Abstract
A single recombinant immunoglobulin G1 (IgG1) anti-RhD antibody (MonoRho) was compared with a currently used polyclonal anti-RhD product (Rhophylac) in a phase 1 study for safety, efficacy of Rhesus D (RhD)–positive red blood cell (RBC) clearance, and prevention of RhD immunization in RhD-negative men challenged with 15 mL RhD-positive RBCs. Both the polyclonal product and recombinant anti-RhD effectively cleared RhD-positive RBCs after intravenous and intramuscular injection. The recombinant anti-RhD demonstrated a slower clearance rate compared with the polyclonal anti-RhD. There was no dose response, and there was considerable variation among subjects who received the same dose of recombinant anti-RhD. Interestingly, RhD-positive RBC clearance rates were strongly associated with Fcγ receptor IIA (FcγRIIA) and FcγIIIA but not with FcγIIIB polymorphisms. Subjects homozygous for FcγRIIA-131H or FcγRIIIA-158V allotypes showed a faster clearance rate compared with both the heterozygote and the corresponding alternative homozygote allotypes. A similar but less marked trend was seen for the polyclonal anti-RhD. Despite the variation in clearance rates there was no evidence of anti-RhD alloantibodies in any of the subjects at +6 months after the RBC challenge.
Introduction
Rhesus prophylaxis to prevent hemolytic disease of the fetus and newborn has been successfully ensured for many years by polyclonal anti–Rhesus D (RhD) products. However, new viral epidemics (eg, severe acute respiratory syndrome), West Nile virus in transfusion products1 and concerns about the transmission of variant Creutzfeldt-Jacob disease illustrate the potential vulnerability of the hyperimmune plasma donor programs that are required for production of current RhD immune globulin products. To avoid the necessity for hyperimmune plasma, a recombinant anti-RhD antibody has been developed (MonoRho) and produced in a stable Chinese hamster ovary (CHO) cell line, MDJ8s.2 Comparison of MonoRho with a polyclonal anti-RhD product (Rhophylac) for specificity and Fc function gave similar results in vitro. The next major question concerns the clinical efficacy and safety of a single recombinant antibody compared with a polyclonal antibody preparation.
A phase 1 clinical trial was designed to assess MonoRho and Rhophylac for their comparative safety, efficacy of RhD-positive red blood cell (RBC) clearance, and prevention of RhD immunization in RhD-negative men challenged with 15 mL RhD-positive (R1r) RBCs followed 24 hours later by anti-RhD immune globulin. A large 15-mL challenge of RhD-positive RBCs was chosen because it represents a worst-case scenario and because a single standard dose of polyclonal anti-RhD (approximately 300 μg) contains sufficient anti-RhD to suppress the immune response to 15 mL RhD-positive RBCs. A hemorrhage of 5 mL or greater occurs in only 0.6% of pregnancies.3 The parameters measured included the following: concentration of RhD-positive RBCs in volunteers over time; elimination rate; saturation of RhD binding sites with anti-RhD; serum concentration of anti-RhD; genotyping for the Fcγ receptor IIa (FcγRIIa), FcγRIIIa, and FcγRIIIb polymorphisms; presence of anti-RhD alloantibodies and anti-MonoRho at 3 and 6 months after challenge; and routine clinical laboratory assessments.
As previously reported4-7 there is a fundamental difference between the methods for determination of anti-RhD content in human plasma-derived products and recombinant or other monoclonal anti-RhD antibodies such as MonoRho. The former are based on the European Pharmacopoeia “AutoAnalyzer” assay,8 which measures agglutinating activity, while the latter are based on determination of purified protein by biochemical means. Our unpublished data (H.A., July 2002) indicate that MonoRho is underestimated by a factor of 4 to 5 in the AutoAnalyzer assay. Thus, an essential part of this study was evaluation of an escalating dose range of MonoRho in vivo in order to estimate which dose of MonoRho would be comparable with the standard dose of plasma-derived anti-RhD.
This phase 1 study showed that a single recombinant human immunoglobulin G1 (IgG1) anti-RhD antibody prevented primary immunization by RhD-positive RBCs.
Patients, materials, and methods
Volunteers
Healthy RhD-negative male volunteers (aged 18-45 years, n = 46) were enrolled after giving voluntary written informed consent. Subjects were excluded if they had blood group alloantibodies, had a history of anaphylactic or other severe systemic reaction to immune globulins, were IgA deficient, had been administered anti-RhD previously, or were previously transfused with RhD-positive blood or any blood-borne products 6 months prior to enrollment.
Anti-RhD
MonoRho is a recombinant human IgG1/kappa antibody produced in the CHO cell line MDJ8s. Its development was based on RhD-specific phage isolated from phage display libraries and subsequent construction of the full-length human IgG1.2,9 MonoRho recognizes a discontinuous epitope on loops 3, 4, and 6 of the RhD protein (Miescher et al2 ; and S.M., unpublished data, July 2002). Clinical material was produced in a 200-L batch-fermentation process, purified, and supplied in ready-to-use syringes containing 300 μg antibody in 1 mL solution. No CHO host cell proteins were detectable, and more than 95% of the antibody was monomeric IgG with less than 1% aggregates and less than 4% fragments. The production process in CHO cells contained validated virus inactivation and nanofiltration steps and complies with current regulatory requirements.
Fab and Fc functions of MonoRho tested in vitro were all comparable with the plasma-derived anti-RhD product.2 Polyclonal anti-RhD (Rhophylac; ZLB Bioplasma, Bern, Switzerland)10 was supplied in ready-to-use syringes containing 1500 international units (IU) (300 μg) anti-RhD determined by AutoAnalyzer.
RhD-positive RBCs
RhD-positive (R1r), Kell-negative, group O RBCs were obtained from accredited regular blood donors from Transfusion Medicine, University Clinics Charité, Berlin, Germany. Donor selection and testing of RBC concentrates were performed according to German guidelines.11,12 RBCs were cryopreserved, thawed, and washed according to Good Manufacturing Practice guidelines.13
Study design and treatment
The study was an open-label phase 1 trial performed in accordance with the Declaration of Helsinki (revised version of Edinburgh, Scotland, 2000) and approved by the ethics committee of the Medical Board of Berlin. Subjects were allocated to receive either a single standard dose of Rhophylac or one of various doses of MonoRho in consecutive cohorts. The study was performed at the Institute of Clinical Pharmacology, Berlin, Germany.
Each volunteer received 15 mL RhD-positive RBCs by intravenous administration and 24 hours later, a single injection of anti-RhD. Of the subjects, 25 received one of various doses (300-1800 μg) of MonoRho by intravenous administration, and 6 received 1200 μg MonoRho by intramuscular administration. Other subjects received 1500 IU Rhophylac by intravenous (n = 9) or intramuscular (n = 6) administration (Table 1).
Overview of subjects, FcγR polymorphism, clinical data, and results
Subject no. and dose of anti-RhD immune globulin, (administration route) . | FcγRIIA . | FcγRIIIA . | FcγRIIIB . | Rescue, d . | RhD+ RBC t1/2, h . | Anti-D after 3 mo . | Anti-D after 6 mo . |
|---|---|---|---|---|---|---|---|
| Rhophylac, 1500 IU/300 μg (IV) | |||||||
| 101 | RR | FF | NA1NA2 | - | 1.59 | Weak | Neg |
| 102 | ND | ND | ND | - | 1.39 | Aggl* | Neg |
| 103 | HH | VF | NA2NA2 | - | 2.26 | Aggl* | Neg |
| 104 | HH | FF | NA1NA2 | - | 0.90 | Neg | Neg |
| 105 | RR | FF | NA1NA2 | - | 3.47 | Neg | Neg |
| 106 | RR | VF | NA1NA2 | - | 0.72 | Neg | Neg |
| 107 | HH | VF | NA1NA2 | - | 0.91 | Pos | Neg |
| 108 | ND | ND | ND | - | 0.93 | Neg | Neg |
| 109 | HR | VF | NA2NA2 | - | 0.98 | Pos | Neg |
| MonoRho, 300 μg (IV) | |||||||
| 201 | HR | FF | NA1NA1 | - | 7.45 | Neg | Neg |
| 202 | HR | FF | NA1NA1 | 7 | 17.33 | Pos | Neg |
| 211 | RR | FF | NA2NA2 | - | 15.25 | Neg | Neg |
| 212 | HR | FF | NA1NA2 | - | 6.31 | Neg | Neg |
| MonoRho, 600 μg (IV) | |||||||
| 301 | RR | FF | NA2NA2 | 7 | 55.64 | Pos | Neg |
| 302 | RR | VF | NA1NA2 | 7 | 37.46 | Pos | Neg |
| 303 | HR | VF | NA2NA2 | - | 5.23 | Neg | Neg |
| 311 | RR | FF | NA2NA2 | - | 78.52 | Neg | Neg |
| 312 | HR | VF | NA2NA2 | - | 2.95 | Neg | Neg |
| 313 | HR | FF | NA1NA1 | - | 31.22 | Neg | Neg |
| MonoRho, 900 μg (IV) | |||||||
| 401 | ND | ND | ND | - | 4.25 | Neg | Neg |
| 402 | RR | VF | NA1NA2 | - | 5.82 | Neg | Neg |
| 403 | HR | VF | NA2NA2 | - | 6.13 | Neg | Neg |
| 404 | HH | VV | NA2NA2 | - | 2.01 | Neg | Neg |
| 405 | ND | ND | ND | - | 2.87 | Neg | Neg |
| 406 | HH | VV | NA1NA2 | - | 4.83 | Neg | Neg |
| MonoRho, 1200 μg (IV) | |||||||
| 501 | HR | FF | NA2NA2 | 7 | 16.33 | Pos | Neg |
| 502 | HR | VF | NA1NA2 | 7 | 14.33 | Pos | Neg |
| 503 | RR | FF | NA1NA2 | 7 | 21.64 | Aggl* | Neg |
| MonoRho, 1800 μg (IV) | |||||||
| 1101 | RR | FF | NA1NA2 | 11 | 203.35 | Pos | Neg |
| 1102 | RR | VF | NA1NA2 | - | 9.00 | Pos | Neg |
| 1103 | HH | VF | NA1NA2 | - | 2.26 | Neg | Neg |
| 1104 | HH | VF | NA1NA2 | - | 4.38 | Neg | Neg |
| 1105 | HR | VF | NA2NA2 | - | 44.55 | Neg | Neg |
| 1106 | HH | FF | NA1NA2 | - | 29.12 | Aggl* | Neg |
| Rhophylac, 1500 IU (IM) | |||||||
| 601 | HH | VV | NA1NA2 | - | 4.91 | Aggl* | Neg |
| 602 | HR | FF | NA1NA2 | - | 30.15 | Pos | Neg |
| 603 | HH | VV | NA2NA2 | - | 5.98 | Weak | Neg |
| 604 | HR | VF | NA2NA2 | - | 7.88 | Pos | Neg |
| 605 | RR | FF | NA2NA2 | - | 15.24 | Neg | Neg |
| 606 | HR | FF | NA1NA1 | - | 9.55 | Neg | Neg |
| MonoRho, 1200 μg (IM) | |||||||
| 1001 | HH | VF | NA1NA2 | - | 8.65 | Pos | Neg |
| 1002 | HR | FF | NA1NA2 | - | 23.61 | Aggl* | Neg |
| 1003 | HH | VF | NA1NA2 | - | 25.33 | Neg | Neg |
| 1004 | HH | FF | NA1NA1 | - | 36.13 | Pos | Neg |
| 1005 | RR | FF | NA1NA2 | - | 50.28 | Neg | Neg |
| 1006 | RR | FF | NA1NA2 | - | 131.43 | Neg | Neg |
Subject no. and dose of anti-RhD immune globulin, (administration route) . | FcγRIIA . | FcγRIIIA . | FcγRIIIB . | Rescue, d . | RhD+ RBC t1/2, h . | Anti-D after 3 mo . | Anti-D after 6 mo . |
|---|---|---|---|---|---|---|---|
| Rhophylac, 1500 IU/300 μg (IV) | |||||||
| 101 | RR | FF | NA1NA2 | - | 1.59 | Weak | Neg |
| 102 | ND | ND | ND | - | 1.39 | Aggl* | Neg |
| 103 | HH | VF | NA2NA2 | - | 2.26 | Aggl* | Neg |
| 104 | HH | FF | NA1NA2 | - | 0.90 | Neg | Neg |
| 105 | RR | FF | NA1NA2 | - | 3.47 | Neg | Neg |
| 106 | RR | VF | NA1NA2 | - | 0.72 | Neg | Neg |
| 107 | HH | VF | NA1NA2 | - | 0.91 | Pos | Neg |
| 108 | ND | ND | ND | - | 0.93 | Neg | Neg |
| 109 | HR | VF | NA2NA2 | - | 0.98 | Pos | Neg |
| MonoRho, 300 μg (IV) | |||||||
| 201 | HR | FF | NA1NA1 | - | 7.45 | Neg | Neg |
| 202 | HR | FF | NA1NA1 | 7 | 17.33 | Pos | Neg |
| 211 | RR | FF | NA2NA2 | - | 15.25 | Neg | Neg |
| 212 | HR | FF | NA1NA2 | - | 6.31 | Neg | Neg |
| MonoRho, 600 μg (IV) | |||||||
| 301 | RR | FF | NA2NA2 | 7 | 55.64 | Pos | Neg |
| 302 | RR | VF | NA1NA2 | 7 | 37.46 | Pos | Neg |
| 303 | HR | VF | NA2NA2 | - | 5.23 | Neg | Neg |
| 311 | RR | FF | NA2NA2 | - | 78.52 | Neg | Neg |
| 312 | HR | VF | NA2NA2 | - | 2.95 | Neg | Neg |
| 313 | HR | FF | NA1NA1 | - | 31.22 | Neg | Neg |
| MonoRho, 900 μg (IV) | |||||||
| 401 | ND | ND | ND | - | 4.25 | Neg | Neg |
| 402 | RR | VF | NA1NA2 | - | 5.82 | Neg | Neg |
| 403 | HR | VF | NA2NA2 | - | 6.13 | Neg | Neg |
| 404 | HH | VV | NA2NA2 | - | 2.01 | Neg | Neg |
| 405 | ND | ND | ND | - | 2.87 | Neg | Neg |
| 406 | HH | VV | NA1NA2 | - | 4.83 | Neg | Neg |
| MonoRho, 1200 μg (IV) | |||||||
| 501 | HR | FF | NA2NA2 | 7 | 16.33 | Pos | Neg |
| 502 | HR | VF | NA1NA2 | 7 | 14.33 | Pos | Neg |
| 503 | RR | FF | NA1NA2 | 7 | 21.64 | Aggl* | Neg |
| MonoRho, 1800 μg (IV) | |||||||
| 1101 | RR | FF | NA1NA2 | 11 | 203.35 | Pos | Neg |
| 1102 | RR | VF | NA1NA2 | - | 9.00 | Pos | Neg |
| 1103 | HH | VF | NA1NA2 | - | 2.26 | Neg | Neg |
| 1104 | HH | VF | NA1NA2 | - | 4.38 | Neg | Neg |
| 1105 | HR | VF | NA2NA2 | - | 44.55 | Neg | Neg |
| 1106 | HH | FF | NA1NA2 | - | 29.12 | Aggl* | Neg |
| Rhophylac, 1500 IU (IM) | |||||||
| 601 | HH | VV | NA1NA2 | - | 4.91 | Aggl* | Neg |
| 602 | HR | FF | NA1NA2 | - | 30.15 | Pos | Neg |
| 603 | HH | VV | NA2NA2 | - | 5.98 | Weak | Neg |
| 604 | HR | VF | NA2NA2 | - | 7.88 | Pos | Neg |
| 605 | RR | FF | NA2NA2 | - | 15.24 | Neg | Neg |
| 606 | HR | FF | NA1NA1 | - | 9.55 | Neg | Neg |
| MonoRho, 1200 μg (IM) | |||||||
| 1001 | HH | VF | NA1NA2 | - | 8.65 | Pos | Neg |
| 1002 | HR | FF | NA1NA2 | - | 23.61 | Aggl* | Neg |
| 1003 | HH | VF | NA1NA2 | - | 25.33 | Neg | Neg |
| 1004 | HH | FF | NA1NA1 | - | 36.13 | Pos | Neg |
| 1005 | RR | FF | NA1NA2 | - | 50.28 | Neg | Neg |
| 1006 | RR | FF | NA1NA2 | - | 131.43 | Neg | Neg |
IV indicates intravenously; -, not required; Neg, negative; ND, not done; Aggl, agglutination; Pos, positive; and IM, intramuscularly.
Blood was tested with a panel of 11 test RBC types. Some of them showed a very weak agglutination, but a specific antibody could not be identified. Agglutination was scored by visual assessment from ++++ (strong agglutination) descending to - (no agglutination) as defined by the DiaMed ID gel scale. The positive agglutination was rated as a 1+ reaction on the ID gel scale
In view of this large challenge, prevention of accidental immunization of the volunteers receiving MonoRho was safeguarded by incorporating a rescue dose of Rhophylac if the RBC clearance rate had not reached predefined limits based on previous experience with polyclonal products.14 Due to results accumulating during the course of the study, the criterion for satisfactory clearance was changed twice. The first 12 subjects treated with MonoRho were administered Rhophylac on day 7 only if less than 92.5% of the RhD-positive RBCs were cleared from the circulation on day 3. The 100% level was set as the RhD-positive RBC concentration measured at 23.5 hours (ie, 30 minutes prior to anti-RhD injection). For the following 6 subjects, the Rhophylac administration was moved to day 11 if the desired clearance level was not reached by day 7. For the remaining 11 subjects, Rhophylac was to be given on day 11 if less than 50% of RhD-positive RBCs were cleared by day 7.
In vivo clearance of RhD-positive RBCs
Peripheral blood samples were obtained from all subjects up to at least 72 hours after administration of RhD-positive RBCs and in some subjects up to a maximum of 17 days. The concentration of RhD-positive RBCs was measured by fluorescence activated cell sorter (FACS) analysis after first removing leukocytes and platelets by dextran sedimentation. RBCs (5 × 107) were incubated with 200 μL of saturating amounts of anti-RhD (MonoRho or Rhophylac) for 30 minutes at 37° C to engage all RhD antigen sites on RhD-positive RBCs. The samples were washed twice followed by addition of 100 μL phycoerythrin (PE) goat anti–human IgG F(ab′)2 (Jackson Immunoresearch, West Grove, PA) and incubated for 30 minutes at 4° C. After washing, the samples were taken up in 1 mL phosphate-buffered saline. Of each sample, 4 × 100 μL was added to 4 staining tubes and incubated with Thiazolorange (Retic-COUNT kit; Becton Dickinson, Basel, Switzerland) for 30 minutes at room temperature. A total of 250 000 events was counted in each tube (ie, 1 million events per sample). RhD-positive RBCs were defined by gating PE-positive and Thiazolorangenegative events. The percentage of RhD-positive RBCs in relation to the total RBC number was calculated. Using this sensitive method, less than 0.005% RhD-positive RBCs could be reliably detected.
Elimination half-life of RhD-positive RBCs
The elimination half-life of RhD-positive RBCs following intramuscular or intravenous administration of anti-RhD was calculated using results of RhD-positive RBC concentrations in RhD-negative blood. The disposition rate constant (λz) was calculated by unweighted log-linear regression of the RhD-positive RBC concentration-time curve. The half-life (t½) was calculated as t½ = ln2/λz.
Saturation of RhD-positive RBCs with anti-RhD IgG
In a subset of subjects the percentage of RhD-positive antigen sites occupied by anti-RhD IgG was determined. The same FACS method as described for the clearance measurements was used, but in addition the same samples were also analyzed without anti-RhD treatment during the staining procedure. The percentage of saturation was calculated as 100 times the ratio of the median PE fluorescence obtained of samples without and with anti-RhD treatment during staining. Only samples with RhD-positive RBC counts of more than 200/million total RBCs were used for calculations.
Concentration of anti-RhD IgG in serum
The serum anti-RhD IgG concentration was measured up to 48 hours after anti-RhD injection. A sensitive assay was developed using a modification of the European Pharmacopoeia FACS assay.6 Briefly, 1.25 × 105 RhD-positive RBCs (R2R2) were incubated with test or standard serum samples containing known concentrations of MonoRho and Rhophylac in human AB serum. After washing, samples were incubated in saturating amounts of fluorescein isothiocyanate goat antihuman IgG Fab (Jackson Immunoresearch). Controls included samples with RhD-negative RBCs and spike samples with low, intermediate, and high anti-RhD content, which were assessed in every experiment and had to be within 25% of the theoretical anti-RhD concentration. The quantitation limit was 0.39 ng anti-RhD/mL.
Serologic detection of anti-RhD
Serum samples obtained at the screening visit and 3 and 6 months after the challenge with RhD-positive RBCs were tested for blood cell alloantibodies by the indirect hemagglutination test (ID gel agglutination test; DiaMed, Cressier, Switzerland). Serum was tested with a panel of 11 test RBCs. Our sensitive FACS method could not be used due to the presence of recombinant antibody still circulating at low levels.
Detection of antibody responses to MonoRho
The predose and the 3- and 6-month samples were checked for the presence of antibodies to MonoRho using an adaptation of the Particle Gel Immuno-Assay system (ID-PaGIA; DiaMed).15 In brief, 10 μL of the serum sample was incubated with 50 μL MonoRho-coated red polystyrene beads on top of the ID gel card for 5 minutes at room temperature then centrifuged and read macroscopically. Positive reactions were recognized by agglutination of beads. The assay was validated with rabbit anti-MonoRho antiserum, as no human antiserum against MonoRho is available. The limit of detection for anti-MonoRho antibody was between 25 and 50 ng/mL.
Safety evaluations
Blood pressure, sublingual body temperature, and heart rate were measured before and at frequent intervals during the 72-hour period following administration of RhD-positive RBCs and after 7 and 30 days. A physical examination was performed at baseline and after 72 hours, 7 days, and 30 days; an electrocardiogram was performed at baseline and after 72 hours. Hematologic parameters (hemoglobin, hematocrit, red blood cell counts, white blood cell counts, differential counts, and platelet counts), serum chemistry (glucose, creatinine, total bilirubin, urea, total protein, aspartate aminotransferase, and alanine aminotransferase), and dipstick urinalysis (protein, glucose, blood, and pH) were measured at baseline and after 23.5 hours, 72 hours, 7 days, and 30 days. Adverse events were recorded throughout the study.
FcγR analysis
Genotyping for the FcγRIIA, FcγRIIIA, and FcγRIIIB polymorphisms was performed as previously described with polymerase chain reaction (PCR)–based allele-specific primer amplification.16
Statistical analysis
The half-lives of the RBCs were log-transformed to achieve normal distribution of the data and equality of variance across groups. To evaluate the effect of the FcγR polymorphisms on the elimination of RhD-positive RBCs after intravenous administration of MonoRho, an analysis of variance (ANOVA) with the factors FcγRIIA, FcγRIIIA, and FcγRIIIB (without interactions) was performed
Results
Anti-RhD IgG serum concentrations
The serum concentrations of anti-RhD IgG measured after intravenous administration of MonoRho were dependent on the dose given (Figure 1). One hour after administration, the mean anti-RhD IgG concentrations ranged between 23 ng/mL (300-μg dose) and 300 ng/mL (1800-μg dose). In comparison, 1 hour after intravenous administration of 1500 IU Rhophylac, the mean concentration was 47 ng/mL.
Anti-RhD IgG serum concentration (mean ± SD) after administration of anti-RhD immune globulin. Rhophylac was administered both intravenously and intramuscularly at the standard dose of 1500 IU (300 μg) anti-RhD as determined by the AutoAnalyzer. MonoRho was administered both intravenously (IV) and intramuscularly (IM) at different doses defined by measurement of purified anti-RhD antibody (optical density [OD], 280 nm). Anti-RhD IgG levels in serum were measured using a sensitive FACS assay as described in “Patients, materials, and methods.”
Anti-RhD IgG serum concentration (mean ± SD) after administration of anti-RhD immune globulin. Rhophylac was administered both intravenously and intramuscularly at the standard dose of 1500 IU (300 μg) anti-RhD as determined by the AutoAnalyzer. MonoRho was administered both intravenously (IV) and intramuscularly (IM) at different doses defined by measurement of purified anti-RhD antibody (optical density [OD], 280 nm). Anti-RhD IgG levels in serum were measured using a sensitive FACS assay as described in “Patients, materials, and methods.”
The anti-RhD IgG concentrations decreased slightly faster after MonoRho than after Rhophylac administration. After 2 days, compared with the concentrations measured at 1 hour, serum anti-RhD had decreased by 80% for the 300-μg dose of MonoRho and by 60% to 65% for the higher doses of MonoRho. In the case of Rhophylac (1500 IU), serum anti-RhD had decreased by 40%. After intramuscular administration, the anti-RhD IgG serum concentrations gradually increased, reaching 10.2 ± 5.9 ng/mL (Rhophylac, 1500-IU dose) and 0.9 ± 1.3 ng/mL (MonoRho, 1200-μg dose). The variability of anti-RhD IgG serum concentrations among subjects receiving the same dose of anti-RhD immune globulin by the intramuscular route was much greater than after intravenous administration.
Clearance of RhD-positive RBCs
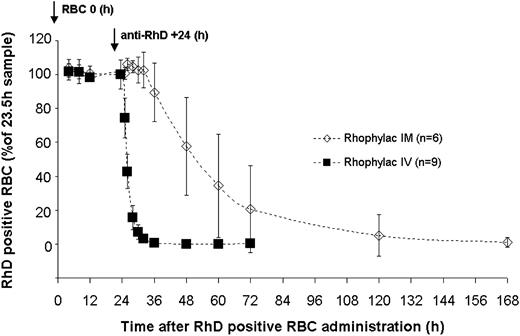
The clearance rate of 15 mL RhD-positive RBCs following administration of Rhophylac and MonoRho is shown in Figures 2, 3 and Table 1. In all subjects, the RhD-positive RBC concentration in blood remained relatively stable (∼ 0.35%) for the 24-hour period until anti-RhD was injected. Following intravenous administration of Rhophylac, RhD-positive RBCs disappeared with a mean half-life of 1.46 ± 0.89 hours. On average more than 95% of RhD-positive RBCs were eliminated from the circulation within 8 hours. After intramuscular administration of Rhophylac, an average of 12 hours was required before approximately 10% of RhD-positive RBCs were cleared and 4 days before 95% of RhD-positive RBCs were cleared.
Kinetics of concentration of RhD-positive RBCs (mean ± SD) measured in blood following intravenous (IV) or intramuscular (IM) administration of 1500 IU (300 μg) Rhophylac. The percentage of RhD-positive RBCs remaining in the blood at different times was calculated according to the 100% value determined at +23.5 hours after RBC administration by FACS assay.
Kinetics of concentration of RhD-positive RBCs (mean ± SD) measured in blood following intravenous (IV) or intramuscular (IM) administration of 1500 IU (300 μg) Rhophylac. The percentage of RhD-positive RBCs remaining in the blood at different times was calculated according to the 100% value determined at +23.5 hours after RBC administration by FACS assay.
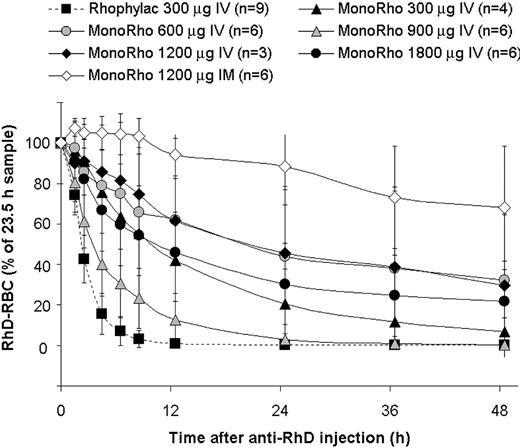
Elimination of RhD-positive RBCs (concentration, mean ± SD) following intravenous administration of either MonoRho at different doses or 1500 IU Rhophylac (300 μg). RhD-positive RBCs (15 mL) were administered 24 hours prior to the anti-RhD injection. The percentage of RBCs remaining in the blood was calculated as for Figure 2.
Elimination of RhD-positive RBCs (concentration, mean ± SD) following intravenous administration of either MonoRho at different doses or 1500 IU Rhophylac (300 μg). RhD-positive RBCs (15 mL) were administered 24 hours prior to the anti-RhD injection. The percentage of RBCs remaining in the blood was calculated as for Figure 2.
The mean half-life of RhD-positive RBC elimination was almost 4 times longer after intramuscular administration of 1200 μg MonoRho than after intramuscular administration of 1500 IU Rhophylac (45.9 ± 44.1 hours versus 12.3 ± 9.5 hours, respectively). The clearance rate differed significantly among subjects receiving MonoRho intramuscularly, with half-lives ranging from 8.6 days to 131.4 days (Table 1). The mean remaining RhD-positive RBCs at 120 hours and 168 hours after anti-RhD administration was 32% (range, 0.02%-59.04%) and 6.4% (range, 0.00%-15.84%), respectively.
The RhD-positive RBC clearance rate showed no correlation to the dose of MonoRho administered intravenously and also varied considerably among subjects who received the same dose (Figure 3). Overall, the half-life of RhD-positive RBC disappearance after MonoRho administered intravenously ranged from 2 to 203 hours, irrespective of the dose (Table 1).
There were 6 subjects who failed to meet the predefined “satisfactory” RhD-positive RBC clearance criteria (see “Patients, materials, and methods”) and who received 1500 IU Rhophylac intravenously either on day 7 or on day 11 (Table 1). In 2 of these subjects the concentrations of RhD-positive RBCs were measured shortly prior to the Rhophylac administration and 4 days (subject no. 503) or 6 days (subject no. 1101) thereafter. Surprisingly, in both subjects the Rhophylac administration did not cause an accelerated clearance of RhD-positive RBCs, rather the concentration of RhD-positive RBCs continued to decrease at approximately the same rate as before. For example, in subject no. 503, 69.2% of RhD-positive RBCs were cleared on day 7 and 86.3%, on day 11. In subject no. 1101, 51.8% of RhD-positive RBCs were cleared on day 11 and 69.4%, on day 17. In subject nos. 501 and 502, who had cleared about 90% of RhD-positive RBCs at 72 hours, more than 99% were already cleared prior to Rhophylac administration on day 7. The percentages of eliminated RhD-positive RBCs for the other 3 subjects who all received the Rhophylac administration on day 7 were as follows at 72 hours and on day 13, respectively: subject no. 202 (87.7%, 99.8%), subject no. 301 (51.1%, 97.5%), and subject no. 302 (64.4%, 100%).
In summary, both MonoRho and Rhophylac after intravenous or intramuscular administration cleared RhD-positive RBCs from the circulation but at different rates. Overall, MonoRho was slower than Rhophylac and showed no dose response with respect to the clearance rate.
Saturation of antibody binding sites on RhD-positive RBCs
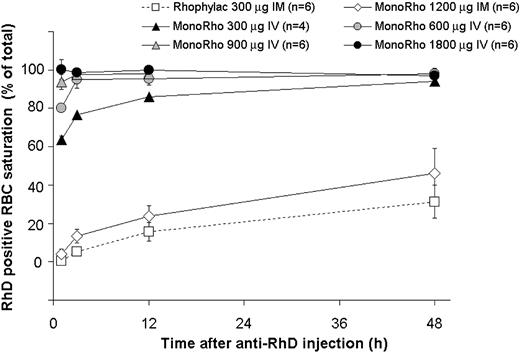
The kinetics of antibody binding to RhD-positive RBCs was measured in all subjects who received anti-RhD by intramuscular administration, the 1800-μg MonoRho dose by intravenous administration and also in some subjects from the 300-μg, 600-μg, and 900-μg MonoRho intravenous treatment groups (Figure 4). Within 1 hour after intravenous injection of MonoRho and before the elimination of RhD-positive RBCs, the 300-μg, 600-μg, 900-μg, and 1800-μg doses were sufficient to saturate a mean of 64%, 80%, 94%, and 100%, respectively, of the RhD-positive binding sites. Within 3 hours and 12 hours, the saturation levels also increased up to more than 80% in the 600-μg and 300-μg doses, respectively.
Kinetics of saturation of RhD-positive RBCs with anti-RhD IgG according to the dose of MonoRho and Rhophylac at 1500 IU (300 μg). RhD-positive RBCs (15 mL) were administered 24 hours prior to the anti-RhD injection. Saturation of RBCs at the indicated time points was measured using a FACS assay on samples treated without and with additional anti-RhD treatment (to allow for saturation of binding) during staining.
Kinetics of saturation of RhD-positive RBCs with anti-RhD IgG according to the dose of MonoRho and Rhophylac at 1500 IU (300 μg). RhD-positive RBCs (15 mL) were administered 24 hours prior to the anti-RhD injection. Saturation of RBCs at the indicated time points was measured using a FACS assay on samples treated without and with additional anti-RhD treatment (to allow for saturation of binding) during staining.
Administration of anti-RhD by the intramuscular route, either MonoRho or Rhophylac, resulted in a slower saturation of binding sites, reaching levels of 20% to 67% after 48 hours (Figure 4). However, this did not jeopardize removal of RhD-positive RBCs, as clearance began when approximately 20% of the binding sites displayed bound anti-RhD (data not shown).
Fcγ receptor analysis
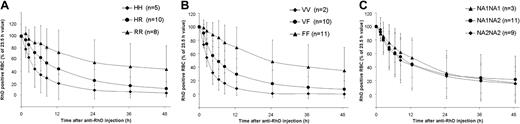
The majority of subjects were typed for the FcγRIIA-131H/R, FcγIIIA-158V/F, and FcγRIIIB NA1/NA2 polymorphisms as follows: 23 subjects received MonoRho intravenously; 6 received MonoRho intramuscularly; 7 of 9 received Rhophylac intravenously; and 6 received Rhophylac intramuscularly (Table 1). The MonoRho intravenous data revealed an association of FcγRIIA (P = .05) and FcγRIIIA (P = .05) allotypes on the RBC clearance rate, while no dependence on FcγRIIIB polymorphisms was seen (P = .87) (Figure 5A-C). The borderline degree of significance obtained for FcγRIIA and FcγRIIIA using this rigorous analysis is related to the small sample size, but a clear trend is shown (Figure 5). Subjects homozygous for FcγRIIA-131H or FcγRIIIA-158V allotypes showed a faster clearance rate compared with both the heterozygotes, FcγRIIA-H/R and FcγRIIIA-V/F, and the alternative homozygotes, FcγRIIA-RR and FcγRIIIA-FF. The RBC clearance rates for subjects receiving Rhophylac intravenously were all fast, and no statistical analysis could be performed (Table 1).
Influence of different FcγR polymorphisms on the clearance rate of RhD-positive RBCs after MonoRho intravenous administration. The polymorphisms were analyzed on DNA extracted from peripheral blood samples. RhD-positive RBCs (15 mL) were administered 24 hours prior to the anti-RhD injection. (A) Influence of FcγRIIa-131 H/R polymorphisms. (B) Influence of FcγRIIIa-158 V/F polymorphisms. (C) Influence of FcγRIIIb-NA1/NA2 polymorphisms. All values are mean ± SD.
Influence of different FcγR polymorphisms on the clearance rate of RhD-positive RBCs after MonoRho intravenous administration. The polymorphisms were analyzed on DNA extracted from peripheral blood samples. RhD-positive RBCs (15 mL) were administered 24 hours prior to the anti-RhD injection. (A) Influence of FcγRIIa-131 H/R polymorphisms. (B) Influence of FcγRIIIa-158 V/F polymorphisms. (C) Influence of FcγRIIIb-NA1/NA2 polymorphisms. All values are mean ± SD.
Administration of anti-RhD by the intramuscular route resulted in slower RBC clearance rates compared with the intravenous route. Despite the smaller number of subjects in the intramuscular groups, a similar but not so marked trend of correlation with FcγR polymorphisms was seen (Table 1). In both the Rhophylac and MonoRho intramuscular treatment groups the presence of FcγRIIA-131R and FcγRIIIA-158F was correlated with a slower removal of antibody-coated cells; for example, the 2 subjects (nos. 1005 and 1006) homozygous for both allotypes IIA-RR and IIIA-FF had the longest RhD-positive RBC elimination half-lives (50.3 and 131.4 hours, respectively) (Table 1).
In summary, these results showed that for both polyclonal and recombinant antibodies there were faster RBC clearance rates after intravenous administration than intramuscular. In particular, for MonoRho administered intravenously the RBC clearance rate was not dependent on the dose of anti-RhD used but instead showed an interesting association with the FcγRIIA and FcγRIIIA polymorphisms.
Prevention of RhD sensitization
The serologic follow-up assessments revealed that after 3 months, weak anti-RhD activity was found in serum samples of 3 of the 9 subjects who were treated with Rhophylac intravenously and in 3 of the 6 subjects who were treated with Rhophylac intramuscularly. In the MonoRho treatment group, 9 of 31 subjects, including 6 of the 7 subjects who received Rhophylac “rescue” medication, also had detectable anti-RhD. The other 3 subjects had received MonoRho only, either 1200 μg by intramuscular administration or 1800 μg by intravenous administration. After 6 months, serum samples from all 46 subjects were negative for anti-RhD (Table 1).
Safety
The administration of RhD-positive RBCs and anti-RhD immune globulins was well tolerated and had no effect on routine laboratory parameters. There were no adverse events in any of the volunteers. The 3- and 6-month serum samples of all 31 subjects who were administered MonoRho contained no detectable antibodies to MonoRho.
Discussion
Recent years have seen an increasing application of therapeutic recombinant monoclonal antibodies in many different clinical situations.17 In order to maximize their effects, much has still to be learned concerning their mechanisms of action and interactions with other effector pathways of the immune system. This is also true for candidate recombinant antibodies projected to be used for Rhesus prophylaxis. In this case, there is an added threshold to overcome, as any potential recombinant antibody must be at least as good as the current polyclonal anti-RhD products. A key question is whether a single IgG1 antibody can replace a polyclonal product containing primarily anti-RhD of IgG1 and IgG3 isotypes and recognizing multiple epitopes on the RhD antigen. Here we report on a successful phase 1 clinical study of a single recombinant IgG1 anti-RhD antibody (MonoRho) designed to assess the safety and efficacy of RhD-positive RBC clearance and prevention of RhD immunization in RhD-negative male volunteers.
Other clinical studies using a mix of IgG1 and IgG3 monoclonal anti-RhD antibodies have been reported but these studies used challenge RBC volumes of no more than 5 mL.18-20 Our study aimed to simulate a worst-case scenario in a first-time pregnancy such that the anti-RhD was given 24 hours after a 15-mL RBC challenge. In 24 subjects treated with MonoRho only and another 7 subjects who received MonoRho and a rescue dose of Rhophylac there was no evidence of immunization tested at 6 months out from the original challenge. This is the first time a single IgG1 recombinant anti-RhD has shown prevention of RhD primary immunization after such a large (15 mL) RBC challenge. A control arm of nontreatment was not feasible due to ethical considerations, but well-documented data from historical controls indicate that in an unprotected challenge 50% of subjects exposed to 12.6 to 14.6 mL RBCs developed anti-RhD antibodies.21
After intravenous administration of MonoRho there was a clear correlation with the dose given and a rapid decline of serum concentration probably due to rapid distribution of antibody into the extravascular space and/or binding to RhD-positive RBCs, which occurs very quickly. After intramuscular administration of both anti-RhD immune globulin preparations, peak concentrations were not reached within the 48-hour postdose observation period. This finding is consistent with results of pharmacokinetic studies performed in healthy RhD-negative male volunteers with MonoRho (J.B., unpublished results, July 2003) and with Rhophylac in RhD-negative pregnant women22 where maximum anti-RhD IgG serum levels were reached after a mean of 3.4 days and 5.5 days, respectively.
There are many unresolved questions concerning Rhesus prophylaxis, including the mechanism of action and whether a single antibody or a mix of antibodies of different epitope specificities and isotypes is an absolute requirement. There is extensive literature on in vitro functional analysis of Fc-mediated effector functions of monoclonal anti-RhD antibodies via FcγR interactions, their utility in predicting prophylactic efficacy, and the relative merits of IgG1 versus IgG3 isotypes with respect to phagocytosis and cytolysis.23-34
Rh prophylaxis is thought to be successful due to the efficient clearance of RhD-positive RBCs from the circulation and phagocytosis of anti-RhD–coated RBCs by macrophages in the spleen.35 Accelerated clearance of coated RBCs and a relation between the rate of clearance and the degree of coating were observed many years ago.36 Results from a decreasing dose response trial indicated that a dose of 100 μg polyclonal anti-RhD was an adequate lower limit.37 Recommendations today vary in different countries and include the addition of antenatal prophylaxis but remain mostly based on these early studies. We considered an escalating dose range of MonoRho intravenously compared with the standard dose of polyclonal anti-RhD as essential due to the previously mentioned discrepancies, which are seen when monoclonal antibodies are quantitated using the European Pharmacopoeia AutoAnalyser assay. The RBC clearance rate as an early indicator of efficacy showed no correlation with the intravenous doses of MonoRho. Clearance was detected starting at approximately 20% saturation of RBCs, but in some subjects the RBC clearance rate was initially slow compared with the polyclonal product and for safety reasons they received a rescue administration of polyclonal anti-RhD. However, this did not speed up the rate of RhD-positive RBC clearance, probably because the RBCs were already saturated to more than 90% with anti-RhD even at the lowest dose of 300 μg, and thus the polyclonal anti-RhD could not bind. In support of this hypothesis, it has been shown in vitro that MonoRho can competitively inhibit the binding of Rhophylac to the RhD antigen on RBCs.9 Our results confirm other clinical data demonstrating that monoclonal antibodies generated a slower RBC clearance rate than polyclonal anti-RhD and that the speed of RBC clearance was not correlated with the ability to prevent RhD immunization.38
Clearance of RhD-positive RBCs from the circulation implies interactions with FcγRs on effector cells of the immune system. It is known that polymorphisms of the leukocyte receptors FcγRIIA, FcγRIIIA, and FcγRIIIB influence the IgG binding capacity of the receptor.39,40 The NA1 isoform has been reported to induce a higher rate of phagocytosis of IgG-sensitized particles, presumably because of its high affinity for both IgG1 and IgG3.31 We found no correlation with the FcγRIIIB NA1/NA2 polymorphism. However, subjects homozygous for the FcγRIIIA-158V isoform had the fastest RBC clearance rates, particularly in the MonoRho intravenous group. It may be relevant in this context that the 158V isoform shows higher binding capacity for IgG1, IgG3, and IgG4 than the 158F variant.41-43 Also, recent studies on the therapeutic activity of the chimeric IgG1 anti-CD20 antibody, rituximab, have shown a greater probability of response linked with the homozygous FcγRIIIA-158V patients, which is thought to be due to the increased antibody-dependent cell cytotoxicity activity on B-lymphoma cells.44
Interestingly, the RBC half-life was also shorter in subjects with the homozygous FcγRIIA-131H genotype. Previous in vitro functional assays had shown no effect of FcγRIIA polymorphisms on IgG1-RBC immune complexes but instead only an effect on IgG3-mediated immune reactions, with the FcγRIIA-131H showing some higher affinity for IgG3.31
The variability in clearance rate in subjects protected with the polyclonal product that contains anti-RhD of the IgG1 and IgG3 subclasses was less pronounced than in the MonoRho-treated subjects. Nevertheless, those subjects in the Rhophylac intramuscular treatment group with the FcγRIIA-131H and FcγRIIIA-158V alleles also tended to have faster RBC clearance rates. This finding agrees with results from a previous study in 13 patients suffering from lupus nephritis, where the half-life of RBCs coated with polyclonal anti-RhD was significantly prolonged in subjects homozygous for the FcγRIIA-131R genotype, but does not agree with respect to FcγRIIIA where no difference was observed.45,46 Another study reported that the FcγRIIA-131R genotype may contribute to impaired removal of circulating immune complexes in patients with lupus nephritis,47 in analogy with the slow RBC clearance seen in this study. In contrast, the results from a clinical trial of monoclonal anti-RhD antibodies from subjects with FcγRIIA and FcγRIIIA polymorphisms have seemingly opposite results, as the RBC clearance rate was more rapid in subjects homozygous for FcγRIIIA-158F than in those expressing the FcγRIIIA-158V allele and no association with FcγRIIA genotypes.48 However these results apply to an IgG3 anti-RhD antibody, whose functional profile in vitro is strikingly different from comparable IgG1 anti-RhD antibodies.34,49,50
While there is an accumulating literature on the clinical impact of the FcγR polymorphisms, this study shows they had no effect on the clinical end point of prevention of immunization. Interestingly, a recent paper51 seems to indicate that phagocytosis of anti-RhD–coated RBCs is initially stimulated and then down-regulated in a time period where RBCs would still be circulating. The saturating levels of MonoRho on RBCs after intravenous administration may indicate that other mechanisms (eg, antigen masking) may play an important role. However, antigen masking is still controversial because studies with polyclonal and monoclonal antibodies, including our MonoRho and Rhophylac intramuscular administration, showed nonsaturating levels of RhD immune globulin on RBCs.19,52 Recent studies have shown that more than 90% of the antibody response in transgenic mice lacking the known receptors for IgG was suppressed53 and that F(ab)2 fragments as well as IgE are efficient suppressors of antibody responses.53,54 These findings strongly suggest that IgG is able to efficiently suppress antibody responses independently of the Fc part and favor an important role for antigen masking.55 Additionally, it has been claimed that there is an Fc dependence for suppression of primary antibody responses based on lack of suppression by F(ab)2 fragments56-58 and nonepitope specificity of suppression.59 These mechanisms would also not exclude a role for inhibition of specific B cells by FcγRIIB signaling once the RBCs are cleared from the circulation.60 The precise mechanism of action of Rh prophylaxis remains unclear and may depend on multiple additional pathways (reviewed in Kumpel20 ; Kumpel and Elson52 ; and Urbaniak and Greiss61 ).
Our study has demonstrated that a single human recombinant IgG1 antibody expressed in CHO cells effectively prevented RhD immunization in male volunteers after a large RBC challenge of a volume not previously tested in other clinical trials of monoclonal anti-RhD antibodies. The encouraging results of this study suggest that MonoRho warrants further development as a safe and efficacious alternative to plasma-derived anti-RhD immune globulin products for Rhesus prophylaxis.
Prepublished online as Blood First Edition Paper, February 19, 2004; DOI 10.1182/blood-2003-11-3929.
S.M., M.O.S., H.A., A.H., I.A., R.M.M., and J.B. are employed by ZLB Bioplasma AG, whose potential product is studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Nicole Eicher (Blood Transfusion Service, CH-3008 Bern, Switzerland); Stefan Bombard (Diamed AG, CH-1785 Cressier sur Morat, Switzerland); Oliver Fuhrmann, project manager, and Jose Banke-Bochita, MD, for administrative and clinical assistance (PAREXEL, Berlin, Germany); and Sé-bastien Déjardin, Andreas Hofmann, and Thomas Iff (ZLB Bioplasma AG, Bern, Switzerland) for their collaboration and excellent technical assistance.

![Figure 1. Anti-RhD IgG serum concentration (mean ± SD) after administration of anti-RhD immune globulin. Rhophylac was administered both intravenously and intramuscularly at the standard dose of 1500 IU (300 μg) anti-RhD as determined by the AutoAnalyzer. MonoRho was administered both intravenously (IV) and intramuscularly (IM) at different doses defined by measurement of purified anti-RhD antibody (optical density [OD], 280 nm). Anti-RhD IgG levels in serum were measured using a sensitive FACS assay as described in “Patients, materials, and methods.”](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/11/10.1182_blood-2003-11-3929/6/m_zh80110462340001.jpeg?Expires=1769852804&Signature=Df2R~kqMr6p9PrBSRe2vDHwVMj9hcxmXSLLJRshP5guCfB2RqnjlePSqQx6Lsa7~S3kzPswzvLGK4WUCyVPhLltguiO5M0YZg0y5Q1QK5onV9xWb4tHFCnZs5g8Y5HUE6EF9Z0doDk5GkfNaEe4Psmp7p~Y~FhGImpaLsyPtNUSaNsQ8CF5FkP~yNc6krXbq8H4yLyxMbAsOaU5kMmKXwi2IyHthYG-kxBkI6JLuYSUZxyn2NC3-zK5hugGJcYufjvyv8RX3jzS1UI7jc1RUrDIs6pv98GWYGKHSLmP0yEKLkz9-kBJ7XWC6IhBQ-fwcRoR2dKNUcK9KkkUKhStS0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal