Abstract
The low-density lipoprotein (LDL) receptor–related protein (LRP) has a well-established role in the hepatic removal of atherogenic apolipoprotein E (APOE)–rich remnant lipoproteins from plasma. In addition, LRP recognizes multiple distinct pro- and antiatherogenic ligands in vitro. Here, we investigated the role of hepatic LRP in atherogenesis independent of its role in removal of APOE-rich remnant lipoproteins. Mice that allow inducible inactivation of hepatic LRP were combined with LDL receptor and APOE double-deficient mice (MX1Cre+LRPflox/floxLDLR–/–APOE–/–). On an LDLR–/–APOE–/– background, hepatic LRP deficiency resulted in decreased plasma cholesterol and triglycerides (cholesterol: 17.1 ± 5.2 vs 23.4 ± 6.3 mM, P = .025; triglycerides: 1.1 ± 0.5 vs 2.2 ± 0.8 mM, P = .002, for MX1Cre+LRPflox/flox-LDLR–/–APOE–/– and control LRPflox/flox-LDLR–/–APOE–/– mice, respectively). Lower plasma cholesterol in MX1Cre+LRPflox/flox-LDLR–/–APOE–/– mice coincided with increased plasma lipoprotein lipase (71.2 ± 7.5 vs 19.1 ± 2.4 ng/ml, P = .002), coagulation factor VIII (4.4 ± 1.1 vs 1.9 ± 0.5 U/mL, P = .001), von Willebrand factor (2.8 ± 0.6 vs 1.4 ± 0.3 U/mL, P = .001), and tissue-type plasminogen activator (1.7 ± 0.7 vs 0.9 ± 0.5 ng/ml, P = .008) compared with controls. Strikingly, MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice showed a 2-fold higher atherosclerotic lesion area compared with controls (408.5 ± 115.1 vs 219.1 ± 86.0 103μm2, P = .003). Our data indicate that hepatic LRP plays a clear protective role in atherogenesis independent of plasma cholesterol, possibly due to maintaining low levels of its proatherogenic ligands.
Introduction
The low-density lipoprotein (LDL) receptor–related protein (LRP) is a large cell-surface receptor that is ubiquitously expressed in a variety of tissues and is present on a wide range of different cell types, including hepatocytes, monocytes, and smooth muscle cells (SMCs).1 The LRP, together with the LDL receptor, has a well-established role in the hepatic removal of proatherogenic remnants of chylomicrons and very low-density lipoproteins (VLDLs) from the circulation.2 Apolipoprotein E (APOE) serves as the ligand for LRP-mediated hepatic uptake of remnant lipoproteins.3
Recently, it has been demonstrated that LRP plays a role not only in atherosclerosis at the level of the liver by removing atherogenic lipoproteins from the circulation, but also has a clear role in atherosclerosis extrahepatically via controlling SMC platelet–derived growth factor (PDGF) receptor activation.4 However, the role of LRP in atherosclerosis may not be restricted to lipoprotein removal and PDGF receptor activation. In vitro studies show that LRP binds a wide range of distinct ligands.5 Hepatic LRP is suggested to be an important determinant of the levels of its ligands in plasma. Some of these ligands have been indicated to play a clear role in modulating atherogenesis, including tissue-type plasminogen activator (t-PA),6 urokinase tissue-type plasminogen activator (u-PA),7 plasminogen activator inhibitor-1 (PAI-1),8 tissue factor pathway inhibitor (TFPI),9 coagulation factor VIII (FVIII),10 and lipoprotein lipase (LPL).11
In the present study, we hypothesize that hepatic LRP may play a role in atherogenesis independent of its well-known role in the plasma removal of atherogenic APOE-rich remnant lipoproteins. To this end, we further investigated the role of LRP in atherogenesis by using mice conditionally lacking hepatic LRP (MX1Cre+LRPflox/flox) on an LDL receptor– and APOE-deficient background (LDLR–/–APOE–/–). Whereas APOE deficiency will exclude removal of APOE-rich lipoproteins via both LDL receptor and LRP, LDL receptor deficiency will also exclude possible removal of lipoproteins mediated by the APOB100 pathway.
Our data show that MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice have slightly though statistically significant lower plasma cholesterol and triglyceride levels compared with control LRPflox/floxLDLR–/–APOE–/– mice. As expected, MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice had higher plasma levels of the LRP ligands LPL, FVIII, and t-PA. Nevertheless, despite the lower plasma cholesterol, MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice show increased atherosclerosis. Thus, our data indicate that hepatic LRP plays a clear protective role in atherogenesis independent of plasma cholesterol, possibly due to maintaining low levels of its proatherogenic ligands.
Materials and methods
Transgenic animals
The experimental animals were obtained by crossbreeding LDLR–/–APOE–/– mice12 with MX1Cre+LRPflox/floxLDLR–/– mice2 resulting in mice deficient for LRP, LDLR, and APOE (MX1Cre+LRPflox/floxLDLR–/–APOE–/–) and control littermates that lack both LDLR and APOE (LRPflox/floxLDLR–/–APOE–/–). Mice were genotyped by polymerase chain reaction (PCR).2,12 For experiments, 6-week-old male transgenic MX1Cre+-LRPflox/floxLDLR–/–APOE–/– (n = 15) and male littermate control LRPflox/floxLDLR–/–APOE–/– (n = 16) mice were used. MX1Cre+LRPflox/floxLDLR–/–APOE–/– (n = 12) and control LRPflox/floxLDLR–/–APOE–/– (n = 13) mice were induced for LRP deficiency by intraperitoneal injection of a polyinosinic: polycytidylic ribonucleic acid (pI:pC; Sigma, St Louis, MO).2 Included as extra uninduced controls were 3 mice of each genotype that did not receive pI:pC injections. In addition, MX1Cre+LRPflox/flox mice were combined with reporter mice carrying a conditional β-galactosidase gene that allows monitoring of Cre-recombinase–mediated DNA excisions.13 Transgenic offspring were induced with pI:pC parallel with the experimental animals. All mice were fed a standard chow diet (SRM-A, Hope Farms, the Netherlands). The institutional committee on animal welfare of Leiden University Medical Center approved all animal experiments.
Plasma parameters
Blood was collected by tail bleeding. Cholesterol and triglycerides were measured in EDTA (ethylenediaminetetraacetic acid)–plasma enzymatically using available kits no. C0534 and no. 337-B (Sigma), respectively. Plasma high-density lipoprotein (HDL) cholesterol was determined using phosphotungstic acid.14 Lipoprotein distribution was determined by fast performance liquid chromatography (FPLC) size fractionation.14
Plasma mouse APOB (B48 and B100) and APOAI concentrations were determined by immunoblotting using polyclonal rabbit-antisera against mouse APOB and APOAI (TNO, Leiden, the Netherlands). Peroxidase-labeled polyclonal goat antirabbit antibody (Nordic, Tilburg, the Netherlands) was used as secondary antibody, and bound peroxidase was visualized using BM-blue peroxidase substrate (Roche Diagnostics, Mannheim, Germany). Protein bands were scanned and subsequently analyzed using TINA version 2.09 software (Raytest Isotopenmessgerate, Straubenhardt, Germany).
Tissue analysis
Tissues of MX1Cre+LRPflox/flox transgenic mice that were combined with Cre reporter mice were frozen, cryosectioned (10 μm), and stained for β-galactosidase activity.
Livers, hearts, and aortas from MX1Cre+LRPflox/floxLDLR–/–APOE–/– and LRPflox/floxLDLR–/–APOE–/– mice were fixed in phosphate-buffered 4% formaldehyde, dehydrated, and embedded in paraffin. In addition, fresh parts of livers (3 random animals per group) were used for detection of LRP.2 Cross-sections (5 μm) of the descending aorta were stained for LRP and PDGF receptor.4 Hearts were cross-sectioned (5 μm) throughout the entire aortic root area. Per mouse, 4 sections with 30-μm intervals were used for quantification of atherosclerotic lesion area.18 Sections were routinely stained with hematoxylin-phloxine-saffron (HPS). Lesion area was determined using Leica Qwin image analysis software (EIS, Asbury, NJ).18
Sections of the aortic root area were stained with rabbit antimouse macrophages antibody (AIA-31240, dilution 1:3000; Accurate Chemical and Scientific, Westbury, NY) and a monoclonal mouse anti-α–SMC actin antibody (clone 1A4, dilution 1:800; Sigma). Biotinylated donkey antirabbit antibody (dilution 1:300; Vector Laboratories, Burlingame, CA) and horse antimouse antibody (dilution 1:400; Vector Laboratories) were used as secondary antibodies, followed by incubation with horseradish peroxidase–labeled avidin-biotin complex. Peroxidase activity was visualized with Nova Red (Vector Laboratories). Macrophage (AIA-31240–positive area) and SMC (1A4-positive area) lesion areas were quantified using Leica Qwin image analysis software (EIS) at the level of 11 individual lesions overlapping in size ranging from 50 000 to 200 000 μm2 and expressed as a percentage of the size of the individual lesion.
Collagen and elastin were stained using Sirius Red and Resorcin-Fuchsin (Chroma-Gesellschaft, Stuttgart, Germany), respectively.
Statistical analysis
All data are presented as mean ± SD. Data were analyzed using the Mann-Whitney U test. P values less than .05 were regarded as statistically significant.
Results
Induction of LRP deficiency
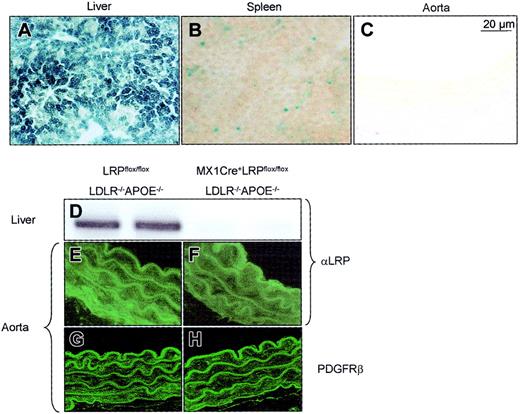
We15 and others2,19 have demonstrated that the inducible MX1Cre transgene achieves near-complete recombination of conditional alleles mainly in the liver. This was verified by crossbreeding MX1Cre transgenics with a reporter strain that allows monitoring Cre-recombinase–mediated DNA excisions in mice. PI:pC treatment of 6-week-old offspring and subsequent tissue analysis for β-galactosidase activity confirmed a complete recombination in the liver 12 weeks after pI:pC induction (Figure 1A). MX1Cre-mediated recombination was, to a lesser extent, also detected at the level of the spleen (Figure 1B). Using this reporter approach, no MX1Cre-mediated recombination was found in heart, muscle, fat, stomach, intestine, brain, and importantly also not in the vasculature as analyzed at the level of the descending aorta (Figure 1C), aortic arch, and aortic root. Like pI:pC-induced MX1Cre+LRPflox/flox mice,15 pI:pC-induced MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice had no detectable LRP in liver membrane extracts as determined by immunoblotting with antibodies directed against the 85-kDa subunit of LRP (Figure 1D). In the same mice, however, aortic LRP protein could be detected using an SMC-specific immunofluorescent staining (Figure 1E-F). Moreover, no increased expression of aortic PDGF receptor was observed, a feature typical for vascular LRP deletion4 (Figure 1G-H). Collectively, these data indicate successful deletion of hepatic LRP in MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice, whereas aortic LRP expression remains unaffected.
MX1Cre-mediated recombination of the conditional alleles. MX1Cre-mediated recombination was determined 12 weeks after pI:pC induction: (1) by detecting β-galactosidase activity on cryosections of liver (A), spleen (B), and aorta (C) of MX1Cre transgenic mice that had been combined with Cre-reporter mice; (2) by immunoblotting membrane proteins isolated from livers of LRPflox/floxLDLR–/–APOE–/– (D, left 2 lanes) and MX1Cre+LRPflox/floxLDLR–/–APOE–/– (D, right 2 lanes) mice and subsequent staining with a polyclonal rabbit anti-LRP; and (3) by immunofluorescent detection of LRP and PDGF receptor in the descending aorta of LRPflox/floxLDLR–/–APOE–/– (E,G) and MX1Cre+LRPflox/floxLDLR–/–APOE–/– (F,H) mice.
MX1Cre-mediated recombination of the conditional alleles. MX1Cre-mediated recombination was determined 12 weeks after pI:pC induction: (1) by detecting β-galactosidase activity on cryosections of liver (A), spleen (B), and aorta (C) of MX1Cre transgenic mice that had been combined with Cre-reporter mice; (2) by immunoblotting membrane proteins isolated from livers of LRPflox/floxLDLR–/–APOE–/– (D, left 2 lanes) and MX1Cre+LRPflox/floxLDLR–/–APOE–/– (D, right 2 lanes) mice and subsequent staining with a polyclonal rabbit anti-LRP; and (3) by immunofluorescent detection of LRP and PDGF receptor in the descending aorta of LRPflox/floxLDLR–/–APOE–/– (E,G) and MX1Cre+LRPflox/floxLDLR–/–APOE–/– (F,H) mice.
Plasma lipid and lipoprotein
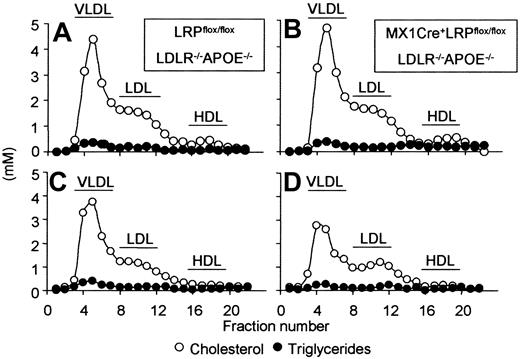
Since LDL receptor and APOE double-deficient mice are unable to clear lipoproteins via the APOE as well as the APOB100 pathway, one would expect no effect of LRP deficiency on plasma lipid levels in MX1Cre+LRPflox/floxLDLR–/–APOE–/– animals compared with LRPflox/floxLDLR–/–APOE–/– mice. Uninduced MX1Cre+LRPflox/floxLDLR–/–APOE–/– and control LRPflox/floxLDLR–/–APOE–/– mice had identical plasma cholesterol, triglyceride levels, and lipoprotein distribution (Table 1; Figure 2A-B). At 4 weeks after induction of LRP deficiency by pI:pC, MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice remarkably showed significant lower plasma cholesterol (P = .025) and plasma triglycerides (P = .002) compared with controls (Table 1). Lower plasma lipids in MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice compared with controls persisted for at least 12 weeks, that is, until the end of the study (cholesterol: 17.1 ± 2.9 vs 24.6 ± 5.0 mM, P = .001; triglycerides: 0.8 ± 0.4 vs 1.5 ± 0.5 mM, P = .008, respectively). The lowering in plasma cholesterol was mainly confined to the VLDL/LDL-sized lipoprotein fraction (Figure 2C-D). There was no effect of LRP deficiency on plasma HDL cholesterol (0.45 ± 0.23 mM and 0.38 ± 0.17 mM, P = .200, for controls and MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice, respectively). In addition, plasma apolipoproteins were not detectably affected by LRP status (relative amounts of plasma: APOB48: 100 ± 18% vs 119 ± 24%, P = .240; APOB100: 100 ± 7% vs 98.9 ± 19.2%, P = .485; APOAI: 100 ± 43% vs 107 ± 25%, P = .320, for controls and MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice, respectively).
Plasma lipids
Genotype . | Cholesterol, mM . | Triglycerides, mM . |
|---|---|---|
| Uninduced | ||
| LRPflox/floxLDLR-/-APOE-/- | 25.9 ± 4.5 | 2.5 ± 1.1 |
| MX1Cre+LRPflox/floxLDLR-/-APOE-/- | 28.1 ± 6.2 | 2.6 ± 1.1 |
| pl:pC induced | ||
| LRPflox/floxLDLR-/-APOE-/- | 23.4 ± 6.3 | 2.2 ± 0.8 |
| MX1Cre+LRPflox/floxLDLR-/-APOE-/- | 17.1 ± 5.2* | 1.1 ± 0.5* |
Genotype . | Cholesterol, mM . | Triglycerides, mM . |
|---|---|---|
| Uninduced | ||
| LRPflox/floxLDLR-/-APOE-/- | 25.9 ± 4.5 | 2.5 ± 1.1 |
| MX1Cre+LRPflox/floxLDLR-/-APOE-/- | 28.1 ± 6.2 | 2.6 ± 1.1 |
| pl:pC induced | ||
| LRPflox/floxLDLR-/-APOE-/- | 23.4 ± 6.3 | 2.2 ± 0.8 |
| MX1Cre+LRPflox/floxLDLR-/-APOE-/- | 17.1 ± 5.2* | 1.1 ± 0.5* |
Data are shown as means ± SD.
P < .05, significantly different from LRPflox/floxLDLR-/-APOE-/- mice at 4 weeks after pl:pC induction.
Lipoprotein distribution. Plasma was obtained from uninduced (A,B) and 4-week induced (C,D) LRPflox/floxLDLR–/–APOE–/– (A,C) and MX1Cre+LRPflox/floxLDLR–/–APOE–/– (B,D) mice. Lipoproteins were size-fractionated by FPLC; cholesterol (○) and triglyceride (•) content of the individual fractions was determined.
Lipoprotein distribution. Plasma was obtained from uninduced (A,B) and 4-week induced (C,D) LRPflox/floxLDLR–/–APOE–/– (A,C) and MX1Cre+LRPflox/floxLDLR–/–APOE–/– (B,D) mice. Lipoproteins were size-fractionated by FPLC; cholesterol (○) and triglyceride (•) content of the individual fractions was determined.
Plasma LPL mass and activity
LPL binds to LRP in vitro.5 To investigate whether LRP plays a role in LPL processing in vivo, we measured both plasma LPL mass and activity levels. Uninduced MX1Cre+LRPflox/floxLDLR–/–APOE–/– and control LRPflox/floxLDLR–/–APOE–/– mice did not differ in plasma LPL levels (Table 2). However, pI:pC-induced MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice had significant (P = .002) 3.7-fold higher plasma LPL levels compared with control LRPflox/floxLDLR–/–APOE–/– mice. Surprisingly, increased LPL levels did not coincide with increased plasma LPL activity (Table 2). MX1Cre+LRPflox/flox LDLR–/–APOE–/– and control mice had similar plasma LPL activity, which was also similar to plasma LPL activity observed in uninduced MX1Cre+LRPflox/floxLDLR–/–APOE–/– and control mice. These data indicate that hepatic LRP deficiency results in accumulation of inactive plasma LPL.
Plasma LPL mass and activity, FVIII, VWF, and t-PA
Genotype . | LPL mass, ng/mL* . | LPL activity, μmol FFA/h/mL* . | FVIII, U/mL* . | VWF, U/mL* . | t-PA, ng/mL* . |
|---|---|---|---|---|---|
| Uninduced | |||||
| LRPflox/floxLDLR-/-APOE-/- | 19.6 ± 2.2 | 11.0 ± 1.4 | 1.8 ± 0.2 | 1.1 ± 0.1 | 1.0 ± 0.3 |
| MX1Cre+LRPflox/floxLDLR-/-APOE-/- | 21.3 ± 5.1 | 9.9 ± 1.6 | 2.0 ± 0.2 | 1.1 ± 0.3 | 1.2 ± 0.2 |
| pl:pC induced | |||||
| LRPflox/floxLDLR-/-APOE-/- | 19.1 ± 2.4 | 9.4 ± 0.9 | 1.9 ± 0.5 | 1.4 ± 0.3 | 0.9 ± 0.5 |
| MX1Cre+LRPflox/floxLDLR-/-APOE-/- | 71.2 ± 7.5‡ | 9.5 ± 1.7 | 4.4 ± 1.1‡ | 2.8 ± 0.6‡ | 1.7 ± 0.7‡ |
Genotype . | LPL mass, ng/mL* . | LPL activity, μmol FFA/h/mL* . | FVIII, U/mL* . | VWF, U/mL* . | t-PA, ng/mL* . |
|---|---|---|---|---|---|
| Uninduced | |||||
| LRPflox/floxLDLR-/-APOE-/- | 19.6 ± 2.2 | 11.0 ± 1.4 | 1.8 ± 0.2 | 1.1 ± 0.1 | 1.0 ± 0.3 |
| MX1Cre+LRPflox/floxLDLR-/-APOE-/- | 21.3 ± 5.1 | 9.9 ± 1.6 | 2.0 ± 0.2 | 1.1 ± 0.3 | 1.2 ± 0.2 |
| pl:pC induced | |||||
| LRPflox/floxLDLR-/-APOE-/- | 19.1 ± 2.4 | 9.4 ± 0.9 | 1.9 ± 0.5 | 1.4 ± 0.3 | 0.9 ± 0.5 |
| MX1Cre+LRPflox/floxLDLR-/-APOE-/- | 71.2 ± 7.5‡ | 9.5 ± 1.7 | 4.4 ± 1.1‡ | 2.8 ± 0.6‡ | 1.7 ± 0.7‡ |
Data are shown as mean ± SD. As determined at 4 weeks* and 12 weeks† after pl:pC injection.
P < .05, significantly different from LRPflox/floxLDLR-/-APOE-/- mice.
Plasma FVIII, VWF, and t-PA
Among the postulated ligands for LRP is the coagulation factor VIII (FVIII).5 Recently, we observed that FVIII indeed accumulates in the plasma of MX1Cre+LRPflox/flox mice.16 Von Willebrand factor (VWF), the carrier protein of FVIII,20 also accumulates in plasma of these mice.16 In the current study, uninduced MX1Cre+LRPflox/floxLDLR–/–APOE–/– and control LRPflox/floxLDLR–/–APOE–/– mice did not differ in plasma FVIII (P = .400) and VWF (P = 1.000) levels (Table 2). However, 4 weeks after induction, MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice had significant 2-fold higher plasma FVIII (P = .010) and VWF (P = .001) compared with LRPflox/floxLDLR–/–APOE–/– mice (Table 2). El-evated levels of FVIII and VWF persisted at least until 12 weeks after pI:pC induction (FVIII: 1.4 ± 0.3 vs 3.7 ± 0.4 U/mL, P < .001; VWF: 1.4 ± 0.4 vs 3.2 ± 0.6 U/mL, P = .002, for LRPflox/floxLDLR–/–APOE–/– and MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice, respectively).
To investigate whether plasma t-PA is also affected in our animal model, we measured plasma t-PA in uninduced mice and in mice 12 weeks after induction of LRP deficiency. As shown in Table 2, uninduced MX1Cre+LRPflox/floxLDLR–/–APOE–/– and control LRPflox/floxLDLR–/–APOE–/– mice did not differ in plasma t-PA levels (P = 1.000). However, 12 weeks after induction of LRP deficiency, MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice showed a significant (P = .008) 1.9-fold increase in plasma t-PA levels compared with control LRPflox/floxLDLR–/–APOE–/– mice.
Thus, LRP deficiency on an LDLR–/–APOE–/– background leads to increased plasma FVIII, its carrier protein VWF, and t-PA in vivo.
Atherosclerosis
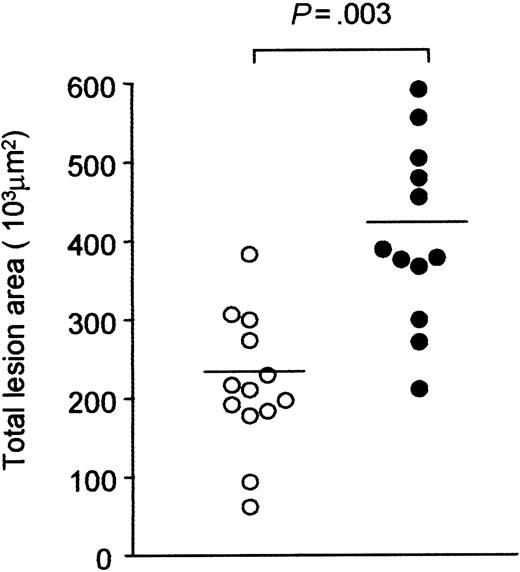
LRP deficiency resulted in lower levels of the proatherosclerotic VLDL/LDL-sized lipoproteins (Table 1; Figure 2) but had an opposite effect on some of its (pro) atherosclerotic ligands (Table 2). Therefore, we investigated whether hepatic LRP deficiency in an LDLR–/–APOE–/– setting has an effect on atherosclerosis development as analyzed 12 weeks after pI:pC induction. Quantitative assessment of the atherosclerotic lesions area revealed that both uninduced LRPflox/floxLDLR–/–APOE–/– and MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice did not differ in atherosclerotic lesion area (175.3 ± 92.5 103μm2 vs 224.8 ± 76.7 103μm2, P = .70, respectively). However, pI:pC-induced MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice displayed a significant (P = .003) 1.9-fold larger atherosclerotic lesion area compared with the control LRPflox/floxLDLR–/–APOE–/– mice (Figure 3).
Quantitative assessment of atherosclerosis. At 23 weeks after pI:pC induction, the extent of atherosclerosis in LRPflox/floxLDLR–/–APOE–/– (○) or MX1Cre+LRPflox/floxLDLR–/–APOE–/– (•) mice was quantified at the level of the aortic root. Each data point represents the mean lesion area per mouse. Significant differences are indicated; P < .05.
Quantitative assessment of atherosclerosis. At 23 weeks after pI:pC induction, the extent of atherosclerosis in LRPflox/floxLDLR–/–APOE–/– (○) or MX1Cre+LRPflox/floxLDLR–/–APOE–/– (•) mice was quantified at the level of the aortic root. Each data point represents the mean lesion area per mouse. Significant differences are indicated; P < .05.
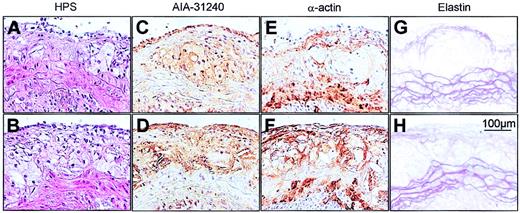
To investigate whether LRP deficiency, in addition to size, also affects the composition of the atherosclerotic lesions, we determined the percentage of macrophages and SMCs in individual lesions of LRPflox/floxLDLR–/–APOE–/– and MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice that were similar in size (ie, 50 000-200 000 μm2). As shown in Table 3 and Figure 4, LRPflox/floxLDLR–/–APOE–/– and MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice had individual lesions with similar percentage of lesion area positive for the antimouse macrophage polyclonal antibody AIA-31240 (Figure 4C-D) and similar percentage of lesion area positive for the antimouse monoclonal antimuscle α-actin antibody 1A4 (Figure 4E-F), indicating no difference in macrophage or SMC content. The non–AIA-31240– and non-1A4–positive areas of the size-matched individual lesions of both groups consisted predominantly of Sirius red–positive (ie, collagen-rich) material and necrosis. Gross inspection revealed no difference in collagen content between the 2 groups (figure not shown). In addition, there were no signs of disrupted elastic layers, increased SMC proliferation, or aneurysm formation in (nondiseased) descending aorta of LRPflox/floxLDLR–/–APOE–/– and MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice (a feature typical for SMC-specific LRP deletion4 ). However, for both genotypes we did find disruption of the elastic layer in 6the aortic root at the level of the media underlying atherosclerotic plaques, which corresponded with lesion severity and not LRP status.
Composition of atherosclerotic lesions
Genotype . | LRPflox/floxLDLR-/-APOE-/- . | MX1Cre+LRPflox/floxLDLR-/-APOE-/- . |
|---|---|---|
| Total lesion area, 103μm2 | 219 ± 86 | 408 ± 115* |
| AIA-31240-positive area, % of total lesion area | 31.5 ± 9.7 | 29.3 ± 11.8 |
| 1A4-positive area, % of total lesion area | 25.3 ± 7.4 | 28.5 ± 12.2 |
| Non-AIA-31240- and non-1A4-positive area, % of total lesion area | 43.6 ± 9.1 | 43.4 ± 16.6 |
Genotype . | LRPflox/floxLDLR-/-APOE-/- . | MX1Cre+LRPflox/floxLDLR-/-APOE-/- . |
|---|---|---|
| Total lesion area, 103μm2 | 219 ± 86 | 408 ± 115* |
| AIA-31240-positive area, % of total lesion area | 31.5 ± 9.7 | 29.3 ± 11.8 |
| 1A4-positive area, % of total lesion area | 25.3 ± 7.4 | 28.5 ± 12.2 |
| Non-AIA-31240- and non-1A4-positive area, % of total lesion area | 43.6 ± 9.1 | 43.4 ± 16.6 |
Areas positively stained with AIA-31240 (macrophages) or 1A4 (smooth muscle cells) were quantified at the level of individual lesions ranging in size from 50 000 to 200 000 μm2 (11 individual lesions per group) and are expressed as percentages of total lesion area. Data are shown as mean ± SD.
P < .05, significantly different from LRPflox/floxLDLR-/-APOE-/- mice.
Photomicrographs of representative atherosclerotic lesions. Size-matched lesions of LRPflox/floxLDLR–/–APOE–/– (upper panels) and MX1Cre+LRPflox/floxLDLR–/–APOE–/– (lower panels) mice. Sections were stained with either HPS (A-B), the macrophage-specific antibody AIA-31240 (C-D), the SMC–α-actin–specific antibody (E-F), or with Resorcin-Fuchsin (elastin; G-H).
Photomicrographs of representative atherosclerotic lesions. Size-matched lesions of LRPflox/floxLDLR–/–APOE–/– (upper panels) and MX1Cre+LRPflox/floxLDLR–/–APOE–/– (lower panels) mice. Sections were stained with either HPS (A-B), the macrophage-specific antibody AIA-31240 (C-D), the SMC–α-actin–specific antibody (E-F), or with Resorcin-Fuchsin (elastin; G-H).
Discussion
LRP has a well-established role in the hepatic removal of APOE-rich remnant lipoproteins from the plasma compartment and thereby has strong antiatherogenic potential.5 In the present study, we investigated the role of hepatic LRP in atherosclerosis development, independent of its role in the metabolism of atherogenic APOE-rich remnant lipoproteins. We have addressed this question using the Cre/LoxP homologous recombination system to inactivate the hepatic LRP gene in an LDL receptor and APOE double-deficient background. On this background, LRP deficiency resulted in slightly lower plasma cholesterol, coinciding with higher plasma lipoprotein lipase, coagulation factor VIII, von Willebrand factor, and tissue-type plasminogen activator. Despite the lower plasma cholesterol, LRP deficiency resulted in 2-fold higher atherosclerosis. Hence, hepatic LRP is atheroprotective independent of plasma cholesterol, possibly due to maintaining low levels of its proatherogenic ligands.
Recently, mice carrying the conditional LRP allele have also been combined with mice that express Cre recombinase under control of the SMC-specific SM22 promotor.4 In these mice, it has been demonstrated that LRP plays a major role in determining vessel wall integrity and atherosclerosis via controlling SMC platelet–derived growth factor (PDGF) receptor activation.4 Although we cannot exclude LRP deletion at single cell level in the vessel wall of our mice, several independent lines of evidence indicate that increased atherosclerosis in MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice is not due to SMC-specific LRP deletion and the consequent dysregulation of the PDGF receptor signaling pathway. First, using the MX1Cre-mediated recombination system, we had no indication of recombination at the level of the vessel wall, either for the conditional reporter β-galactosidase allele (Figure 1C) or for LRP itself (Figure 1E-F). Second, we did not observe differences in PDGF receptor staining in MX1Cre+LRPflox/flox LDLR–/–APOE–/– mice compared with LRPflox/floxLDLR–/–APOE–/– mice (Figure 1G-H). Third, no signs of disrupted elastic layers, increased SMC proliferation, or aneurysm formation were observed in (nondiseased) descending aortas of our experimental mice. It should be mentioned, however, that our reporter studies demonstrate β-galactosidase positivity in the spleen (< 5% of the cells; Figure 1B), indicating MX1Cre-mediated recombination at the level of splenocytes possibly including macrophages. Therefore, we cannot fully exclude the possibility that LRP deletion at the level of (spleen and/or plaque) macrophages contributes to the observed phenotype.
The use of mice that lack hepatic LRP on an LDL receptor and APOE double-deficient background allowed us to investigate the role of LRP in atherogenesis independent of its role in the metabolism of APOE-rich lipoproteins. Whereas APOE deficiency will exclude removal of APOE-rich lipoproteins via both LDL receptor and LRP, LDL receptor deficiency will also exclude possible removal of lipoproteins mediated by the APOB100 pathway.21 Thus, one would expect plasma cholesterol levels to be similar in MX1Cre+LRPflox/floxLDLR–/–APOE–/– and control LRPflox/floxLDLR–/–APOE–/– mice. Why plasma cholesterol levels decrease upon deletion of LRP on an LDLR–/–APOE–/– background (Table 1; Figure 2), therefore, remains an intriguing question. This observation may be related to the elevated levels of plasma LPL in hepatic LRP–deficient mice. In vitro and in vivo studies show that LPL may perform a bridging function between lipoprotein particles and extracellular heparan sulfate proteoglycans (HSPGs),22 thereby stimulating the uptake of lipoproteins by cells, including hepatocytes and macrophages.23 The fact that accumulation of LPL coincided with decreased plasma cholesterol levels let us speculate that LPL might also stimulate uptake of lipoproteins in our animal model. If true, this uptake would be independent of LDL receptor, APOE, and LRP. Alternatively, we cannot exclude that LRP deficiency slightly affects plasma cholesterol through other mechanisms. These mechanisms may include an effect of LRP on VLDL production, which has previously been demonstrated for the LDL receptor.24
The plasma LPL measurements in MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice provide the first in vivo evidence that LRP is involved in LPL removal and confirm the in vitro data on the direct interaction between LPL and LRP.5 Our data further indicate that hepatic LRP contributes to the plasma removal of catalytically inactive LPL, but not its active variant (Table 2). It should be noted that our plasma LPL mass assay does not distinguish between monomeric, dimeric, and potential multimeric LPL forms that occur in plasma. Therefore, it remains speculative which LPL form is cleared from the circulation by hepatic LRP. As active LPL largely consists of dimers,25 it seems conceivable that the hepatic LRP–dependent removal of LPL is restricted to its monomeric variant.
LPL elevation in MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice may be one of the mechanisms through which LRP deficiency may become a proatherosclerotic condition. Elevation of catalytically inactive LPL may not only promote beneficial lipoprotein uptake in the liver by binding of lipoproteins to HSPGs, but may also cause retention and uptake of lipoproteins in the vessel wall.11,26 Therefore, elevated LPL, as a consequence of LRP deficiency, may become a potent proatherosclerotic factor.
Previously, we have demonstrated that conditional disruption of the LRP gene in mice results in the accumulation of plasma FVIII levels that coincided with an increase of plasma VWF.16 These findings were confirmed in the present study for hepatic LRP–deficient mice on an APOE and LDL receptor double-deficient background (Table 2). Immunohistochemical studies on human atherectomy specimens revealed the presence of FVIII in the vicinity of macrophages and SMCs in atheromatous regions with massive deposits of oxidized LDL (oxLDL).10 Studies in VWF and LDL receptor double-deficient mice show that VWF recruits platelets/leukocytes to the lesion and that VWF deficiency protects against atherosclerosis.27 These observations are in favor of a role for FVIII and VWF in promoting plaque formation in our MX1Cre+LRPflox/floxLDLR–/–APOE–/– mouse model.
In vitro studies have shown that LRP plays an important role in t-PA binding.5 In the present study, we provide clear in vivo evidence that LRP is involved in determining plasma levels of t-PA (Table 2). t-PA deficiency reduces atherosclerosis as found in studies using t-PA–deficient atherosclerosis-susceptible APOE*3-Leiden mice.6 Whether (plasma) t-PA elevation can account for the accelerated atherosclerosis observed in our LRP-deficient mice remains unclear. t-PA is suggested to play a role in atherosclerosis by affecting cellular migration and proliferation of SMCs.6 Since LRP deficiency did not affect lesion composition, including the SMC content, t-PA seems not to play a dominant role in the accelerated atherosclerosis in MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice.
The data provided by this study do not allow identification of the exact mechanism by which hepatic LRP affects the development of atherosclerosis in MX1Cre+LRPflox/floxLDLR–/–APOE–/– mice. Of the many LRP postulated ligands, we were able to measure only a limited number in our mouse model. Other LRP ligands that are related to atherosclerosis include urokinase tissue-type plasminogen activator (u-PA),7 plasminogen activator inhibitor-1 (PAI-1),8 and tissue factor pathway inhibitor (TFPI).9 In the future it will be of interest to crossbreed mice deficient for u-PA, PAI-1, TFPI, FVIII, VWF, or t-PA on a MX1Cre+LRPflox/floxLDLR–/–APOE–/– background. This will further advance our understanding on the mechanism by which hepatic LRP modulates atherosclerosis.
Prepublished online as Blood First Edition Paper, February 5, 2004; DOI 10.1182/blood-2003-11-4051.
Supported by grants from the Royal Netherlands Academy of Arts and Sciences (B.J.M.v.V.), the European Union project QLK1-CT-1999-498 (S.M.S.E.S.), and the Netherlands Heart Foundation project 2000.099 (G.G. and K.W.v.D.) and project 2000.051 (L.S.M.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Marijke Voskuilen, Ria van den Hoogen, Erik Offerman, and Kristan Melford from Cornell University, New York, for their excellent technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal