Abstract
Tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL) is a member of the TNF superfamily exerting cytotoxic activities toward tumor cells. Herein, we demonstrate that therapeutic concentrations of interferon α (IFNα) stimulate the expression of high levels of TRAIL mRNA and the release of elevated amounts of a soluble bioactive form of TRAIL (sTRAIL) in both human neutrophils and monocytes. Supernatants harvested from IFNα-treated neutrophils/monocytes elicited, on TRAIL-sensitive leukemic cell lines, proapoptotic activities that were significantly reduced by either a combination of TRAIL-R1/Fc and TRAIL-R2/Fc chimeras or neutralizing anti-TRAIL, anti–TRAIL-R1, and anti–TRAIL-R2 antibodies, suggesting that they were mediated by released sTRAIL acting on both TRAIL receptors. Since diseases such as chronic myeloid leukemia (CML) and melanoma are effectively treated with IFNα,we also demonstrate that CML neutrophils and peripheral blood mononuclear cells (PBMCs) cultured with IFNα at therapeutic concentrations retain the capacity of releasing sTRAIL, suggesting that CML leukocytes, in vivo, might represent an important source of sTRAIL. In this regard, we show that sTRAIL serum levels as well as leukocyte-associated TRAIL significantly increase in melanoma patients following IFNα administration. Collectively, these findings indicate that sTRAIL released by IFNα-activated neutrophils and monocytes contributes not only to the immunoregulatory actions but also to the therapeutic activities of IFNα.
Introduction
Tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL/Apo-2 ligand) is a member of the TNF superfamily identified on the basis of the homology of its extracellular domain with Fas/Apo-1 ligand.1,2 Although primarily expressed as a type II transmembrane protein, TRAIL may exist in a soluble form (sTRAIL) either generated through enzymatic shedding or released in association with microvesicles.3-6 TRAIL exerts its activity by interacting with a complex system of 2 death receptors (DR4/TRAIL-R1 and DR5/TRAIL-R2) and 3 decoy receptors (DcR1/TRAIL-R3, DcR2/TRAIL-R4, and osteoprotegerin/OPG).7,8 Although these receptors are characterized by a high sequence homology in their extracellular domains, only DR4/TRAIL-R1 and DR5/TRAIL-R2 contain a functionally active cytoplasmic death domain that allows an apoptotic response upon TRAIL stimulation.9,10 The biologic significance of TRAIL as mediator of innate and specific immunity against transformed and virus-infected cells has been clearly documented by several reports.11-18 In particular, it has been demonstrated that activated monocytes, dendritic cells, natural killer cells, and T lymphocytes may exert tumoricidal activities through membrane-bound TRAIL.5,8,12-15 Evidence that TRAIL-deficient mice are more susceptible to experimental and spontaneous tumor initiation and metastasis further support the fundamental role of this molecule in cancer immunosurveillance.19 Moreover, in recent years, it has been suggested that the immunomodulatory activity of interferons (IFNs) in controlling cancer development might also be attributable to their capacity of inducing membrane-bound TRAIL expression in different effector cells.14,15,18,20-22 In the light of the tumor-selective proapoptotic activity of membrane-bound TRAIL, the potential use of recombinant soluble forms of this molecule as cancer therapeutics is presently being exploited in several preclinical and preliminary clinical studies.23
TRAIL is expressed on the surface of different activated cells of the immune system such as IFNγ-, IFNα-, or lipopolysaccharide (LPS)–stimulated monocytes5 and/or dendritic cells12,13 ; CD4+ T lymphocytes upon specific T-cell receptor (TCR) engagement or IFNα stimulation14 ; and IFNγ-stimulated natural killer cells.15 More recently, induction of membrane-bound TRAIL has been also shown on measles virus–stimulated dendritic cells16 and Newcastle disease virus–stimulated monocytes.17 Even though neutrophils are well known to produce ligands of the TNF family, such as TNFα, Fas ligand (FasL), CD30L24 , and B-lymphocyte stimulator (BLyS),25 it has not yet been investigated whether they eventually express membrane-bound TRAIL or release sTRAIL.
In this work, we show that peripheral blood neutrophils and mononuclear cells up-regulate TRAIL mRNA and release significant amounts of functionally active sTRAIL in response to IFNα. Given the use of IFNα in the treatment of diseases such as chronic myeloid leukemia (CML)26 and melanoma, we also demonstrate that neutrophils and mononuclear cells isolated from CML patients efficiently release sTRAIL after treatment with IFNα. Furthermore, we report that administration of low-dose IFNα (3 million units) to patients with stage IV metastatic melanoma results in a dramatic increase of sTRAIL serum levels and leukocyte-associated TRAIL. Taken together, our data suggest that the release of sTRAIL by IFNα-treated peripheral blood cells represents a novel mechanism whereby therapeutic concentrations of IFNα might exert immunomodulatory and antitumor activities.
Patients, materials, and methods
Cell purification and culture
Hghly purified granulocytes (neutrophils > 96.5%, eosinophils < 3%), peripheral blood mononuclear cells (PBMCs), monocytes, and lymphocytes were isolated under endotoxin-free conditions.27 Immediately after purification, cells were suspended in RPMI 1640 supplemented with 10% low-endotoxin fetal calf serum (FCS; < 0.5 endotoxin units (EU)/mL; Biowhittaker Europe, Verviers, Belgium) and cultured at 5 × 106/mL in the absence or in the presence of 100 to 1000 U/mL IFNα (Roche Laboratories, Nutley, NJ), 100 ng/mL LPS (Sigma, St Louis, MO), 10 ng/mL granulocytemacrophage colony-stimulating factor (GM-CSF; Schering-Plough, Kenilworth, NJ), 10 nM fMLP (formyl-methionyl-leucyl-phenylalanine; Sigma), or 1000 U/mL G-CSF (Chugai Pharmaceutical, London, United Kingdom). After the desired incubation period, cells were collected and spun at 350g for 5 minutes. The resulting supernatants were immediately frozen in liquid nitrogen and stored at –70° C. The corresponding pellets were either extracted for total RNA or thawed in phosphate-buffered saline (PBS) containing 0.5% nonidet P-40 (NP40), 5 mM EDTA (ethylenediaminetetraacetic acid), 1 mM phenylmethylsulfonyl fluoride (PMSF), and 5 μg/mL leupeptin/pepstatin A and then spun (14 000g, 5 min) to remove cell debris. Peripheral blood leukocytes and bone marrow samples from patients affected by CML were obtained, after signed informed consent, during routine diagnosis at the Hematology Unit of the University of Verona. Approval was obtained by the University of Verona institutional review board. Bone marrow samples from CML patients in chronic phase underwent the same procedures used to purify peripheral blood neutrophils, allowing us to obtain a mixed cell population composed of myeloblasts, promyelocytes, myelocytes, and metamyelocytes, along with some band and segmented neutrophils. MEG-01 and KU-812 cell lines, derived from CML patients in megakaryocytic and myeloid blast crisis, respectively (obtained from DSMZ, Braunschweig, Germany), and Fas+/DR4+ Jurkat cells (J32 clone; kindly provided by Dr L. Zamai, Institute of Morphological Sciences, University of Urbino, Italy)28 were cultured in RPMI 1640 supplemented with 10% FCS.
RNA isolation and Northern blot analysis
Flow cytometry for membrane-bound TRAIL and TRAIL receptors
Briefly, freshly isolated or cultured cells were centrifuged, suspended in 100 μL of PBS containing 0.2% albumin and 0.2% sodium azide, and then incubated for 15 minutes with 10% complement-inactivated human serum. Cells were then incubated with 10 μg/mL monoclonal antibodies (mAbs) raised against human TRAIL (TNFSF10; R&D Systems, Minneapolis, MN), human TRAIL-R1, -R2, -R3, -R4 (Apotech Corporation, Epalinges, Switzerland), or control mouse immunoglobulin G1 (IgG1; Sigma), followed by biotinylated secondary sheep antimouse IgG (Sigma). Immunolabeling was revealed using phycoerythrin-conjugated streptavidin (Becton Dickinson, Mountain View, CA). Analysis was performed with FACScan using CellQuest software (Becton Dickinson). For each sample, the mean fluorescence intensity (MFI) was calculated by subtracting the MFI of the corresponding immunolabeled isotype control.25
Western blot analysis
Supernatants from IFNα-activated neutrophils and PBMCs were analyzed by Western blot under reducing, nonreducing, and native conditions. Supernatants were first concentrated by a Centricon Plus 20 device (Amicon, Beverly, MA) and then analyzed for total protein concentration by Lowry assay (Bio-Rad Laboratories, Hercules, CA). Under native conditions, equal amounts of protein with additional 10% of glycerol were separated on a 6% phenoxyacetic acid (PAA) gel. Under nonreducing and reducing conditions, equal amounts of protein for each sample were separated on a 4% to 12% Bis-Tris gel (Invitrogen SRL, Milan, Italy). Before loading, samples were denatured in NuPage lithium dodecyl sulfate (LDS) sample buffer (Invitrogen SRL) at 60° C (nonreducing conditions) or at 95° C (reducing conditions, containing NuPAGE Sample Reducing Agent; Invitrogen) for 15 minutes. After electrophoresis, proteins were electroblotted onto a polyvinylidene fluoride (PVDF) membrane (Amersham Pharmacia Biotech Italia, Milan, Italy). Membranes were stained using 1 μg/mL of antihuman TRAIL mAb (clone B35-1; BD Biosciences, Calderara, Italy), developed with a secondary mouse antihuman IgG conjugated to horseradish peroxidase (BD Biosciences), and detected by SuperSignal detection system (Pierce Chemical, Rockford, IL). In all Western blot experiments soluble nondisulfide-linked trimeric recombinant human soluble TRAIL (rhsTRAIL; R&D Systems) was used as a positive control.
ELISA
Concentrations of sTRAIL and sFas ligand in cell-free supernatants, cells pellets, and sera obtained from healthy donors and melanoma patients were measured by using commercial enzyme-linked immunosorbent assay (ELISA) kits (soluble TRAIL/Apo2L and soluble FasL ELISA KITs; Diaclone Research, Besancon, France), according to the manufacturer's instructions.
Apoptosis assessment
Apoptotic rate of neutrophils was determined by propidium iodide staining, as previously described,29 whereas apoptosis of leukemic cell lines was measured by annexin-V–FLUOS Staining kit (Roche Diagnostic, Mannheim, Germany). Neutrophils were preincubated with or without 100 U/mL IFNα for 4 hours and then cultured in the absence or in the presence of 1 to 20 ng/mL of SuperKillerTRAIL (Apotech Corporation) for additional 20 hours. In other experiments, normal or CML neutrophils were cultured with or without 100 U/mL IFNα in the presence of up to 500 nM imatinib mesylate (Novartis Pharma AG, Basel, Switzerland) and then harvested after 24 hours. MEG-01, Jurkat J32 (both TRAIL sensitive), and KU-812 (TRAIL insensitive) cell lines were seeded at a density of 1 × 106 cells/mL in the presence of media conditioned by IFNα alone or by neutrophils/monocytes cultured with IFNα. Supernatants from neutrophils were concentrated by Centricon Plus 20 device (Amicon) as described29 and then used at a final concentration of 5 ng/mL sTRAIL (as measured by ELISA). The following reagents were used to neutralize TRAIL-induced apoptosis of leukemic cell lines: Fc chimera TRAIL-R1 and Fc chimera TRAIL-R2 (R&D Systems) at 100 ng/mL; mAb 2E5 (anti-TRAIL; Alexis, San Diego, CA) at 3 μg/mL; mAb HS101 and mAb HS201 (anti–TRAIL-R1 and anti–TRAIL-R2, respectively; Alexis) at 5 μg/mL.
Patients
After signed informed consent was obtained, blood was collected from 6 healthy subjects and 6 patients with stage IV metastatic melanoma treated at the Istituto Nazionale Tumori of Milano. Patients were enrolled in clinical protocols of vaccine therapy that scheduled subcutaneous administration of IFNα. Blood samples were collected prior to and 24 hours after the first administration of 3 million units (MU) IFNα (IFNα2b, Schering-Plough Segrate, Milan, Italy; IFNα, Alfa Wasserman Alanno, Pescara, Italy). Serum was immediately separated from blood by centrifugation at 860g for 10 minutes at 4° C and stored at –80° C. PBMCs were also isolated from these blood samples and lysed for TRAIL measurement in cell-associated pellets.
Statistical analysis
Data are expressed as means ± SEM. Statistical evaluation was performed by the Student t test for paired data.
Results
Human neutrophils incubated with IFNα up-regulate TRAIL mRNA expression
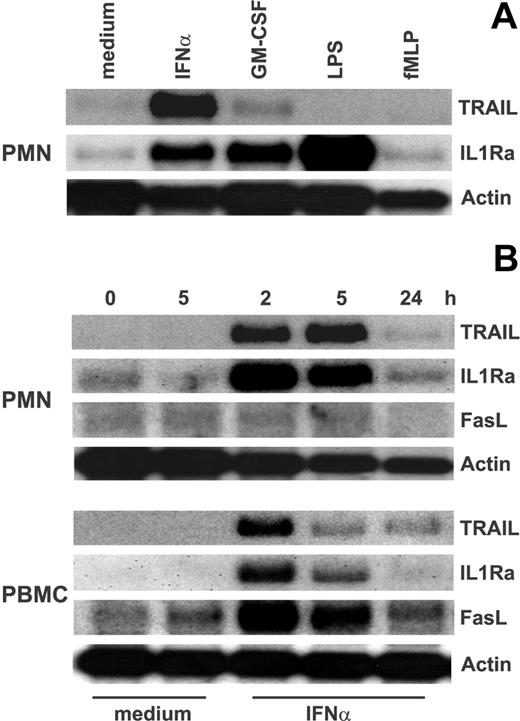
To investigate TRAIL mRNA expression in neutrophils, these cells were cultured with a variety of mediators and then processed for Northern blot analysis. Figure 1 shows that resting neutrophils express constitutive levels of TRAIL mRNA that persisted for up to 24 hours of culture. TRAIL mRNA expression was not substantially increased by the addition of GM-CSF, LPS, fMLP (Figure 1A), or G-CSF (not shown) to the cultures of neutrophils, even though these stimuli up-regulated the IL-1ra gene expression (Figure 1A).24 By contrast, IFNα-treated neutrophils exhibited a considerably high accumulation of TRAIL mRNA (Figure 1A), with maximum expression levels reached after 5 hours of incubation (Figure 1B). The genuine ability of neutrophils to express TRAIL mRNA was further confirmed by the Northern blot experiments performed with PBMCs (Figure 1B), which displayed a different time course of TRAIL mRNA accumulation and which selectively expressed FasL mRNA in response to IFNα.30,31 Finally, TNFα gene was expressed neither in resting nor in IFNα-treated leukocytes (Figure 1B).
Expression of TRAIL mRNA in IFNα-stimulated neutrophils and PBMCs. (A) Neutrophils were stimulated for 3 hours with 100 U/mL IFNα, 10 ng/mL GM-CSF, 100 ng/mL LPS, and 10 nM fMLP before total RNA extraction and analysis of TRAIL, IL-1ra, and actin mRNA expression by Northern blotting. (B) Neutrophils and PBMCs, purified from the same donor, were cultured for the times indicated with or without 100 U/mL IFNα and then subjected to Northern blot analysis for TRAIL, FasL, IL-1ra, and actin mRNA expression. Time 0 indicates RNA extraction in freshly isolated leukocytes. Data are representative of at least 2 independent experiments for each panel.
Expression of TRAIL mRNA in IFNα-stimulated neutrophils and PBMCs. (A) Neutrophils were stimulated for 3 hours with 100 U/mL IFNα, 10 ng/mL GM-CSF, 100 ng/mL LPS, and 10 nM fMLP before total RNA extraction and analysis of TRAIL, IL-1ra, and actin mRNA expression by Northern blotting. (B) Neutrophils and PBMCs, purified from the same donor, were cultured for the times indicated with or without 100 U/mL IFNα and then subjected to Northern blot analysis for TRAIL, FasL, IL-1ra, and actin mRNA expression. Time 0 indicates RNA extraction in freshly isolated leukocytes. Data are representative of at least 2 independent experiments for each panel.
Soluble TRAIL release by IFNα-stimulated leukocytes
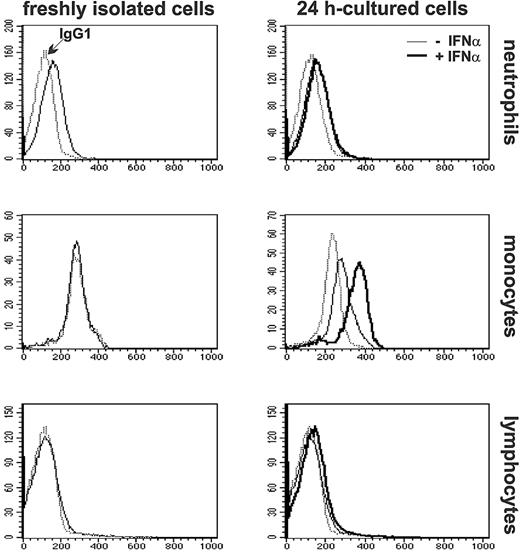
To determine whether up-regulation of TRAIL mRNA correlated with an increased accumulation of the related membrane-bound protein, expression of surface TRAIL was analyzed by flow cytometry in either freshly isolated or cultured neutrophils and PBMCs. As demonstrated by the representative experiment shown in Figure 2, low levels of constitutive TRAIL expression were found in freshly isolated neutrophils (38 ± 11 MFI, n = 5) but not on freshly isolated monocytes or lymphocytes from the same donors. As previously reported,11 expression of membrane-bound TRAIL increases in 24-hour cultured monocytes (35 ± 13 MFI, n = 5) and is further augmented by IFNα (66 ± 11 MFI), whereas in lymphocytes remains negative, regardless of the presence of IFNα in the incubation medium20 (Figure 2). Surprisingly, while the levels of surface TRAIL did not change in 24-hour cultured neutrophils (25 ± 7 MFI) compared with freshly isolated cells, they did not increase at all upon treatment with IFNα (24 ± 8 MFI; Figure 2), even if the cytokine was used at 1000 U/mL (data not shown).
Expression of membrane-bound TRAIL in freshly isolated and IFNα-stimulated leukocytes. Neutrophils and PBMCs, purified from the same donor, were analyzed by flow cytometry immediately after isolation or after incubation for 24 hours in the absence or in the presence of 100 U/mL IFNα. Monocytes and lymphocytes were identified in the total PBMC population according to forward light scatter and side scatter parameters. Staining with anti-TRAIL mAb are indicated with normal and bold histograms for cells cultured in the absence or in the presence of IFNα, respectively. Dotted histograms represent staining with isotype control IgG1 mAbs. These profiles are representative of analyses performed in 5 healthy donors.
Expression of membrane-bound TRAIL in freshly isolated and IFNα-stimulated leukocytes. Neutrophils and PBMCs, purified from the same donor, were analyzed by flow cytometry immediately after isolation or after incubation for 24 hours in the absence or in the presence of 100 U/mL IFNα. Monocytes and lymphocytes were identified in the total PBMC population according to forward light scatter and side scatter parameters. Staining with anti-TRAIL mAb are indicated with normal and bold histograms for cells cultured in the absence or in the presence of IFNα, respectively. Dotted histograms represent staining with isotype control IgG1 mAbs. These profiles are representative of analyses performed in 5 healthy donors.
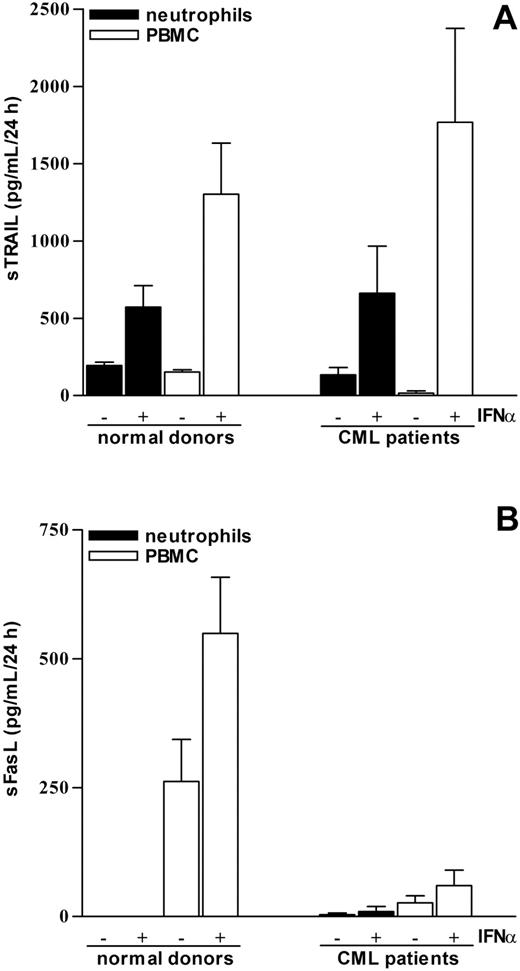
To explain such discrepancy, we investigated whether, at least in neutrophils, TRAIL was released as a soluble protein (sTRAIL) instead of being expressed on the cell surface. For this purpose, neutrophils, PBMCs, or, alternatively, Percoll-purified monocytes were cultured for 24 hours in the absence or the presence of 100 U/mL IFNα to analyze their capacity to release sTRAIL into the cell-free supernatants. Figure 3A shows that while untreated leukocytes constitutively released small but detectable amounts of sTRAIL, both neutrophils and PBMCs cultured with IFNα secreted significant amounts of sTRAIL. On a per cell basis, PBMCs resulted consistently more effective than neutrophils (Figure 3A), with monocytes contributing for 85% ± 6% (n = 5) of the total sTRAIL release by PBMCs. Time-course experiments revealed that sTRAIL was released as early as after 3 hours of incubation with IFNα, progressively accumulating into the supernatants for up to 48 hours (data not shown). The genuine release of sTRAIL by neutrophils was substantiated by the findings that sFasL was present only in supernatants harvested from cultured PBMCs but not from activated neutrophils (Figure 3B), in line with the Northern blot data (Figure 1) and with earlier studies.31 Importantly, concentrations of IFNα ranging from 100 to 1000 U/mL were equally effective in inducing sTRAIL release by either neutrophils or PBMCs (data not shown). Measurement of total TRAIL production by leukocytes (ie, released sTRAIL in parallel with cell-associated TRAIL) demonstrated that treatment with IFNα for 24 hours induced approximately a 5-fold increase of total TRAIL synthesis relative to cells treated with medium only in both neutrophils and PBMCs (data not shown).
Release of sTRAIL by IFNα-stimulated neutrophils and PBMCs of healthy donors and CML patients. Neutrophils and PBMCs isolated from healthy donors or CML patients were incubated for 24 hours in the absence or in the presence of 100 U/mL IFNα. The yields of sTRAIL (A) and sFasL (B) were determined in the corresponding cell-free supernatants by ELISA. The mean values ± SEM of cytokine release from 3 to 4 independent experiments are shown.
Release of sTRAIL by IFNα-stimulated neutrophils and PBMCs of healthy donors and CML patients. Neutrophils and PBMCs isolated from healthy donors or CML patients were incubated for 24 hours in the absence or in the presence of 100 U/mL IFNα. The yields of sTRAIL (A) and sFasL (B) were determined in the corresponding cell-free supernatants by ELISA. The mean values ± SEM of cytokine release from 3 to 4 independent experiments are shown.
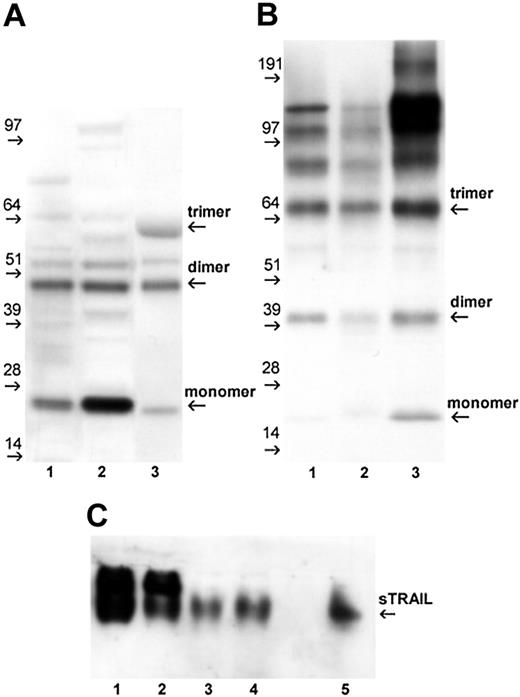
To further confirm the release of sTRAIL by IFNα-activated neutrophils/PBMCs as detected by ELISA, we initially performed Western blot analysis of concentrated supernatants under reducing conditions. As reported in Figure 4A, sTRAIL was detectable as 2 major bands of approximately 24 kDa and 48 kDa in both neutrophils and PBMC supernatants, likely representing the monomeric and dimeric forms of the protein, respectively. A recombinant bioactive soluble TRAIL (rhsTRAIL), produced as a nondisulfide-linked homotrimer, showed a similar band pattern with an additional band at about 64 kDa, representing the trimeric conformation of the protein. In the attempt to investigate the aggregation state of the bioactive sTRAIL released by activated cells, Western blot analysis was performed under nonreducing and native conditions. As shown in Figure 4B (nonreducing), a band of approximately 66 kDa was detectable in supernatants from both activated neutrophils and PBMCs and rhsTRAIL preparations. This band likely represents the trimeric form of the protein, which maintains aggregation stability in the experimental setting used (denaturation at 60° C). Moreover, higher aggregated forms (multimers) of sTRAIL, as well as dimers and monomers, were detectable. Western blot analysis under native conditions (Figure 4C) further confirms the similarity of the migration patterns displayed by the bioactive rhsTRAIL and the sTRAIL present in cell supernatants. Altogether, these results suggest that sTRAIL released by IFNα-activated leukocytes is characterized by a similar aggregation state as the one observed with the proapoptotic homotrimeric rhsTRAIL.
Western blot analysis of sTRAIL released by IFNα-stimulated neutrophils and PBMCs. Supernatants harvested from neutrophils and PBMCs, cultured for 24 hours with 100 U/mL IFNα, were concentrated and analyzed for the presence of sTRAIL by Western blot, as described in “Patients, materials, and methods.” Data are representative of analyses performed in 2 healthy donors. (A) Concentrated supernatants (50 μg/sample) from neutrophils (lane 1) and PBMC (lane 2) were run under reducing conditions and stained with anti-TRAIL mAb. The rhsTRAIL (nondisulfide-linked homotrimer) at 100 ng/sample (lane 3) was included as positive control. (B) The rhsTRAIL (lane 1), supernatants of activated neutrophils (lane 2), and PBMCs (lane 3) were analyzed by Western blot under nonreducing conditions; samples were heated at 60° C for 15 minutes before loading. (C) Freshly concentrated supernatants of activated PBMCs (lanes 1-2) and neutrophils (lane 3-4) were analyzed under native conditions in comparison with rhsTRAIL (lane 5).
Western blot analysis of sTRAIL released by IFNα-stimulated neutrophils and PBMCs. Supernatants harvested from neutrophils and PBMCs, cultured for 24 hours with 100 U/mL IFNα, were concentrated and analyzed for the presence of sTRAIL by Western blot, as described in “Patients, materials, and methods.” Data are representative of analyses performed in 2 healthy donors. (A) Concentrated supernatants (50 μg/sample) from neutrophils (lane 1) and PBMC (lane 2) were run under reducing conditions and stained with anti-TRAIL mAb. The rhsTRAIL (nondisulfide-linked homotrimer) at 100 ng/sample (lane 3) was included as positive control. (B) The rhsTRAIL (lane 1), supernatants of activated neutrophils (lane 2), and PBMCs (lane 3) were analyzed by Western blot under nonreducing conditions; samples were heated at 60° C for 15 minutes before loading. (C) Freshly concentrated supernatants of activated PBMCs (lanes 1-2) and neutrophils (lane 3-4) were analyzed under native conditions in comparison with rhsTRAIL (lane 5).
IFNα protects neutrophils from TRAIL-induced apoptosis
Recently, a recombinant TRAIL preparation that has been engineered to self-associate spontaneously into stable structures with high biologic activity, called leucine zipper (LZ)–TRAIL, was demonstrated to accelerate the constitutive apoptotic rate of human neutrophils.32 In our hands, increasing concentrations (1-20 ng/mL) of rhsTRAIL His-tag CC-mutant (a multimeric soluble recombinant form of TRAIL characterized by a biologic activity higher than LZ-TRAIL) confirmed that soluble forms of recombinant TRAIL significantly increase the spontaneous neutrophil apoptosis in a 24-hour culture period (from 30% ± 6% to 52% ± 6% with 20 ng/mL rhsTRAIL; P < .01, n = 5). In light of the ability of IFNα to induce the release of an endogenous sTRAIL, we investigated whether type I IFN could eventually promote neutrophil apoptosis. However, this appeared not to be the case, since 100 U/mL IFNα not only reduced the constitutive apoptosis of neutrophils (to 20% ± 5%; P < .01) but also prevented that induced by 20 ng/ml rhsTRAIL (24% ± 10%; P < .01). Similarly to neutrophils, PBMCs remained more viable (by 10% ± 5%, n = 3) when cultured in the presence of IFNα. In agreement with the data reported by Renshaw et al,32 flow cytometry analysis of TRAIL receptors in freshly isolated neutrophils confirmed a low expression of TRAIL-R2 (38.7 ± 9.5 MFI, n = 5), a high expression of TRAIL-R3/DcR1 (332.1 ± 8.3 MFI), and the absence of both TRAIL-R1 and TRAIL-R4.
Neutrophils and PBMCs isolated from CML patients release sTRAIL upon stimulation with therapeutic concentrations of IFNα
Having observed that leukocytes from healthy donors release significant amounts of sTRAIL upon treatment with concentrations of IFNα (100 U/mL), which were therapeutically reached in plasma of CML patients,33 we investigated the release of sTRAIL by leukocytes isolated from these subjects. For this purpose, peripheral blood neutrophils and PBMCs isolated from chronic-phase CML patients at the diagnosis were cultured for 24 hours in the absence or presence of 100 U/mL IFNα before harvesting their supernatants for sTRAIL measurement. As depicted in Figure 3A, IFNα-stimulated neutrophils and PBMCs isolated from CML patients release sTRAIL at levels similar to those of normal leukocytes. Strikingly, elevated levels of sTRAIL were also measured in supernatants harvested from cultures of a mixture of immature myeloid cells (myeloblasts, promyelocytes, myelocytes, and metamyelocytes) containing some bands and segmented neutrophils isolated from the bone marrow of CML patients. In fact, when incubated in the presence of IFNα, these cells released increased amounts of sTRAIL (268 ± 53 pg/mL versus 136 ± 142 pg/mL, respectively; n = 2) indicating that even immature CML myeloid elements are responsive to IFNα and contribute to sTRAIL release in their microenvironment. Similarly to what was observed in healthy donors, sFasL was detected only in supernatants of CML PBMCs treated with IFNα (Figure 3B). However, the yields of sFasL recovered in these samples were lower than those recovered from normal PBMCs. Additional experiments demonstrated that the patterns of membrane-bound TRAIL and TRAIL receptor expression in freshly isolated CML neutrophils were substantially similar to those of healthy donors (data not shown). In contrast, CML neutrophils displayed a constitutive apoptotic rate lower than normal neutrophils, remaining, however, susceptible to the proapoptotic effect of rhsTRAIL His-tag CC-mutant (by 10.5% ± 4%; n = 2). Collectively, these data demonstrate that the capacity of leukemic leukocytes to release sTRAIL in response to IFNα is unaltered.
Neutrophil- and monocyte-derived supernatants induce apoptosis of TRAIL-sensitive leukemia cells
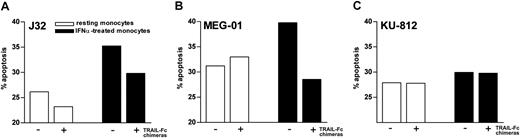
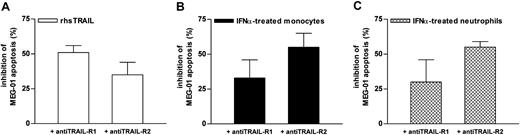
To ascertain the biologic activity of sTRAIL released by IFNα-treated phagocytes, we tested the ability of supernatants from IFNα-treated neutrophils and monocytes to induce apoptosis of TRAIL-sensitive cell lines. For this purpose, we used Fas+/DR4+ Jurkat (J32 clone), a well-characterized TRAIL-sensitive T-acute lymphoblastic leukemia cell line,28 and MEG-01, a Philadelphia chromosome–positive (Ph+) CML cell line displaying susceptibility to recombinant TRAIL. We also used KU-812, another Ph+ CML cell line, which, unlike MEG-01, was resistant to rhsTRAIL His-tag CC-mutant. Flow cytometric analysis of TRAIL receptor expression revealed a positive staining for TRAIL-R1 and TRAIL-R2 in MEG-01 and KU-812 cells and for only TRAIL-R2 in J32 cells, the latter finding in agreement with previous observations.34,35 Preliminary experiments revealed that viability of J32, MEG-01, and KU-812 cells was unaffected by doses of IFNα of up to 10 000 U/mL for 72 hours. Basal apoptosis rates of J32, MEG-01, and KU-812 cells detected in a 40-hour culture period were 9% ± 1%, 20% ± 1%, and 22% ± 5% (n = 5), respectively, as measured by annexin-V binding. These percentages variably increased if the cells were cultured in the presence of medium conditioned by supernatants harvested from resting monocytes (Figure 5) or neutrophils. Remarkably, apoptosis of MEG-01 and J32, but not of KU-812, greatly augmented if leukemia cells were cultured in the presence of medium containing supernatants harvested from monocytes stimulated with IFNα (Figure 5). That the latter enhancement was specifically provoked by endogenous sTRAIL was demonstrated by the observations that it was greatly reduced either by a combination of TRAIL-R1/Fc and TRAIL-R2/Fc chimeras (Figure 5) or by neutralizing anti-TRAIL mAb 25E (data not shown), whereas it was not influenced by neutralizing anti-IFNα mAb (data not shown). Supernatants of IFNα-treated neutrophils also increased the apoptosis rate of MEG-01 cells (from 52% ± 5% to 67% ± 6%; n = 3), such effect being partially neutralized (by 59% ± 4%) by TRAIL inhibitors. Anti-TRAIL mAb 25E or TRAIL-R1/Fc and TRAIL-R2/Fc chimeras completely suppressed the proapoptotic effect of 20 ng/mL rhsTRAIL of 82% ± 5%, 42% ± 3%, and 24% ± 5% in J32, MEG-01, and KU-812 cells, respectively. By using specific TRAIL-R1– and TRAIL-R2–neutralizing antibodies, we could also demonstrate that the proapoptotic activities of supernatants from IFNα-treated neutrophils and monocytes on MEG-01 cells are initiated through the involvement of both receptors, TRAIL-R2 appearing to signal more efficiently (Figure 6). The rhsTRAIL-induced apoptosis of MEG-01 cells was also mediated by the engagement of both TRAIL receptors, but, in this case, TRAIL-R1 appeared to play a more important role than TRAIL-R2 (Figure 6). Interestingly, the inhibitory action of TRAIL-R1 and TRAIL-R2 antibodies on the proapoptotic effects exerted by either leukocyte-derived supernatants or rhsTRAIL was only partial (Figure 6) and did not augment if the 2 reagents were used in combination instead of singularly (data not shown).
The sTRAIL present in supernatants of IFNα-treated monocytes enhances apoptosis in human leukemic cell lines. Apoptosis rate of J32, MEG-01, and KU-812 leukemic cell lines was assessed by annexin-V–FLUOS staining after a 2-day culture in the presence of conditioned medium prepared from resting (□) and IFNα-stimulated (▪) monocytes. Cell incubation was carried out in the absence or the presence of a combination of 100 ng/mL TRAIL-R1/Fc and TRAIL-R2/Fc chimeras. The experiment depicted is representative of 3.
The sTRAIL present in supernatants of IFNα-treated monocytes enhances apoptosis in human leukemic cell lines. Apoptosis rate of J32, MEG-01, and KU-812 leukemic cell lines was assessed by annexin-V–FLUOS staining after a 2-day culture in the presence of conditioned medium prepared from resting (□) and IFNα-stimulated (▪) monocytes. Cell incubation was carried out in the absence or the presence of a combination of 100 ng/mL TRAIL-R1/Fc and TRAIL-R2/Fc chimeras. The experiment depicted is representative of 3.
Effect of anti–TRAIL-R1 and anti–TRAIL-R2 neutralizing antibodies on MEG-01 apoptosis-induced by supernatants harvested from IFNα-treated leukocytes. MEG-01 cells were pretreated for 30 minutes with anti–TRAIL-R1 or anti–TRAIL-R2 neutralizing antibodies (5 μg/mL for each one) before addition of conditioned medium prepared from resting and IFNα-stimulated monocytes and neutrophils or 20 ng/mL rhsTRAIL. Apoptosis rate of MEG-01 cells was then assessed after a 2-day culture. Means ± SEM of the percentage of apoptotic inhibition exerted by anti–TRAIL-R1 or anti–TRAIL-R2 neutralizing antibodies calculated from 3 to 5 independent experiments are shown.
Effect of anti–TRAIL-R1 and anti–TRAIL-R2 neutralizing antibodies on MEG-01 apoptosis-induced by supernatants harvested from IFNα-treated leukocytes. MEG-01 cells were pretreated for 30 minutes with anti–TRAIL-R1 or anti–TRAIL-R2 neutralizing antibodies (5 μg/mL for each one) before addition of conditioned medium prepared from resting and IFNα-stimulated monocytes and neutrophils or 20 ng/mL rhsTRAIL. Apoptosis rate of MEG-01 cells was then assessed after a 2-day culture. Means ± SEM of the percentage of apoptotic inhibition exerted by anti–TRAIL-R1 or anti–TRAIL-R2 neutralizing antibodies calculated from 3 to 5 independent experiments are shown.
Detection of increased sTRAIL levels in sera of patients treated with IFNα
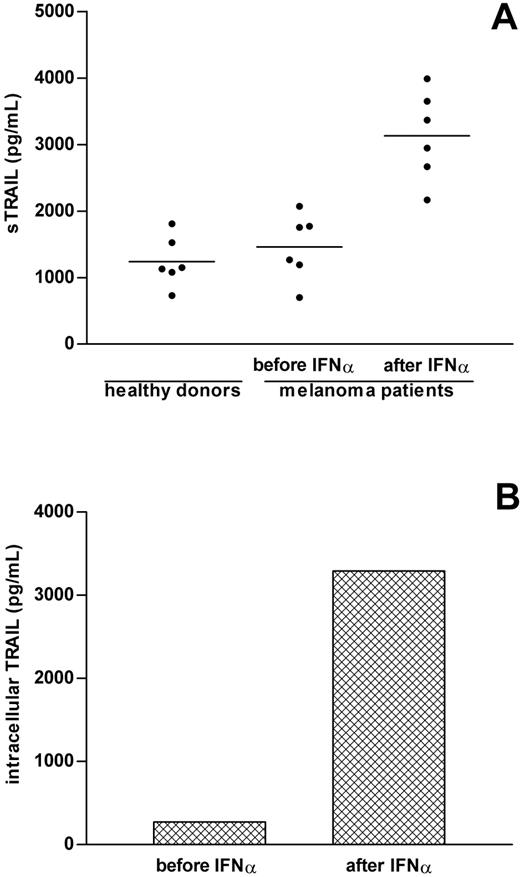
To assess whether release of sTRAIL takes place in vivo, we analyzed the levels of sTRAIL in sera of 6 stage IV metastatic melanoma patients undergoing treatment with low-dose (3 MU) IFNα. Serum samples were collected prior to and 24 hours after IFNα administration. The amounts of sTRAIL present in these sera before treatment with IFNα (1460 ± 203 pg/mL) were substantially comparable to those found in healthy donors (1239 ± 153 pg/mL; Figure 7A). Remarkably, sTRAIL serum levels dramatically increased in all patients after IFNα treatment (3133 ± 273 pg/mL; P = .0006; Figure 7A), whereas those of FasL did not significantly change (39 ± 11 vs 54 ± 14 pg/mL; P = .1). Analysis of the intracellular levels of TRAIL demonstrated that the accumulation of leukocyte-associated TRAIL dramatically augmented following IFNα exposure (Figure 7B), consistent with a TRAIL de novo synthesis. Collectively, these data demonstrate that serum levels of sTRAIL are increased following systemic IFNα administration and that peripheral blood leukocytes greatly contribute to this sTRAIL release in vivo.
Detection of increased sTRAIL levels in sera of IFNα-treated patients. (A) Serum samples obtained from 6 healthy donors and 6 stage IV metastatic melanoma patients were collected either prior to or 24 hours after IFNα administration and then analyzed for sTRAIL content by ELISA. The figure shows the mean values of averaged duplicate determinations. (B) PBMCs obtained from 2 different patients (collected either prior to or 24 hours after IFNα infusion) were lysed as described in “Patients, materials, and methods” and the levels of intracellular TRAIL determined by ELISA. The mean values of TRAIL levels associated to PBMC pellets are shown.
Detection of increased sTRAIL levels in sera of IFNα-treated patients. (A) Serum samples obtained from 6 healthy donors and 6 stage IV metastatic melanoma patients were collected either prior to or 24 hours after IFNα administration and then analyzed for sTRAIL content by ELISA. The figure shows the mean values of averaged duplicate determinations. (B) PBMCs obtained from 2 different patients (collected either prior to or 24 hours after IFNα infusion) were lysed as described in “Patients, materials, and methods” and the levels of intracellular TRAIL determined by ELISA. The mean values of TRAIL levels associated to PBMC pellets are shown.
Discussion
In this study, we demonstrate that upon incubation with therapeutic concentrations of IFNα, human neutrophils and monocytes are induced to accumulate TRAIL mRNA and release a soluble form of TRAIL (sTRAIL) that fully retains proapoptotic activities toward TRAIL-sensitive leukemic cell lines. Other agonists such as fMLP, LPS, G-CSF, and GM-CSF failed to up-regulate TRAIL gene expression and soluble protein release. Since additional experiments proved that IFNγ, at lower levels than IFNα, also promotes sTRAIL release by neutrophils (C.T., M.A.C., unpublished observations, December 2003), we could conclude that IFNs act as specific stimuli for sTRAIL production by neutrophils. Membrane-bound TRAIL was expressed on the surface of freshly isolated neutrophils at very low levels, without, however, increasing in response to either IFNα or IFNγ (C.T., M.A.C., unpublished observations, December 2003). In contrast, surface TRAIL was induced by IFNα in monocytes, confirming the observations made by Griffith and colleagues.11 These findings suggest that in neutrophils and monocytes, TRAIL surface expression and release are probably regulated by different mechanisms. It will be interesting to verify if neutrophils are exclusively committed to release TRAIL, similarly to what was previously observed in the case of BlyS.25
Release of endogenous sTRAIL by activated neutrophils did not correlate with an increased apoptosis of the same cells, as IFNα resulted in significantly prolong neutrophil survival. These data apparently contradict those reported by Renshaw and colleagues,32 who have recently shown that a recombinant soluble version of TRAIL strongly resembling the membranebound form (LZ-TRAIL) accelerates neutrophil apoptosis in the absence of additional proinflammatory stimuli. Indeed, even in our hands, rhsTRAIL His-tag CC-mutant slightly augmented neutrophil apoptosis. It must be, however, specified that all commercial recombinant TRAIL preparations are specifically designed to exert potent proapoptotic activities and, in the studies mentioned previously,32 such preparations have been used at concentrations much higher than those released by activated neutrophils. Nonetheless, sTRAIL present as homotrimeric or multimeric aggregates in supernatants conditioned by IFNα-stimulated neutrophils or monocytes was biologically functional, since it exerted remarkable cytotoxic activities toward TRAIL-sensitive leukemic cell lines, including Jurkat (J32 clone) and MEG-01 cells. These proapoptotic effects were mediated through TRAIL-R1 and TRAIL-R2, indicating that the soluble form of TRAIL released by IFNα-activated leukocytes can signal via both receptors. Even though the issue of a possible differential activation of TRAIL-R1 and TRAIL-R2 by natural or recombinant sTRAIL has been poorly addressed,36,37 our results are in agreement with those of Clarke et al.36 Collectively, our data demonstrate that endogenous TRAIL can be biologically effective not only as an integral membrane protein12-15 but also as a soluble released cytokine. To our knowledge, detection of TRAIL in a soluble form has been previously demonstrated only in supernatants derived from LPS-activated monocytes/macrophages,5 from reovirus-infected neoplastic cells,36 and from phytohemagglutinin (PHA)–activated and/or CD59-triggered Jurkat cells and normal T-cells blast.3,4,6 According to the latter reports, sTRAIL secretion by lymphocytes represents one of the mechanisms contributing to the activation-induced T-cell death (AICD) process by inducing the apoptosis of the activated lymphocytes themselves.4,6 On the basis of our findings, and given the role of IFNs in innate immunity, it is tempting to speculate that recombinant as well as endogenous IFNs employ, other than membrane-bound TRAIL, sTRAIL derived from peripheral blood neutrophils and mononuclear cells as a potential cytotoxic mediator against transformed and virus-infected cells. Studies are ongoing in our laboratory to understand whether the cytotoxic potential of sTRAIL could be enhanced by its interaction with extracellular matrix, as recently demonstrated for sFasL.38
The observations made in this work have additional clinical implications. IFNα has been proven to be an effective antiviral and antineoplastic drug and its systemic administration is currently employed in the treatment of several malignancies other than in viral diseases.39 For instance, CML is a clonal myeloproliferative expansion of transformed hematopoietic cells in which therapy with IFNα was found to suppress the leukemic clone.40 Interestingly, although the mechanisms whereby IFNα functions have not been elucidated, it has been suggested that, in Ph+ leukemias, the immunomodulatory activity of IFNα might be mediated by membrane-bound TRAIL expressed on activated T lymphocytes.41 In this paper, we show that CML neutrophils and mononuclear cells, upon incubation with therapeutic dosages of IFNα, release sTRAIL into the extracellular environment as efficiently as normal leukocytes. In contrast, sFasL was not expressed or released by normal or CML neutrophils. Regretfully, we could not obtain sera of CML patients subjected to IFNα administration due to the current use of imatinib mesylate as treatment of choice in CML, but we had access to sera of melanoma patients undergoing IFNα therapy. In these samples, we detected serum levels of sTRAIL that were dramatically more elevated than those measured prior to cytokine injection or than those found in healthy donors. The sFasL was instead only marginally increased in sera of IFNα-treated patients. To our knowledge, this is the first evidence showing that IFNα, in vivo, provokes the release of sTRAIL when administered systemically. The latter is further supported by very recent findings on increased sTRAIL levels in sera of patients affected by multiple sclerosis who have been given systemic IFNβ1 administration.42 Although we could not prove it for the reasons explained previously, it seems reasonable to hypothesize that, in chronic-phase CML, which is a disease clinically characterized by an high number of circulating neutrophils belonging to the CML clone, administration of IFNα produces a massive release of sTRAIL into the serum. According to our data and to those published by Plasilova et al,43 who used a recombinant His-tag form of TRAIL, leukocyte-derived sTRAIL would preferentially induce apoptosis of CD34 Ph+ leukemic blasts rather than of leukemic neutrophils themselves. This is further supported by the fact that CD34+ cells isolated from CML patients, but not from healthy donors, express TRAIL-R1 and TRAIL-R2 death receptors (C.T., M.A.C., unpublished observations, December 2003). Consistent with the absence of TRAIL-R1 and TRAIL-R2 on normal CD34+ cells, it has been reported that, following exposure to His-TRAIL, CD34+ cells from healthy donors retain the capacity to repopulate human hematopoiesis when transplanted in sublethally irradiated severe combined immunodeficiency/nonobese diabetic (SCID/NOD) mice.43 Interestingly, preliminary experiments revealed that concentrations of imatinib mesylate up to 500 nM do not interfere with the ability of IFNα to trigger the release of sTRAIL by either normal or CML leukocytes (C.T., M.A.C., unpublished observations, January 2004), thus providing a further rationale for the concurrent use of IFNα and imatinib mesylate in CML.44 Our data also exclude sFasL as a potential molecule mediating the effects of IFNα for the following reasons: (1) it was not expressed and released by CML neutrophils, and (2) it was minimally increased in sera of IFNα-treated patients. In line with this notion, Uno et al41 recently reported that recombinant TRAIL, but not FasL, effectively induces apoptotic cell death in most of the CML and Ph+ acute leukemia cell lines that they examined.
In summary, our data indicate that IFNα can exert its immunomodulatory activities in neoplastic and viral diseases by promoting the release of soluble TRAIL (other than by inducing the expression of the corresponding membrane-bound form) by effector cells of the immune system. Our findings also support the idea that, due to the lack of systemic toxic effects and to its synergistic activity with other chemotherapeutic agents,23,44,45 rhsTRAIL may be an ideal candidate to substitute IFNα in combination regimens.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-08-2806.
Supported by grants from Ministero dell'Istruzione, dell'Università e della Ricerca (COFIN, FIRB, and 60%), Associazione Italiana per la Ricerca sul Cancro, CARIVERONA-2001 and Fondazione del Monte di Bologna e Ravenna.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal