowheads). Note the presence of the same clones in different tissues from the same mouse. Bone marrow cells from a non-infected mouse (WT) and from one of the vector control mice (MSCV) were used as controls.
In vitro and in vivo characterization of the transforming properties of FOP-FGFR1 kinase. (A) Proliferative effect of FOP-FGFR1 on murine Sca-1+ hematopoietic progenitors. Each point represents the mean number (SEM) of total colony-forming cells (CFCs) per 104 cells generated after sequential plating in methylcellulose from three independent transduction experiments. (B) Kaplan-Meier plots of recipient mice survival after transplantation of bone marrow Sca-1+ stem cells infected with FOP-FGFR1 and its mutants as indicated (n=number of mice). Arrows and asterisk indicate the FOP-FGFR1 Y511/F mice that developed a thymic lymphoma and that were killed for characterization (n=10), respectively. (C) Histopathology of mice transplanted with FOP-FGFR1 and derived mutants. Views of spleen (ii, iii), and liver (v, vi) of transplanted mice. (i, iv) Normal tissues in a negative control mouse transplanted with Sca-1+ bone marrow cells retrovirally transduced with MSCV. In the FOP-FGFR1 animals, spleen (ii) and liver (v) showed effacement of normal architecture due to marked extramedullary hematopoiesis with maturing myeloid and erythroid precursors, and numerous megakaryocytes (original magnification × 100, inserts × 400). A slight extramedullary hematopoiesis was also found in the FOP-FGFR1 Y511/F spleens (iii). The liver from FOP-FGFR1 Y511/F mouse (vi) was almost normal, with only a few foci of inflammation. The 5-μm deparaffinized sections were hematoxylin-eosinstained. (D) TCR gene rearrangement in FOP-FGFR1 (F-R1) and FOP-FGFR1Y511/F (Y511/F) transplanted mice. Representative results of blot hybridization with J2 probes of gels containing PCR products from indicated tissues and mice using D2 5′ and J2 3′ PCR primers19 . Purified thymocytes from wild-type (WT) and RAG-1-/- mice were used as positive and negative controls, respectively. Autoradiographs were exposed for 4 hours so that the faint bands corresponding to TCR rearrangements of some mature T cells in spleen samples are not visible. (E) FOP-FGFR1 and FOP-FGFR1 PLC-binding–defective (Y511) are expressed in tissues involved in the myeloproliferative disease. To evaluate expression of the introduced FOP-FGFR1 (left panel) and FOP-FGFR1 Y511/F (right panel) proteins, cell lysates prepared from different tissues as indicated were subjected to immunoprecipitation with anti–C-FGFR1 and analyzed by Western blot with anti–C-FGFR1 and/or antiphosphotyrosine antibodies. Note the strong expression of the mutant in spleen, and thymus (in the animals with thymus hypertrophy) and the faint one in bone marrow and liver, in agreement with the histopathological findings. (F-G) Southern blot detection of provirus integration in tissues of FOP-FGFR1 mice. Genomic DNAs from bone marrow (BM), spleen (S), liver (L), and thymus from several FOP-FGFR1 and FOP-FGFR1 Y511/F transplanted and diseased mice, were digested with either KpnI (F) or BamHI (G) and hybridized with a radioactive probe from the neomycin resistance gene. (F) KpnI cuts in both retroviral LTRs; the single band of expected size demonstrates the presence of provirus. GP+E86 packaging cell line FOP-FGFR1 stably transfected (GP+E FOP-FGFR1), and bone marrow cells from one of the control vector mice (MSCV) were used as controls. Asterisk indicates the thymus sample from a FOP-FGFR1Y511/F mouse that developed a malignant lymphoma 126 days after transplantation. (G) BamHI cuts only once in the provirus; this results in bands of different sizes corresponding to 2 to 4 genomic integration sites (arrowheads). Note the presence of the same clones in different tissues from the same mouse. Bone marrow cells from a non-infected mouse (WT) and from one of the vector control mice (MSCV) were used as controls.
To assess whether FOP-FGFR1 expression could induce hematopoietic malignancy, mice received transplants of Sca-1+ bone marrow cells infected with FOP-FGFR1, kinase-inactive, and MSCVneo vector (12, 14, 15 mice, respectively) (Table 1). Mice receiving either kinase-inactive or vector alone transfected cells did not develop disease after 1 year (Figure 1B), and blood cells were largely composed of mature lymphocytes (Table 1). In contrast, all mice that received transplants of FOP-FGFR1 developed a fatal myeloproliferative-like syndrome within 4 weeks after transplantation (median latency, 32 days; Figure 1B). Their WBCs were elevated compared with control mice (2-8 × 105 versus 0.5-2 × 104) (Table 1) consisting of maturing myeloid cells; blasts were absent and eosinophils were rarely present (not shown).
Summary of bone marrow transplantation experiments with FOP-FGFR1 and related mutants
Constructs . | Affected/total (no. of expts)* . | Latency, d . | Median survival, d . | RBC, mm3 × 106 . | WBC, mm3 . | Peripheral blood formula, % . | Spleen, cm . | Liver . | Phenotypes at autopsy† . |
|---|---|---|---|---|---|---|---|---|---|
| MSCV | 0/15 (5) | NA | > 365 | 7.1 ± 2.2 | 0.5-2 × 104 | Ly: 84 ± 10 | L: 1.3-1.4 | Normal | Normal |
| Gr: 12.4 ± 7.8 | I: 0.3-0.5 | ||||||||
| M: 3.6 ± 3.3 | |||||||||
| Eo: 0-4 | |||||||||
| FOP-FGFR1 | 12/12 (3) | 32-40‡ | 32 | 6.8 ± 3.2 | 2-8 × 105 | Ly: 9.5 ± 6.8 | L: 2.5-3.0 | Hepatomegaly | MPD |
| Gr: 81.2 ± 7.5 | I: 0.8-1.0 | ||||||||
| M: 3.5 ± 3.2 | |||||||||
| Eo: 0-4 | |||||||||
| Ac: 5.7 ± 2.7 | |||||||||
| FOP-FGFR1 | 0/14 (3) | NA | > 365 | 6.7 ± 3.0 | 0.5-2 × 104 | Ly: 90 ± 6.2 | L: 1.3-1.4 | Normal | Normal |
| K259/A | Gr: 8 ± 5 | I: 0.3-0.5 | |||||||
| M: 2 ± 1.2 | |||||||||
| Eo: 0-3 | |||||||||
| FOP-FGFR1 | 2/17 (3) | 144 | 144 | 6.9 ± 2.3 | 3 × 104-2 × 105 | Variable | L: 2.5-3.5 | Normal | Moderate MPD |
| Y511/F | I: 0.7-1.0 | ||||||||
| 10/17 (3) | 112-322 | 241 | 7.0 ± 0.5 | 2-3 × 104 | Variable | L: 1.2-1.3 | Normal | Moderate MPD | |
| I: 0.3-0.4 | |||||||||
| 5/17 (3) | 91-154 | 119 | 7.1 ± 0.7 | Variable | Variable | L: 1.0-1.3 | Normal | Thymic lymphoma | |
| I: 0.3-0.4 |
Constructs . | Affected/total (no. of expts)* . | Latency, d . | Median survival, d . | RBC, mm3 × 106 . | WBC, mm3 . | Peripheral blood formula, % . | Spleen, cm . | Liver . | Phenotypes at autopsy† . |
|---|---|---|---|---|---|---|---|---|---|
| MSCV | 0/15 (5) | NA | > 365 | 7.1 ± 2.2 | 0.5-2 × 104 | Ly: 84 ± 10 | L: 1.3-1.4 | Normal | Normal |
| Gr: 12.4 ± 7.8 | I: 0.3-0.5 | ||||||||
| M: 3.6 ± 3.3 | |||||||||
| Eo: 0-4 | |||||||||
| FOP-FGFR1 | 12/12 (3) | 32-40‡ | 32 | 6.8 ± 3.2 | 2-8 × 105 | Ly: 9.5 ± 6.8 | L: 2.5-3.0 | Hepatomegaly | MPD |
| Gr: 81.2 ± 7.5 | I: 0.8-1.0 | ||||||||
| M: 3.5 ± 3.2 | |||||||||
| Eo: 0-4 | |||||||||
| Ac: 5.7 ± 2.7 | |||||||||
| FOP-FGFR1 | 0/14 (3) | NA | > 365 | 6.7 ± 3.0 | 0.5-2 × 104 | Ly: 90 ± 6.2 | L: 1.3-1.4 | Normal | Normal |
| K259/A | Gr: 8 ± 5 | I: 0.3-0.5 | |||||||
| M: 2 ± 1.2 | |||||||||
| Eo: 0-3 | |||||||||
| FOP-FGFR1 | 2/17 (3) | 144 | 144 | 6.9 ± 2.3 | 3 × 104-2 × 105 | Variable | L: 2.5-3.5 | Normal | Moderate MPD |
| Y511/F | I: 0.7-1.0 | ||||||||
| 10/17 (3) | 112-322 | 241 | 7.0 ± 0.5 | 2-3 × 104 | Variable | L: 1.2-1.3 | Normal | Moderate MPD | |
| I: 0.3-0.4 | |||||||||
| 5/17 (3) | 91-154 | 119 | 7.1 ± 0.7 | Variable | Variable | L: 1.0-1.3 | Normal | Thymic lymphoma | |
| I: 0.3-0.4 |
RBC indicates erythrocytes; NA indicates not applicable; Ly, lymphocytes; Gr, granulocytes; M, monocytes; Eo, eosinophiles; MPD, myeloproliferative disorder; L, length; I, thickness; Ac, atypical cells.
Number of mice analyzed for each construct × number of independent experiments (expts).
MPD phenotype at biopsy; all mice developed a rapid and fatal MPD characterized by high white blood cell counts (WBCs); inverted peripheral blood formula, splenomegaly, and hepatomegaly; myeloid and megakaryocyte hyperplasia in peripheral blood, spleen, and liver. Cachexia, rear-leg paralysis, and paraspinal hemorrhages were observed. Moderate MPD means that some features described appeared with latency and were reduced as follows: no or moderate elevation of WBC counts. Myeloid hyperplasia in bone marrow was eventually observed as well as cachexia. Enlarged spleens showing slight infiltration with maturing granulocytes and rare megakaryocytes are only observed in 2 of 17 cases; hepatomegaly was never observed. Thymic lymphoma was diagnosed by the presence of a greatly enlarged thymus.
Data obtained from 11 mice; the 12th mouse that was terminally ill was killed 88 days after transplantation.
The pathologic changes found at necropsy of FOP-FGFR1 mice were remarkably consistent, including hepatosplenomegaly with extramedullary hematopoiesis. Bone marrows were hypercellular, with mostly maturing myeloid cells. Spleens and livers showed replacement of normal architecture by extensive hematopoiesis with immature and mature granulocytes, foci of erythropoiesis, and megakaryocytes (Figure 1C). There was no evidence of lymphoproliferative disease.
The 17 FOP-FGFR1Y511/F mice developed a fatal disease which was drastically different from FOP-FGFR1–induced disease. First, the disease developed after a much longer latency period (91-322 days; Figure 1B; Table 1). Second, blood cell counts were never as high as those observed in the FOP-FGFR1 disease (Table 1). Third, their bone marrow (BM) showed only mild myeloproliferation, extramedullary hematopoiesis was less important (Figure 1C), and only 2 of them developed enlarged spleens. Fourth, 5 of 17 mice had an enlarged thymus, and one of those mice developed a high-grade malignant lymphoma with massive invasion by neoplastic CD3+ cells and monoclonal rearrangement of the TCRβ locus (Figure 1D).
Expression of FOP-FGFR1 protein was found in all affected tissues (Figure 1E). Integrated and unrearranged provirus was detected by Southern blot analyses in all tissues from any tested mice that received transplants (Figure 1F). Two to 4 prominent bands were observed with BamHI restriction enzyme, suggesting that only a few clones of cells were responsible for the myeloproliferative disease (Figure 1G). The same bands were present in different tissues from the same mouse, demonstrating that different clones did not arise independently in affected tissues.
In summary, the murine MPD induced by FOP-FGFR1 kinase was characterized by marked leukocytosis, hypercellular bone marrow, and hepatosplenomegaly, indicative of myeloid hyperplasia. These features resemble 2 other diseases, the murine model for p190 and p210 breakpoint cluster region–Abelson murine leukemia (BCR-ABL)–induced chronic myeloid leukemia (CML) and the chronic phase of human 8p12 MPD. Common features with murine CML include markedly elevated WBCs with predominance of myelocytes and mature neutrophils, splenomegaly, and multiple organ involvement.21,22 Similarity between CML and 8p12 MPD extends to the presence of a common partner for the FGFR1 and ABL kinases, ie, BCR,23,24 suggesting that the same promoter sequences can lead to both diseases.
We observed 2 differences between murine and human MPDs. First, as already observed for CML,25 the development of the disorder in the mouse was very fast, and the mice died without progression to blast phase. This could be due to overexpression of FOP-FGFR1 from a retroviral promoter rather than expression of FOP-FGFR1 from the endogenous FOP promoter. The actual cause of death in diseased mice is still unclear. For most murine MPD models, the death is related to respiratory insufficiency caused by pulmonary infiltration and/or hemorrhage. In our case lungs were healthy, but cachexia and paraspinal hemorrhage were consistent. Hepatosplenomegaly, which was constant, could also be involved in rear-leg paralysis and hepatic failure. Second, the mice did not develop lymphoproliferative disease. This situation has also been observed for translocated E26 transforming–specific (ETS) leukemia (TEL)–platelet-derived growth factor receptor β (TEL-PDGFRβ), another tyrosine kinase fusion that induces fatal myeloproliferation in mice without lymphoproliferative disease.26 An explanation is that FOP-FGFR1 mice died before overt lymphoma could develop. Data obtained with the Y511 mutant are in favor of this hypothesis. FOP-FGFR1Y511/F mice had a prolonged latency, showing the importance of this main PLCγ binding site in MPD induction. Some FOP-FGFR1Y511/F mice developed lymphoid manifestations, suggesting that FOP-FGFR1 targeted into hematopoietic stem cells and induced MPD, but that lymphoid defects need a longer time to develop and/or become noticeable. These results differ from TEL-TRKC (tyrosine kinase C) fusion for which PLCγ was completely dispensable for disease and had no effect on disease latency.27 Our previous studies showed that Y511 is crucial for cell survival.6 We can argue that Y511/F mutation in mice induced a decrease in some antiapoptotic signals delivered by way of FOP-FGFR1 kinase, leading to a moderate MPD. The murine bone marrow retroviral transduction and transplantation model of MPD will be a powerful tool in the evaluation of novel therapeutic FGFR1 inhibitors against fusion FGFR1-induced diseases.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-05-1690.
Supported in part by INSERM, grants from the Association pour la Recherche sur le Cancer and the Fondation de France (Comité contre la Leucémie), and by the Fondation pour la Recherche Médicale (G.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank C. Bagnis, F. Bardin, C. Chabannon, F. Fiore, and S. Marchetto for their advice during the initiation of this study; R. Hawley (American Red Cross, Rockville, MD) for providing the MSCV vector; M. Malissen for help with TCR rearrangements and for discussions, together with A. Miazek and S. Nunez-Cruz. We gratefully acknowledge P. Gibier for his help regarding maintenance of mice; C. Constant, R. Galindo, and B. Puig for their assistance with mouse irradiations, flow cytometry, and statistical analyses, respectively.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal