Abstract
The effect of the putative iron regulatory peptide hepcidin on iron absorption was investigated in mice. Hepcidin peptide was synthesized and injected into mice for up to 3 days, and in vivo iron absorption was measured with tied-off segments of duodenum. Liver hepcidin expression was measured by reverse transcriptase–polymerase chain reaction. Hepcidin significantly reduced mucosal iron uptake and transfer to the carcass at doses of at least 10 μg/mouse per day, the reduction in transfer to the carcass being proportional to the reduction in iron uptake. Synthetic hepcidin injections down-regulated endogenous liver hepcidin expression excluding the possibility that synthetic hepcidin was functioning by a secondary induction of endogenous hepcidin. The effect of hepcidin was significant at least 24 hours after injection of hepcidin. Liver iron stores and hemoglobin levels were unaffected by hepcidin injection. Similar effects of hepcidin on iron absorption were seen in iron-deficient and Hfe knockout mice. Hepcidin inhibited the uptake step of duodenal iron absorption but did not affect the proportion of iron transferred to the circulation. The effect was independent of iron status of mice and did not require Hfe gene product. The data support a key role for hepcidin in the regulation of intestinal iron uptake.
Introduction
Recent advances in molecular-level understanding of iron absorption regulation have implicated several genes as regulators of iron absorption, 2 of which (Hfe and hepcidin) have received particular attention.1-3 Hepcidin was originally identified as an antimicrobial peptide synthesized in liver, but evidence from knockout mice suggests this peptide is a negative regulator of iron absorption.3,4 Initial work implicated hepcidin as the long-sought “stores regulator” of iron absorption proposed by Finch.5 However, recent work has suggested a wider role for this peptide as it also shows an expression pattern consistent with the “erythroid regulator.”6 A mutation in hepcidin has recently been implicated as a cause of juvenile hemochromatosis.7
Transgenic mice overexpressing hepcidin were found to develop an iron-deficient phenotype, consistent with an effect on placental iron transport and intestinal iron absorption.8 Frazer et al9 provided data that quantitatively relates hepcidin expression to iron absorption rates and expression of duodenal transporters in an iron-deficient rat model. It can be deduced that a similar inverse correlation between hepcidin expression and iron absorption probably exists in humans, based on data provided by Nemeth et al10 relating urinary hepcidin to serum ferritin levels. Thus far, however, no data measuring the direct effect of injecting hepcidin on iron absorption rates is available. We therefore synthesized hepcidin peptide and injected this into normal, iron-deficient, and Hfe knockout mice and measured iron absorption rates.
Materials and methods
Mice (129/Ola-C57BL/6 mixed background strain) with a 2-kb pgk-neor gene flanked by loxP sites replacing a 2.5-kb BglII fragment (see Bahram et al11 for details) were used as Hfe knockouts (KOs). Heterozygotes were mated and wild-type and homozygote Hfe KO littermates were identified at 4 to 5 weeks of age. Mice were fed CRM diet (Scientific Diet Supplies [SDS], Witham, Essex, United Kingdom) ad lib and a mixed group of males and females (2 males and 4 females in each experimental group) was studied at 8 to 12 weeks of age. Other experiments were performed with male CD1 mice aged 6 to 10 weeks. Mice were injected with 0.15 M NaCl or hepcidin dissolved in 0.15 M NaCl by the intraperitoneal route. Iron deficiency was induced by feeding mice an iron-deficient purified diet (see Bahram et al11 for details) for 3 weeks after weaning. Controls received an iron-replete diet identical to the iron-deficient diet except for the addition of 180 mg/kg Fe.11 All animal experiments were performed under the authority of a United Kingdom Home Office license.
Peptide synthesis
Hepcidin (human and mouse sequence, 25 amino acids) was synthesized on a Wang alcohol resin with a loading of 1.30 mmol/g on a Rainin automatic peptide synthesizer (Protein Technologies, Tuscon, AZ) using the standard Fmoc chemistry. Cysteine sulphurs were protected with trityl groups and all other side-chain functions were protected with trifluoroacetic acid (TFA)–labile groups.All the cysteines were introduced as preformed symmetrical anhydrides to prevent enantiomerization during assembly. The completed peptide was deprotected and cleaved from the resin with a mixture of TFA, ethanedithiol, and water (94:3:3). The final product was precipitated with cold diethyl ether, dried, and purified by reverse-phase high-performance liquid chromatography (HPLC) on a Vydac C18 column (Vydac Hessperia, Anaheim, CA).
The lyophilized prepurified reduced hepcidin was dissolved in neat TFA and rotary evaporated so as to give a thin film of peptide on the surface of a quick-fit flask. The peptide was further dried under vacuum for 24 hours. To this dried film, 0.1 M de-gassed ammonium bicarbonate was added and the mixture was stirred for 48 hours while open to atmosphere. The reaction was then analyzed by the Ellman reagent to ascertain complete oxidation. The mixture was lyophilized and purified by reverse-phase HPLC on a Vydac C18 column.
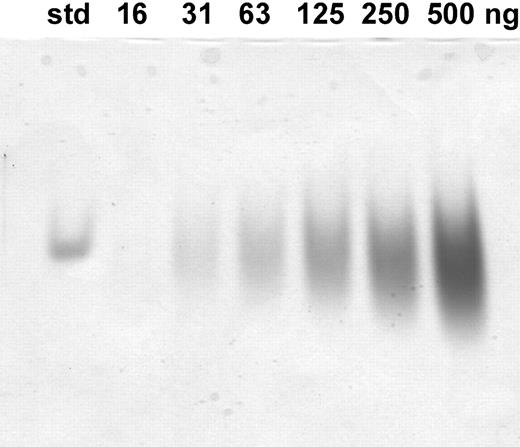
The mass of oxidized peptide was verified by mass spectrometry (Maldi-Tof) and was found to be 2789. The oxidized hepcidin was further analyzed by electrophoresis on a 16.5% tricine sodium dodecyl sulfate (SDS)–polyacrylamide gel. It migrated as a narrow single band with an apparent molecular weight of less than 10 000 in agreement with the apparent molecular weight of native urinary hepcidin when electrophoresed under identical conditions. We further compared our synthetic peptide with standard hepcidin by electrophoresis on acid/urea gel12 (Figure 1); standard hepcidin13 separated as a single band, whereas our synthetic peptide used in this study separated as a broad band, like it was a mixture of multiple forms. However, we found that both standard hepcidin and the synthetic hepcidin were immunoreactive with an antibody raised against urinary hepcidin. We concluded that our synthetic peptide is the correct molecular weight for oxidized hepcidin, contains a form equivalent to urinary hepcidin, but also has forms of hepcidin that migrate differently on acid/urea gel.
Analysis of synthetic hepcidin by 12.5% acid-urea polyacrylamide gel electrophoresis (PAGE). The gel was loaded with the indicated amounts of peptide and after electrophoresis it was stained with Coomassie blue. The standard is 1 μg hepcidin produced synthetically and validated as identical to urinary hepcidin-25 by mass spectrometry, reverse-phase high-performance liquid chromatography on a C18 column, and acid-urea PAGE (courtesy of E. Nemeth and T. Ganz, University of California, Los Angeles).
Analysis of synthetic hepcidin by 12.5% acid-urea polyacrylamide gel electrophoresis (PAGE). The gel was loaded with the indicated amounts of peptide and after electrophoresis it was stained with Coomassie blue. The standard is 1 μg hepcidin produced synthetically and validated as identical to urinary hepcidin-25 by mass spectrometry, reverse-phase high-performance liquid chromatography on a C18 column, and acid-urea PAGE (courtesy of E. Nemeth and T. Ganz, University of California, Los Angeles).
In vivo iron absorption was determined with tied-off duodenal segments in anesthetized mice. 59FeNTA2 medium (250 μM; 1:2 ferric nitrilotriacetate [Fe:NTA] in physiologic medium, 125 mM NaCl, 3.5 mM KCl, 10 mM MgCl2, 1 mM CaCl2, 16 mM Hepes, pH 7.4) was injected into prewashed (0.5 mL warm 0.15 M NaCl) duodenal segments. After 10 minutes, the animal was killed and the duodenal segment removed, opened, flushed with 10 mL 0.15 M NaCl, blotted, and weighed. Blood was drawn from the heart and sampled (5 μL) for hemoglobin assay. The remainder was allowed to clot, spun at 10 000g, and serum was separated from red cells. A liver sample was taken for nonheme iron assay. Radioactivity in the duodenal segment and various samples was determined in a gamma counter (LKB Wallac, Uppsala, Sweden). The carcass was counted in a large volume sample counter.14 The activity of 59Fe present in the intestinal tissue is referred to as mucosal retention (MR), whereas that in the carcass (sum of activity in carcass and all samples taken for assays) is referred to as mucosal transfer (MT). The sum of the mucosal retention and mucosal transfer represents the total mucosal uptake (TMU).14 Hemoglobin and tissue nonheme iron concentrations were determined as described previously.15 Serum iron, unsaturated iron binding capacity, and total iron binding capacity were determined with a Sigma manual assay kit (Sigma Chemical, Poole, United Kingdom).
Expression of specific transcripts for hepcidin were analyzed by real-time PCR. Total liver RNA was extracted using QIAamp RNA Blood Mini Kits (Qiagen Crawley, West Sussex, United Kingdom) and was reverse transcribed into cDNA by the avian myeloblastosis virus (AMV) first-strand cDNA synthesis kit (Roche Diagnostic, Mannheim, Germany). Amplification of messenger RNA was performed using LightCycler Fast Start DNA master SYBR Green 1 (Roche Diagnostic) on a LightCycler real-time PCR instrument (version 3.5; Roche Diagnostic) according to the manufacturer's protocol. The primer pairs used to quantify hepcidin 1 were forward ACCACCTATCTCCATCAAC, reverse GGTCAGGATGTGGCTC. Quantification was obtained by comparing the crossing point (ie, the cycle number at which fluorescence can be detected) of samples against a standard curve constructed from known amounts of PCR product. Levels of mRNA for hepcidin were normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using LightCycler Relative Quantification Software version 1.0.
Statistical analysis
Values are expressed as means plus or minus the standard error of the mean (SEM). Significant differences between more than 2 corresponding groups were analyzed by 2-way analysis of variance (ANOVA; 5% level). ANOVA was performed with Statistical Package for Social Scientists (SPSS, Chicago, IL).
Results
Injecting mice with hepcidin (10 μg or 100 μg) daily for 3 days was found to have no effect on hemoglobin levels or liver nonheme iron levels (Table 1); however, a small decrease in body weight was noted, the effect being significant at the higher dose.
Effect of hepcidin on body weights, iron stores, and hemoglobin in mice
Treatment . | n . | Body weight, g . | Hemoglobin, g/L . | Liver nonheme iron, nmol/mg . |
|---|---|---|---|---|
| Control | 10 | 38.8 ± 0.6 | 156 ± 05 | 1.82 ± 0.19 |
| 10 μg hepcidin | 6 | 37.2 ± 1.4 | 154 ± 07 | 1.67 ± 0.28 |
| 100 μg hepcidin | 6 | 36.4 ± 1.0* | 156 ± 04 | 1.59 ± 0.15 |
Treatment . | n . | Body weight, g . | Hemoglobin, g/L . | Liver nonheme iron, nmol/mg . |
|---|---|---|---|---|
| Control | 10 | 38.8 ± 0.6 | 156 ± 05 | 1.82 ± 0.19 |
| 10 μg hepcidin | 6 | 37.2 ± 1.4 | 154 ± 07 | 1.67 ± 0.28 |
| 100 μg hepcidin | 6 | 36.4 ± 1.0* | 156 ± 04 | 1.59 ± 0.15 |
Mice were injected daily for 3 days with 0.15 M NaCl (controls) or hepcidin dissolved in 0.15 M NaCl.
Data are expressed as means ± SEM for the number of mice per group.
P < .05 compared with appropriate control.
Mucosal uptake of iron by duodenum was found to be decreased (Figure 2) at both hepcidin doses, with a tendency for the effect to increase with an increasing hepcidin dose. The quantity of iron transferred to the carcass was also decreased, but this effect was not statistically significant at the 10-μg dose. To further investigate the effects of dose and time, mice were injected with 50 μg hepcidin once, and then iron absorption was measured after 4 hours or 24 hours (Table 2). Iron absorption was not significantly reduced by either treatment. If mice were dosed twice with 25 μg or 50 μg hepcidin (8-hour gap between doses) then studied 24 hours later, iron absorption was significantly reduced (Table 2).
Effect of hepcidin on iron absorption in mice. Mice were injected daily for 3 days with 0.15 M NaCl (controls; ▪) or hepcidin (10 μg [ ] or 100 μg [□]) dissolved in 0.15 M NaCl. Tied-off segments of duodenum were incubated with 250 μM 59FeNTA2 for 10 minutes. Data show radioiron retained by the duodenal tissue (MR), radioiron transferred to the carcass (MT), and total uptake of radioiron by duodenum (TMU). *P < .05, **P < .01.
] or 100 μg [□]) dissolved in 0.15 M NaCl. Tied-off segments of duodenum were incubated with 250 μM 59FeNTA2 for 10 minutes. Data show radioiron retained by the duodenal tissue (MR), radioiron transferred to the carcass (MT), and total uptake of radioiron by duodenum (TMU). *P < .05, **P < .01.
Effect of hepcidin on iron absorption in mice. Mice were injected daily for 3 days with 0.15 M NaCl (controls; ▪) or hepcidin (10 μg [ ] or 100 μg [□]) dissolved in 0.15 M NaCl. Tied-off segments of duodenum were incubated with 250 μM 59FeNTA2 for 10 minutes. Data show radioiron retained by the duodenal tissue (MR), radioiron transferred to the carcass (MT), and total uptake of radioiron by duodenum (TMU). *P < .05, **P < .01.
] or 100 μg [□]) dissolved in 0.15 M NaCl. Tied-off segments of duodenum were incubated with 250 μM 59FeNTA2 for 10 minutes. Data show radioiron retained by the duodenal tissue (MR), radioiron transferred to the carcass (MT), and total uptake of radioiron by duodenum (TMU). *P < .05, **P < .01.
Effect of different hepcidin dose regimens on iron absorption in mice
Treatment . | n . | Mucosal retention, pmol/mg . | Mucosal transfer, pmol/mg . | Total mucosal uptake, pmol/mg . |
|---|---|---|---|---|
| Intraperitoneal injection* | ||||
| Control | 4 | 17.7 ± 3.2 | 30.4 ± 5.9 | 48.1 ± 5.6 |
| 50 μg hepcidin × 1 | 4 | 17.7 ± 2.4 | 20.4 ± 3.8 | 38.1 ± 6.1 |
| Intraperitoneal injection† | ||||
| Control | 4 | 25.4 ± 2.0 | 21.9 ± 3.4 | 47.4 ± 3.1 |
| 50 μg hepcidin × 1 | 4 | 19.1 ± 2.9 | 18.8 ± 1.6 | 37.8 ± 3.7 |
| Control | 4 | 21.2 ± 3.1 | 27.3 ± 3.3 | 48.5 ± 4.1 |
| 50 μg hepcidin × 2 | 4 | 13.2 ± 2.2 | 19.3 ± 1.9 | 32.5 ± 3.6‡ |
| Subcutaneous injection† | ||||
| Control | 4 | 20.4 ± 0.8 | 30.1 ± 2.6 | 50.6 ± 2.5 |
| 50 μg hepcidin × 1 | 6 | 16.6 ± 0.9‡ | 16.5 ± 0.6§ | 33.1 ± 1.2§ |
Treatment . | n . | Mucosal retention, pmol/mg . | Mucosal transfer, pmol/mg . | Total mucosal uptake, pmol/mg . |
|---|---|---|---|---|
| Intraperitoneal injection* | ||||
| Control | 4 | 17.7 ± 3.2 | 30.4 ± 5.9 | 48.1 ± 5.6 |
| 50 μg hepcidin × 1 | 4 | 17.7 ± 2.4 | 20.4 ± 3.8 | 38.1 ± 6.1 |
| Intraperitoneal injection† | ||||
| Control | 4 | 25.4 ± 2.0 | 21.9 ± 3.4 | 47.4 ± 3.1 |
| 50 μg hepcidin × 1 | 4 | 19.1 ± 2.9 | 18.8 ± 1.6 | 37.8 ± 3.7 |
| Control | 4 | 21.2 ± 3.1 | 27.3 ± 3.3 | 48.5 ± 4.1 |
| 50 μg hepcidin × 2 | 4 | 13.2 ± 2.2 | 19.3 ± 1.9 | 32.5 ± 3.6‡ |
| Subcutaneous injection† | ||||
| Control | 4 | 20.4 ± 0.8 | 30.1 ± 2.6 | 50.6 ± 2.5 |
| 50 μg hepcidin × 1 | 6 | 16.6 ± 0.9‡ | 16.5 ± 0.6§ | 33.1 ± 1.2§ |
Mice were injected with 0.15 M NaCl (controls) or the indicated dose of hepcidin dissolved in 0.15 M NaCl either once or twice with an 8-hour gap between the 2 injections.
Data are expressed as means ± SEM for the number of mice per group.
Iron absorption was studied 4 hours after injections.
Iron absorption was studied 24 hours after injections.
P < .05 compared with appropriate control.
P < .001 compared with appropriate control.
The data suggest that there are dose and time dependencies of the effect of hepcidin. The doses required to give significant effects are, however, very high, suggesting that hepcidin may be rapidly cleared from the circulation, thus providing an explanation for the failure of earlier experiments to consistently find a humoral factor controlling iron absorption.16
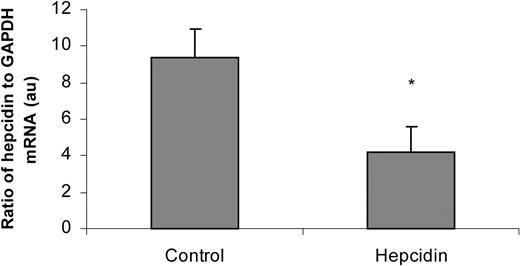
In order to test whether the injected synthetic hepcidin was causing an inflammatory reaction resulting in enhanced endogenous hepcidin production and consequent down-regulation of iron absorption, we measured hepcidin mRNA levels in livers from mice injected for 3 days with 100 μg hepcidin (Figure 3). The data show that the synthetic hepcidin caused a decrease in endogenous hepcidin gene expression. This finding resembles findings made with transgenic mice with constitutive overexpression of hepcidin8 and may reflect developing iron deficiency.
Effect of synthetic hepcidin injections on endogenous liver hepcidin expression. Mice were injected daily for 3 days with 0.15 M NaCl (controls) or hepcidin (100 μg) dissolved in 0.15 M NaCl, then liver samples were analyzed for hepcidin and GAPDH mRNA levels. The ratio of hepcidin to GAPDH is shown (means ± SEM). *P < .05.
Effect of synthetic hepcidin injections on endogenous liver hepcidin expression. Mice were injected daily for 3 days with 0.15 M NaCl (controls) or hepcidin (100 μg) dissolved in 0.15 M NaCl, then liver samples were analyzed for hepcidin and GAPDH mRNA levels. The ratio of hepcidin to GAPDH is shown (means ± SEM). *P < .05.
As there are little data on endogenous levels of hepcidin or its plasma kinetics, it is not possible to say how levels in the mice were altered by the quantities we injected. We tried injecting mice by the subcutaneous route to delay release into the blood, however, this only slightly increased the effect of hepcidin (Table 2; intramuscular injection gave similar results, not shown). We also tried injections of mouse hepcidin (this differs at amino acid positions H3N, G12K, H15N, R16N, K18Q, and M21L compared with human hepcidin); however, the effect was similar to the human peptide (data not shown). Injection of hepcidin (10 μg/mL, approximately 1 μg per segment) directly into the lumen of tied-off segments of duodenum, together with the radioiron solutions used for in vivo iron absorption measurements, did not affect iron absorption (mucosal uptake: 37.0 ± 5.7 compared with 40.6 ± 10.2 [SEM; n = 3; pmol/mg over 10 minutes] for controls).
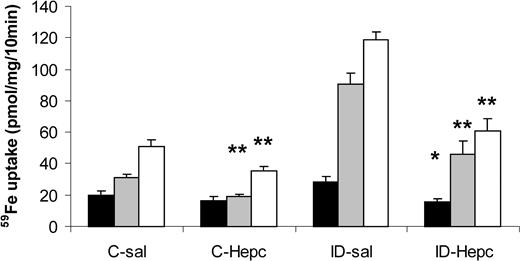
We then measured the effect of hepcidin injection on iron absorption in iron-deficient mice (Figure 4). These mice are expected to have decreased endogenous hepcidin levels.4,9 Figure 4 suggests that hepcidin decreased iron absorption by a similar proportion in iron-deficient and control mice. This was tested by investigating the statistical significance of the effects of diet and hepcidin treatment on iron absorption parameters (Table 3). Significant effects of iron deficiency and hepcidin treatment on iron absorption were found. A significant interaction of iron deficiency and hepcidin was found, suggesting that the 2 effects are not additive. Logarithmic transformation of the data removed this effect, confirming that hepcidin had decreased mucosal uptake or transfer in both iron-deficient and control mice by a similar factor. It is also noteworthy that mucosal uptake and transfer were affected by similar proportions so that the transfer of iron to the carcass, expressed as a percentage of the iron taken up by the mucosa, was not affected by hepcidin. In contrast, proportional mucosal transfer of iron is affected by iron deficiency (Tables 3 and 4).
Effect of hepcidin on iron absorption in iron-deficient mice. Mice were fed an iron-replete (C) or iron-deficient (ID) diet for 3 weeks from weaning and injected with 0.15 M NaCl (sal) or hepcidin (50 μg single injection) on each of 2 consecutive days, 24 hours before measuring iron absorption with tied-off duodenal segments. Data (means ± SEM) shown are mucosal retention (▪), transfer of radioiron to the carcass (▦), and total mucosal uptake of radioiron (□). Significance of effects was analyzed by ANOVA and is shown in Table 3. *P < .05, **P < .01 compared with mice fed the same diet and injected with saline.
Effect of hepcidin on iron absorption in iron-deficient mice. Mice were fed an iron-replete (C) or iron-deficient (ID) diet for 3 weeks from weaning and injected with 0.15 M NaCl (sal) or hepcidin (50 μg single injection) on each of 2 consecutive days, 24 hours before measuring iron absorption with tied-off duodenal segments. Data (means ± SEM) shown are mucosal retention (▪), transfer of radioiron to the carcass (▦), and total mucosal uptake of radioiron (□). Significance of effects was analyzed by ANOVA and is shown in Table 3. *P < .05, **P < .01 compared with mice fed the same diet and injected with saline.
Analysis of variance of effects of hepcidin and iron deficiency on iron absorption
Effect . | Mucosal retention . | Mucosal transfer . | Total mucosal uptake . | % Mucosal transfer . | Wt . | Hb . |
|---|---|---|---|---|---|---|
| Iron deficiency | .131 | 3.09 × 10-8 | 7.64 × 10-8 | .00019 | .625 | .00046 |
| Hepcidin | .00141 | 7.40 × 10-5 | 2.32 × 10-5 | .637 | .995 | .598 |
| Interaction | .0391 | .00677 | .00301 | .304 | .763 | .613 |
Effect . | Mucosal retention . | Mucosal transfer . | Total mucosal uptake . | % Mucosal transfer . | Wt . | Hb . |
|---|---|---|---|---|---|---|
| Iron deficiency | .131 | 3.09 × 10-8 | 7.64 × 10-8 | .00019 | .625 | .00046 |
| Hepcidin | .00141 | 7.40 × 10-5 | 2.32 × 10-5 | .637 | .995 | .598 |
| Interaction | .0391 | .00677 | .00301 | .304 | .763 | .613 |
Results shown are P values for nontransformed data. Logarithmic transformation of data eliminated the significant interactions for mucosal retention, mucosal transfer, and total mucosal uptake, but not the significant effects of iron deficiency or hepcidin.
Wt indicates body weight; and Hb, hemoglobin level.
Effect of dietary iron level and hepcidin on proportional mucosal transfer of iron
Diet . | Treatment . | Mucosal transfer, % of total uptake . |
|---|---|---|
| Iron replete | Control | 63.4 ± 3.9 |
| Iron replete | Hepcidin | 57.2 ± 6.8 |
| Iron deficient | Control | 80.3 ± 1.4 |
| Iron deficient | Hepcidin | 82.6 ± 0.7 |
Diet . | Treatment . | Mucosal transfer, % of total uptake . |
|---|---|---|
| Iron replete | Control | 63.4 ± 3.9 |
| Iron replete | Hepcidin | 57.2 ± 6.8 |
| Iron deficient | Control | 80.3 ± 1.4 |
| Iron deficient | Hepcidin | 82.6 ± 0.7 |
Iron deficiency was induced by feeding the mice a low iron diet for 3 weeks. Iron-replete controls received the same diet supplemented with iron. Mice were injected with 0.15 M NaCl (controls) or hepcidin (50 μg single injection) on each of 2 consecutive days, 24 hours before they were killed. Statistical analysis is shown in Table 3.
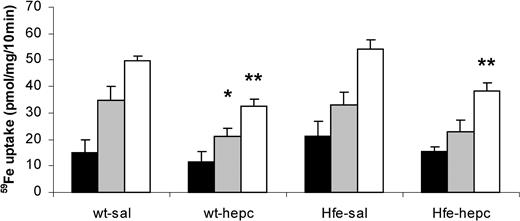
Finally, we tested whether the presence of the Hfe gene was necessary for the effect of hepcidin on iron absorption by injecting the peptide into Hfe KO and wild-type mice (Figure 5). Iron absorption was increased in Hfe KO mice compared with wild-type mice, but only to a small extent in the genetic background we used. The effect of hepcidin was similar in Hfe KO and wild-type mice (Table 5). As before, hepcidin had no effect on the percentage transfer of iron from gut to carcass.
Effect of hepcidin on iron absorption in Hfe KO mice. Wild-type (wt) or Hfe knockout (Hfe-KO) mice were injected with 0.15 M NaCl (sal) or hepcidin (50 μg single intraperitoneal injection) on each of 2 consecutive days, 24 hours before measuring iron absorption with tied-off duodenal segments. Data shown are mucosal retention (▪), transfer of radioiron to the carcass (▦), and total mucosal uptake of radioiron (□). Significance of effects was analyzed by ANOVA and is shown in Table 5. *P < .05, **P < .01 compared with mice of the same genotype injected with saline.
Effect of hepcidin on iron absorption in Hfe KO mice. Wild-type (wt) or Hfe knockout (Hfe-KO) mice were injected with 0.15 M NaCl (sal) or hepcidin (50 μg single intraperitoneal injection) on each of 2 consecutive days, 24 hours before measuring iron absorption with tied-off duodenal segments. Data shown are mucosal retention (▪), transfer of radioiron to the carcass (▦), and total mucosal uptake of radioiron (□). Significance of effects was analyzed by ANOVA and is shown in Table 5. *P < .05, **P < .01 compared with mice of the same genotype injected with saline.
Analysis of variance for effects of hepcidin and Hfe gene knockout on iron absorption
Effect . | Mucosal retention . | Mucosal transfer . | Total mucosal uptake . | % Mucosal transfer . | Wt . | Hb . |
|---|---|---|---|---|---|---|
| Genotype | .248 | .981 | .074 | .335 | .428 | .588 |
| Hepcidin | .304 | .013 | 9.65 × 10-6 | .700 | .655 | .167 |
| Interaction | .777 | .680 | .839 | .956 | .902 | .569 |
Effect . | Mucosal retention . | Mucosal transfer . | Total mucosal uptake . | % Mucosal transfer . | Wt . | Hb . |
|---|---|---|---|---|---|---|
| Genotype | .248 | .981 | .074 | .335 | .428 | .588 |
| Hepcidin | .304 | .013 | 9.65 × 10-6 | .700 | .655 | .167 |
| Interaction | .777 | .680 | .839 | .956 | .902 | .569 |
Results shown are P values for nontransformed data.
Discussion
The present data show that hepcidin injection causes reduced iron absorption in mice. Our preparation of synthetic hepcidin was shown to contain forms that are equivalent to native urinary hepcidin; however, on acid urea gel it was found also to contain additional forms of hepcidin. Despite this impurity, our preparation of hepcidin evoked biologic responses and probably contained one or more active forms. The nature of the active form of hepcidin will only be known once the form in which it circulates and binds its receptor has been identified. We found the effect was not secondary to enhanced synthesis of endogenous hepcidin, suggesting that the effect did not result from a proinflammatory response to the injected peptide. The decrease occurs primarily at the mucosal uptake (ie, presumably brush border membrane) step, with transfer of iron from the duodenum to the animal being decreased in proportion. This finding implies that hepcidin has little direct effect on the basolateral transfer proteins for iron, with decreases in transfer being a consequence of decreased uptake. This is in agreement with previous kinetic findings on the adaptation of uptake and transfer in iron-deficient rats, where the fraction of iron available for transfer across the basolateral membrane increased in parallel to the available fraction of iron when “uptake” and “transfer” are in a steady state.17 It cannot however, be ruled out that the decreased transfer of iron is due to a coordinated decrease in the iron absorption pathway. The latter possibility is consistent with the observations of Frazer et al9 in iron-deficient rats. Hepcidin had no effect on iron stores or hemoglobin levels, therefore the effect may be a direct action on the gut. There was no immediate effect of coinjection of radioiron and hepcidin into the lumen of tied-off segments of duodenum, however, suggesting an endocrine effect of hepcidin, mediated via alterations in gene expression, as shown by Frazer et al.9 The effect of hepcidin was independent of iron stores (and presumably endogenous hepcidin levels) in keeping with our previous finding that hypoxia affects iron absorption by a similar proportional factor in mice with normal or decreased iron stores.18 The inhibition of iron absorption by hepcidin was unaffected by knockout of the Hfe gene. Hepcidin expression is reported to be decreased in adult Hfe KO mice, despite the latter's elevated iron stores.19,20 These findings are consistent with a direct effect of hepcidin on the iron absorption pathway and suggest that Hfe gene product is either (1) involved in a distinct hepcidin-independent iron absorption regulation mechanism, or (2) involved upstream of the interaction of hepcidin with intestine, as suggested by Ahmad et al19 and Bridle et al.20 It is noteworthy that dietary iron deficiency was found to significantly alter proportional mucosal transfer of iron, whereas hepcidin injection did not affect this parameter. This implies that some additional factor, other than hepcidin, may also be involved in the regulation of mucosal transfer of iron by low-iron diet feeding.
Our data support a role for hepcidin as a hormone that regulates duodenal iron absorption, thereby controlling body iron levels. Further work on the interaction of hepcidin with the duodenum is necessary to elucidate the mechanism of action of this peptide.
Prepublished online as Blood First Edition Paper, January 29, 2004; DOI 10.1182/blood-2003-03-0953.
Supported by the Wellcome Trust, Sir Jules Thorn Charitable Trust, and United Kingdom Medical Research Council. S.B.'s laboratory is supported by the Ministère de la Recherche and INSERM of France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Susan Gilfillan for supply of Hfe KO breeders. We are grateful to Dr Tomas Ganz and Elizabeth Nemeth for performing acid urea gel electrophoresis on our preparation of synthetic hepcidin and for a gift of urinary hepcidin.

![Figure 2. Effect of hepcidin on iron absorption in mice. Mice were injected daily for 3 days with 0.15 M NaCl (controls; ▪) or hepcidin (10 μg [] or 100 μg [□]) dissolved in 0.15 M NaCl. Tied-off segments of duodenum were incubated with 250 μM 59FeNTA2 for 10 minutes. Data show radioiron retained by the duodenal tissue (MR), radioiron transferred to the carcass (MT), and total uptake of radioiron by duodenum (TMU). *P < .05, **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/10/10.1182_blood-2003-03-0953/6/m_zh80100461350002.jpeg?Expires=1767726822&Signature=LuLiWAc08QzG6JYsc15hD2mSYGVZIx8TcQKOADjHD2M29cx~L0sIBbymfbKxkT9995JxHG03Jek0dAh97VNzRaJUAs4B5e9axM-gSCSOZUlpY3NqXTKn-Q2HZv~5T71jLFlwGPTC12RtonwMczZZMCtwm~~3R1M0WpU0-FV7npYS53iNUBACz1uDC4zpdmzkltzr4Zc~8rfh8Q3NB0twK52IdScfDwXPMss4XXW8WLRtLc4gEJQbaJE8MO0J7R8ojvMrPlM6DFZn3fnnWEUETqqnG~0acnuoWjhaSqXDWtG3fS9DBhvWxeapFiGVmoDZqMA0hLDMU9W57-8v6bGZJQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal