Abstract
BCR-ABL and v-ABL are oncogenic forms of the Abl tyrosine kinase that can cause leukemias in mice and humans. ABL oncogenes trigger multiple signaling pathways whose contribution to transformation varies among cell types. Activation of phosphoinositide 3-kinase (PI3K) is essential for ABL-dependent proliferation and survival in some cell types, and global PI3K inhibitors can enhance the antileukemia effects of the Abl kinase inhibitor imatinib. Although a significant fraction of BCR-ABL-induced human leukemias are of B-cell origin, little is known about PI3K signaling mechanisms in B-lineage cells transformed by ABL oncogenes. Here we show that activation of class IA PI3K and downstream inactivation of FOXO transcription factors are essential for survival of murine pro/pre-B cells transformed by v-ABL or BCR-ABL. In addition, analysis of mice lacking individual PI3K genes indicates that products of the Pik3r1 gene contribute to transformation efficiency by BCR-ABL. These findings establish a role for PI3K signaling in B-lineage transformation by ABL oncogenes. (Blood. 2004;103:4268-4275)
Introduction
Altered forms of the tyrosine kinase c-Abl are potent oncogenes. v-ABL is the transforming gene of Abelson murine leukemia virus (Ab-MuLV).1 Neonatal mice infected with Ab-MuLV predominantly develop B-cell leukemias corresponding to the pro-B or pre-B-cell stages. Infection of murine bone marrow or fetal liver also results in preferential transformation of pro/pre-B cells. Expression of the v-ABL oncogene can transform certain other cell types in vitro, including myeloid cells and certain fibroblast lines. BCR-ABL is a fusion oncogene expressed in human leukemia cells with a (9;22) translocation known as the Philadelphia (Ph) chromosome.2,3 Depending on the specific breakpoint, Bcr-Abl proteins exist in p190, p210, or p230 forms that are associated with different clinical syndromes. p210-Bcr-Abl is found in nearly all cases of chronic myelogenous leukemia (CML) and some patients with Ph+ acute lymphoid leukemia (ALL). p190-Bcr-Abl is almost exclusively found in ALL. Retroviral transduction of murine bone marrow cells with p210 or p190 forms can yield either myeloid or B-lymphoid tumors in recipient mice, depending on the experimental model used.4
The Abl tyrosine kinase inhibitor imatinib (Gleevec, STI-571) has been shown to elicit remarkable clinical responses with minimal toxicity in CML and Ph+ ALL patients. Unfortunately, resistance eventually develops and is usually associated with mutations or amplifications of BCR-ABL.5,6 Targeting multiple signaling steps therefore may generate more lasting remissions in patients with ABL-dependent leukemias. Expression of v-Abl or Bcr-Abl activates many of the same signaling intermediates including the small G proteins Ras and Rac, tyrosine kinases of the Janus kinase family, protein kinase C, and phosphoinositide 3-kinase (PI3K).7,8 It is important to note that signaling components downstream of ABL oncogenes have been defined in diverse cell systems. However, it is becoming evident that many of the effects of v-ABL and BCR-ABL are cell-context specific and may not have functional significance in the transformation of primary cells. For example, signal transducer and activator of transcription 5 was shown to be dispensable for v-ABL- or BCR-ABL-mediated transformation and leukemogenesis even though it is phosphorylated in human CML cells and is required for conversion of hematopoietic cell lines to growth factor-independence.9,10 Thus, it is critical that the contribution of specific signaling components to ABL-mediated disease be confirmed in direct transformation assays done in hematopoietic cell types representative of their natural target cells.
The PI3K family of enzymes phosphorylates inositol phospholipids, thereby promoting membrane association of certain cytoplasmic proteins that specifically bind to PI3K lipid products.11,12 There are many subtypes of PI3K with distinct functions in cells. Class IA PI3Ks, the subgroup that functions downstream of activated tyrosine kinases, are heterodimers composed of a catalytic subunit and a regulatory subunit. Signaling through class IA PI3K promotes cell proliferation and survival in many cell types and is strongly associated with transformation and metastasis.13 There are 3 catalytic subunit isoforms (p110α, p110β, and p110δ) and 5 regulatory subunit isoforms (p85α, p55α, p50α, p85β, and p55γ; the first 3 are transcriptional variants of a single gene, Pik3r1).11 We and others have shown that the predominant class IA regulatory isoform expressed in murine B cells, p85α, is required for B-cell proliferation triggered by some but not all mitogens.14,15 In addition, p85α-deficient mice show impaired B-cell development as judged by reduced percentages of pre-B and mature B cells.14,15
Previous work on v-Abl activation of PI3K has been carried out mostly in model cell lines. In fibroblasts, it was shown that the transforming ability of v-Abl variants correlated with the ability to activate PI3K.16 In a mast cell line, a temperature-sensitive allele of v-ABL was used to demonstrate that activation of PI3K, and its downstream effector Akt, contributes to the suppression of apoptosis following growth factor withdrawal.17 PI3K has been implicated downstream of Bcr-Abl mainly from studies of the p210 isoform in mouse models of CML and in myeloid cell lines converted to factor independence.18-22 Most recently, it was shown that PI3K inhibitors synergize with imatinib to inhibit growth of human CML cells.23 It has also been reported that PI3K and Akt are activated in murine B-lineage cells transformed by BCR-ABL.20,22 However, the importance of PI3K signaling, the specific isoforms involved, and the critical downstream targets have not been studied directly in these cells. In this work we investigate whether PI3K activation contributes to v-ABL and p190-BCRABL transformation of immature B-lineage cells. In addition, we test the hypothesis that Pik3r1 gene products, previously shown to be essential for B-cell development, are required for transformation in this system. Finally, we address the roles of downstream effectors of PI3K in proliferation and survival of B cells transformed by ABL oncogenes. We show that PI3K signaling and FOXO inactivation are essential in this system, and that Pik3r1 contributes quantitatively to transformation by BCR-ABL but not v-ABL.
Materials and methods
Antibodies and inhibitors
Primary antibodies used for immunoblot were rabbit antisera specific for p110β (H-198; Santa Cruz Biotechnology, Santa Cruz, CA), p110α and p110δ (gift from Bart Vanhaesebroeck, Ludwig Cancer Research Institute, London, United Kingdom), phosphotyrosine (PY20; Santa Cruz Biotechnology), a monoclonal antibody (mAb) specific for p110α (Transduction Laboratories, Lexington, KY), a rabbit antiserum that recognizes all class IA PI3K regulatory isoforms (anti-pan-p85, 06-195; Upstate Biotechnology, Lake Placid, NY), and a mAb specific for p55γ (V2; Abcam, Cambridge, MA). Rabbit antibodies specific for total and phosphorylated forms of glycogen synthase kinase 3β (GSK3β) (Ser9), Akt (Thr308), and FOXO (Ser256) were from Cell Signaling Technologies (Beverly, MA). To detect p85β, lysates were subjected to partial immunodepletion with a mAb specific for an N-terminal epitope in p85α (clone AB6; Upstate Biotechnology) (3 successive immunoprecipitations) before blotting with anti-pan-p85. Reagents used for flow cytometry (fluorescence activated cell sorting [FACS]) were as follows: Annexin V-phycoerythrin (PE; Caltag, Burlingame, CA), anti-B220-fluorescein isothiocyanate, and Streptavidin-Cychrome (Becton Dickinson, San Jose, CA), anti-Thy1.1-Biotin, and anti-BP-1-PE (eBiosciences, San Diego, CA). Pharmacologic inhibitors (Calbiochem, San Diego, CA) were used at the following final concentration: LY294002, 10 μM; rapamycin, 10 ng/mL; and imatinib mesylate, 10 μM.
Retroviral vectors and generation of virus stocks
Bicistronic retroviral vectors were used to express exogenous genes and a marker gene from the murine stem cell virus (MSCV) long terminal repeat (LTR). pMIG (MSCV-IRES-GFP) was provided by R. Hawley (American Red Cross, Rockville, MD) and pMIT (MSCV-IRES-Thy1.1), by P. Marrack (National Jewish Hospital, Denver, CO). A cDNA encoding p190-Bcr-Abl was subcloned into pMIG, and epitope-tagged cDNAs for FOXO3a and FOXO3a(A3) (gift from B. Burgering, University Medical Center, Utrecht, The Netherlands) and Δp85 (gift from T. Shioi, Beth Israel Deaconess Medical Center, Boston, MA) were subcloned into pMIT. High-titer helper-free retrovirus stocks were prepared by transient cotransfection of 293T cells with pMIT vectors and ψ- ecotropic packaging vector.24 Viral supernatants were collected 24 to 48 hours after transfection, filter-sterilized, and stored at -80°C. Virus titers were determined by transducing murine 3T3 fibroblasts and checking marker gene expression by FACS. In each individual experiment, the same virus stock was used to infect cells of different genotypes.
Colony transformations and generation of transformed cell lines
Animal procedures were approved by the institutional animal care and use committees of University of California (UC) Irvine and UC Los Angeles (UCLA). Pik3r1 heterozygous mice were in a mixed genetic background (129SvEv × C57Bl/6) or were backcrossed with Balb/c (Jackson Laboratories, Bar Harbor, ME) for 8 generations. From timed matings of heterozygous animals, embryonic day-16.5 (E16.5) or E18.5 embryos were obtained and fetal livers isolated. Following preparation of single-cell suspensions in RPMI 1640 with 10% heat-inactivated fetal calf serum (FCS), cells were placed on ice during the polymerase chain reaction genotyping procedure (approximately 6 hours). Pik3r1 null and wild-type samples were then used for v-ABL and BCR-ABL infections. Fetal liver cells were infected with AbMuLV by mixing 2 × 106 cells in 1 mL with 1 mL of P160 AbMuLV stock and polybrene (4 μg/mL). After incubation at 37°C, 5% CO2 for 2 to 4 hours, cells (1 × 105 cells/mL in 24-well plates) were placed in a 1.2% agar medium supplemented with 1 × RPMI 1640, 50 μM β-mercaptoethanol, and 20% FCS. For BCR-ABL, 1 × 106 cells were incubated with retroviral stocks for pMIG or pMIG-p190-BCR-ABL and polybrene (4 μg/mL) for 3 hours at 37°C. Cells were centrifuged at 550g (2000 rpm) at 30°C for 30 minutes. Cells (5 × 104) were then added to methylcellulose METHOCULT M3630 with recombinant human interleukin-7 (rhIL-7; StemCell Technologies, Vancouver, BC, Canada) in duplicate dishes. The remaining fractions of infected cells were used for suspension cultures that were grown initially in pre-B-cell medium (RPMI 1640, 20% heat-inactivated FCS, penicillin [100 units/mL], streptomycin [100 μg/mL], 2 mM l-glutamine, 50 μg/mL gentamicin, 50 μM β-mercaptoethanol). Established lines were later switched to 10% FCS. v-ABL colony assays were counted 9 days after infection by adding the counts from all 24 wells. BCR-ABL colony assays were scored 7 days after transduction by adding the total counts from both dishes.
Retroviral transductions of transformed cell lines
Although the v-ABL-transformed cell lines were established by infection with a replication competent retrovirus (AbMuLV), we found that MSCV-based retroviral vectors could superinfect some v-ABL cells, as well as cell lines established with pMIG-p190-BCR-ABL, and provided the most reliable method of gene delivery. Cells (2 × 106) in 1 mL pre-B-cell medium were mixed with 1 mL virus and 8 μg/mL polybrene and centrifuged in 24-well plates at 33°C for 45 minutes at 450g. After incubation for 20 to 24 hours at 37°C, cells were either analyzed by FACS or expanded into 3 mL fresh medium.
FACS analysis
Cells were analyzed for green fluorescent protein (GFP) expression and stained with various antibodies and/or Annexin V. For cell cycle analysis, cells were fixed in ethanol and stained with propidium iodide as described.14,25 The percentages of cells in different phases of the cell cycle were calculated with ModFit LT software (Verity Software House, Topsham, ME).
In vivo leukemogenesis assay
BCR-ABL-transformed pro/pre-B cells were harvested after 7 days in culture (at which point the live cells were > 90% GFP+) and intravenously introduced into syngeneic (Balb/c.scid) mice, sublethally irradiated at 6 Gy (600 rad). Animals were monitored daily for signs of illness as previously described.26
Cell morphologic analyses and surface marker staining
Peripheral blood, spleen, and bone marrow cells were harvested from overtly ill animals and single-cell suspensions from these tissues were depleted of red blood cells by hypotonic lysis and acquired on FACScan (Becton Dickinson) or cytospun onto microscope slides (Fisher, Hampton, NH). FACS data were analyzed using WinMDI version 2.8 software (Scripps Research Institute, La Jolla, CA). Dead cells were excluded from analysis based on forward- and side-scatter properties. Peripheral blood smears (nonlysed), as well as spleen and bone marrow cytospins, were analyzed by Giemsa/Wright staining using the 2-hydroxyethyl methacrylate (HEMA) solution per the manufacturer's protocol (Biochemical Sciences, Swedesboro, NJ).
Immunoprecipitation/immunoblotting
The indicated numbers of cells were washed in 1 × phosphate-buffered saline (PBS), then lysed in 40 μL for direct immunoblot analysis, or 500 μL for immunoprecipitation, of lysis buffer (50 mM Tris[tris(hydroxymethyl) aminomethane, pH 7.6], 150 mM NaCl, 1% Triton X-100, 10% glycerol). Immunoprecipitates were prepared by sequential one-hour incubations with antibody and protein A-Sepharose. Beads were washed 3 times with lysis buffer before boiling in 1 × sample buffer. Immunoprecipitates or 40 μg lysate was electrophoresed (7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE]) and transferred to nitrocellulose. Immunoblotting was done as described.25
PI3K activity
Lysates from 1 × 106 cells were immunoprecipitated with the pan85 antibody or lysates from 5 × 106 cells with the pTyr antibody. Immune complex PI3K assays were performed as previously described.27 A phosphoimager (Molecular Dynamics, Sunnyvale, CA) was used to quantitate activity.
Results
Contribution of Pik3r1 gene products to PI3K-dependent transformation by p190-BCR-ABL
Addition of the global PI3K inhibitor LY294002 blocked pre-B colony formation in transformation assays with v-ABL or p190-BCR-ABL (data not shown). The compound was added at a concentration (10 μM) that abrogates specific mitogenic signals in B cells without general toxicity.14 These results suggested a role for PI3K in transformation in this system but did not distinguish which isoforms are important. To determine if the predominant class IA regulatory isoform was required for transformation, we studied mice with a disrupted Pik3r1 gene, encoding p85α and the smaller splice variants p55α and p50α. This mutation causes perinatal lethality,28 so bone marrow could not be obtained. Instead, we used fetal liver as a source of hematopoietic progenitors for in vitro transformation assays. For v-ABL transformation, cells were infected with AbMuLV and plated in soft agar. For BCR-ABL, cells were plated in methylcellulose following infection with a replication-defective retrovirus in which the murine stem cell virus long terminal repeat (MSCV LTR) drives expression of p190-Bcr-Abl in a bicistronic mRNA with GFP (pMIG-p190-BCR-ABL).
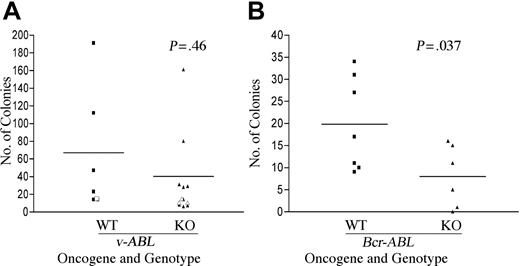
In 4 independent experiments with AbMuLV, we observed no significant reduction in the number of colonies derived from fetal liver of Pik3r1 null compared with wild type (Figure 1A). Clonal cell lines could be established from both genotypes following transfer of colonies into liquid culture, and bulk cell lines could be derived by plating fetal liver in liquid culture immediately after infection. We did observe a significant reduction (approximately 2-fold) in the number of pre-B-cell colonies in methylcellulose seeded with BCR-ABL-transduced fetal liver from Pik3r1 null compared with wild type (Figure 1B). However, cell lines could also be derived from BCR-ABL transformants of both genotypes. The expression of v-Abl and Bcr-Abl in the respective cell lines was confirmed by immunoblotting with a monoclonal antibody to c-Abl (data not shown). Together these results indicated that Pik3r1 gene products are not essential for establishment or maintenance of the transformed phenotype but contribute to maximal efficiency of transformation by BCR-ABL.
Partially impaired transformation of B-cell progenitors from Pik3r1 null fetal liver by BCR-ABL but not v-ABL. Wild-type (WT) and Pik3r1 null (KO) fetal liver cells were infected with Ab-MuLV or pMIG-p190-BCR-ABL. Transformation by v-ABL (A) and BCR-ABL (B) was measured by counting colonies formed in soft agar or methylcellulose. Open symbols are from a single transformation assay done in cells from a mixed genetic background (129SvEv × C57BL6) at embryonic day 16.5, whereas the closed symbols were from 3 independent assays done in cells from a Balb/c background at E18.5. Horizontal bars depict averages: v-ABL WT, 67; v-ABL KO, 40; BCR-ABL WT, 20; and BCR-ABL KO, 8. Data analyzed by paired t test revealed no difference between v-ABL WT and KO (P = .46) but showed a significant decrease in transformation of KO by p190-BCR-ABL (P = .037).
Partially impaired transformation of B-cell progenitors from Pik3r1 null fetal liver by BCR-ABL but not v-ABL. Wild-type (WT) and Pik3r1 null (KO) fetal liver cells were infected with Ab-MuLV or pMIG-p190-BCR-ABL. Transformation by v-ABL (A) and BCR-ABL (B) was measured by counting colonies formed in soft agar or methylcellulose. Open symbols are from a single transformation assay done in cells from a mixed genetic background (129SvEv × C57BL6) at embryonic day 16.5, whereas the closed symbols were from 3 independent assays done in cells from a Balb/c background at E18.5. Horizontal bars depict averages: v-ABL WT, 67; v-ABL KO, 40; BCR-ABL WT, 20; and BCR-ABL KO, 8. Data analyzed by paired t test revealed no difference between v-ABL WT and KO (P = .46) but showed a significant decrease in transformation of KO by p190-BCR-ABL (P = .037).
Given the impairment of B-cell development in Pik3r1 null mice, it was possible that the target cell for transformation differed depending on the genotype of the fetal liver. However, most of the cell lines of both genotypes were positive for the B-lineage marker B220 (Table S1; see the Supplemental Tables link at the top of the online article on the Blood website). Some cell lines contained mixed populations of pro/pre-B cells as determined by staining for BP-1, a marker that distinguishes early pro-B cells (BP-1-) from late pro-B and pre-B cells (BP-1+).29 However, WT and Pik3r1 null lines did not show reproducible differences in the percentages of BP-1+ versus BP-1- cells (Table S1). All cell lines were surface immunoglobulin M-negative (IgM-).
Delayed leukemia development in absence of Pik3r1 gene products
Perinatal lethality in the Pik3r1 null mice hindered our ability to use standard mouse models for leukemia caused by v-ABL or BCR-ABL. Instead, we tested the leukemogenic potential of day-18.5 fetal liver cells following initial transformation in vitro by p190-BCR-ABL. Cells were allowed to expand for 1 week in vitro to ensure efficient disease induction, as fetal liver transformation is less efficient than for bone marrow (Figure 1; Table S2). Equal numbers of transformed wild-type and Pik3r1 null cells were then injected into severe combined immunodeficiency (SCID) mice and monitored for disease progression. Mice receiving wild-type cells died from pro/pre-B-cell leukemia after an average of 17 days, whereas mice receiving Pik3r1 null cells succumbed after 23 days (Figure 2A).Although this delay was significant (P = .018), the phenotype of the leukemia was comparable (Figure 2B and data not shown). Cells from the spleen and bone marrow were removed and stained for B220 and BP-1, and analyzed for expression of GFP. A majority of pro/pre-B-cells from mice injected with wild-type and Pik3r1 null cells were GFP positive (Figure 2B). One difference between the wild-type and Pik3r1 null cells was a lower percentage of BP-1+ cells in the bone marrow and spleen of mice receiving Pik3r1 null cells. This was not due to altered B-cell precursor distribution in the Pik3r1 null fetal liver (Table S2), perhaps indicating a decrease in leukemogenic potential of BP-1+ transformants.
Delayed onset but similar phenotype of leukemic disease in mice that received transplants of p190-BCR-ABL-transduced fetal liver from Pik3r1 null embryos. Wild-type and Pik3r1 null fetal liver transduced with p190-BCR-ABL were allowed to expand one week in vitro prior to transplantation of 1 × 106 cells into SCID mice. (A) Kaplan-Meyer plot showing number of days until leukemia development. Of the mice, 4 received wild-type cells (from 2 pooled embryos) and 4 received Pik3r1 null cells (from one embryo). (B) Bone marrow and spleen cells were cytospun and stained with Giemsa/Wright to show the lymphoblastic phenotype. Cells were also analyzed by FACS for expression of GFP, B220, and BP-1. Original magnifications of the panels are shown below the images.
Delayed onset but similar phenotype of leukemic disease in mice that received transplants of p190-BCR-ABL-transduced fetal liver from Pik3r1 null embryos. Wild-type and Pik3r1 null fetal liver transduced with p190-BCR-ABL were allowed to expand one week in vitro prior to transplantation of 1 × 106 cells into SCID mice. (A) Kaplan-Meyer plot showing number of days until leukemia development. Of the mice, 4 received wild-type cells (from 2 pooled embryos) and 4 received Pik3r1 null cells (from one embryo). (B) Bone marrow and spleen cells were cytospun and stained with Giemsa/Wright to show the lymphoblastic phenotype. Cells were also analyzed by FACS for expression of GFP, B220, and BP-1. Original magnifications of the panels are shown below the images.
Proliferation requires Abl, class IA PI3K, and mTOR but not p85α
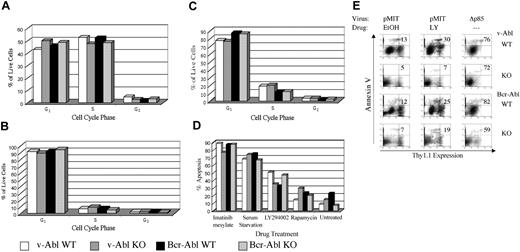
The results from colony assays indicated that Pik3r1 gene products are not essential for the transformation of B-cell progenitors by v-ABL but contribute quantitatively to BCR-ABL transformation. To study growth parameters of established cell lines in more detail, we compared cell size, cell cycle profiles, and cell death by flow cytometry. The results showed no consistent differences among genotypes in the percentages of dead cells, or of live cells in G0/G1, S, or G2/M phases (Figure 3A). In contrast, treatment of either wild-type or Pik3r1 null cell lines with LY294002 for 24 hours resulted in dramatic cell cycle arrest, as judged by decreased S and increased G0/G1 fractions (Figure 3B). LY294002 treatment also increased apoptosis, although this effect varied among cell lines (Figure 3D). The sensitivity of wild-type and Pik3r1 null cells to a range of LY294002 concentrations was comparable (data not shown). These results confirm that PI3K enzyme activity is required for ABL oncogenes to promote cell cycle progression and survival in B-lineage cells. However, Pik3r1 gene products are not solely required for this function.
Comparable cell cycle, survival, and drug sensitivity in wild-type and Pik3r1 null cell lines.BCR-ABL- or v-ABL-transformed cells of the indicated genotypes were cultured for 24 hours in either diluent alone (0.1% EtOH) (A), or treated with LY294002 (B) or rapamycin (C), then fixed and stained with propidium iodide to determine DNA content. The percentages of live cells in G0/G1, S, and G2/M stages of cell cycle were calculated using ModFit LT software. (D) The percentages of apoptotic cells under the indicated conditions were assessed by subdiploid DNA content using Cell Quest plots (BD Biosciences, San Diego, CA) of propidium iodide fluorescence. Graphs are representative of at least 3 experiments per condition and at least 3 different cell lines per genotype. (E) Cell lines were retrovirally infected with pMIT or pMIT-Δp85, then cultured in the presence of LY294002 (LY) or EtOH before analysis by FACS 20 hours after infection. The extent of apoptosis in cells with different levels of Thy1.1 expression was determined by Annexin V staining. The number in the upper-right quadrant represents the percentage of dying cells among the population expressing high levels of the Thy1.1 marker.
Comparable cell cycle, survival, and drug sensitivity in wild-type and Pik3r1 null cell lines.BCR-ABL- or v-ABL-transformed cells of the indicated genotypes were cultured for 24 hours in either diluent alone (0.1% EtOH) (A), or treated with LY294002 (B) or rapamycin (C), then fixed and stained with propidium iodide to determine DNA content. The percentages of live cells in G0/G1, S, and G2/M stages of cell cycle were calculated using ModFit LT software. (D) The percentages of apoptotic cells under the indicated conditions were assessed by subdiploid DNA content using Cell Quest plots (BD Biosciences, San Diego, CA) of propidium iodide fluorescence. Graphs are representative of at least 3 experiments per condition and at least 3 different cell lines per genotype. (E) Cell lines were retrovirally infected with pMIT or pMIT-Δp85, then cultured in the presence of LY294002 (LY) or EtOH before analysis by FACS 20 hours after infection. The extent of apoptosis in cells with different levels of Thy1.1 expression was determined by Annexin V staining. The number in the upper-right quadrant represents the percentage of dying cells among the population expressing high levels of the Thy1.1 marker.
We also examined the effects of rapamycin, an immunosuppressant that targets the mammalian target of rapamycin (mTOR)/S6kinase pathway downstream of PI3K. Rapamycin also promoted cell cycle arrest, but was less effective than LY294002 in promoting apoptosis (Figure 3C). Addition of imatinib had a greater apoptotic effect than LY294002, causing more than 80% of the cells to die (Figure 3D). These results suggest that oncogenic Abl kinases promote survival via both PI3K-dependent and PI3K-independent pathways. Cell survival was also dependent on the presence of serum (Figure 3D).
LY294002 inhibits all classes of PI3K catalytic subunit at the concentrations used. To ascertain if class IA PI3K function was specifically required in these cells, we used a dominant-negative construct (Δp85) that selectively inhibits class IA signaling. Δp85 is a variant of p85α lacking a portion of the p110-binding domain, and has been shown to act as a dominant-negative in other cell systems by competing with endogenous class IA heterodimers (including those containing p85β or p55γ) for binding to signaling complexes.30 Δp85 was inserted into a retroviral vector (pMIT) that drives expression of the test gene from the MSCV LTR along with the surface marker Thy1.1 on a bicistronic mRNA.31 At 20 hours after infection, cells transduced with Δp85 showed markedly lower levels of Thy1.1 marker gene expression compared with cells transduced with empty vector pMIT (Figure 3E, compare right panels with left panels). A selective increase in cell death was observed in the high-expressing population of Δp85-transduced cells, as measured by staining with Annexin V (Figure 3E; note percentages in upper right quadrant). Similar patterns were observed regardless of Pik3r1 genotype (Figure 3E). The finding that cells with high expression of Δp85 undergo cell death suggests that some class IA PI3K function is required for maintenance of the transformed phenotype. The reduced Thy1.1 expression was not a general effect on protein expression caused by PI3K inhibition, as LY294002 did not reduce expression of Thy1.1 in cells transduced with empty vector (Figure 3E, middle panels).
Class IA PI3K signaling output is maintained in transformed cell lines lacking p85α
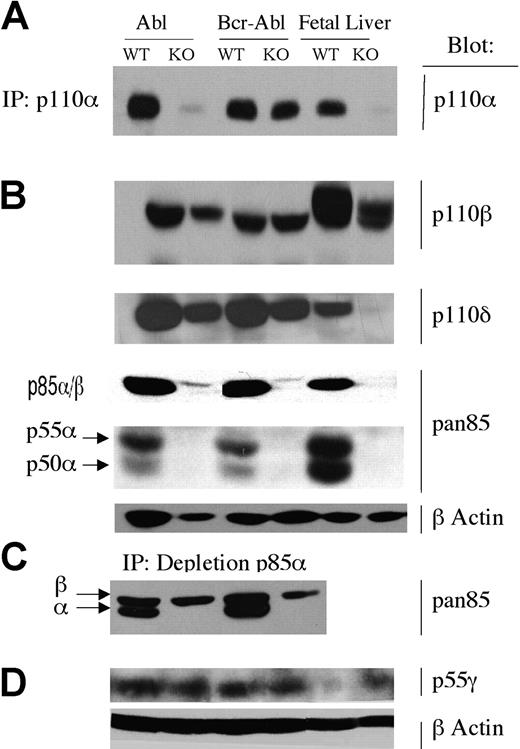
In many tissues in Pik3r1 null mice, loss of p85α, p55α, and p50α is associated with marked reductions in the expression of the class IA catalytic subunit isoforms (p110α, p110β, and p110δ).28,32 This is presumed to be the result of decreased stability of the monomeric catalytic subunits.33 Indeed, protein expression of all 3 catalytic isoforms was markedly reduced in total fetal liver populations from Pik3r1 null embryos (Figure 4, top 2 rows). In most transformed Pik3r1 null lines, however, expression of p110α, p110β, and p110δ was reduced to a lesser degree than in the original fetal liver populations (Figure 4A-B).
Expression of PI3K isoforms. Lysates or immunoprecipitates (IP) from wild-type and Pik3r1 null (WT and KO) cells transformed by v-ABL or BCR-ABL were immunoblotted with the indicated antibodies. (A) p110α was detected by IP and immunoblotting. (B) p110β, p110δ, p85α, p85β, p55α, and p50α were detected by direct immunoblotting; β-actin was used as a loading control. (C) To optimize detection of p85β with anti-pan-p85, lysates were first subjected to partial immunodepletion of p85α using a specific mAb. p55γ was detected by direct immunoblotting; β-actin was used as a loading control. Isoform blots are representative of 2 to 6 cell lines per genotype.
Expression of PI3K isoforms. Lysates or immunoprecipitates (IP) from wild-type and Pik3r1 null (WT and KO) cells transformed by v-ABL or BCR-ABL were immunoblotted with the indicated antibodies. (A) p110α was detected by IP and immunoblotting. (B) p110β, p110δ, p85α, p85β, p55α, and p50α were detected by direct immunoblotting; β-actin was used as a loading control. (C) To optimize detection of p85β with anti-pan-p85, lysates were first subjected to partial immunodepletion of p85α using a specific mAb. p55γ was detected by direct immunoblotting; β-actin was used as a loading control. Isoform blots are representative of 2 to 6 cell lines per genotype.
Catalytic subunit expression could be preserved by the expression of other class IA regulatory isoforms (p85β or p55γ). We have found that the available antibodies are inadequate for specific detection of murine p85β in these cells. However, p85β can be detected weakly with an antiserum raised to the N-terminal Src homology 2 domain of p85α, termed “anti-pan-p85.” Blotting with this antiserum revealed a strong band at 85 kDa in wild-type cells that was greatly reduced in Pik3r1 null cells, as expected (Figure 4C). Similarly, bands corresponding to p55α and p50α were detected in transformed wild-type cell lines but not Pik3r1 null cells (Figure 4B). The residual signal at 85 kDa in cells lacking p85α represents p85β. Although the putative p85β band migrates slightly more slowly than p85α, in wild-type cells it is mostly obscured by the stronger p85α signal. To better distinguish and quantitate the putative p85β band, we analyzed lysates following partial immunodepletion of p85α with a monoclonal antibody (Figure 4C). This strategy revealed comparable p85β expression in Pik3r1 null cells relative to wild type. Similarly, transformed cells of both genotypes expressed comparable amounts of the p55γ isoform. Interestingly, this isoform was only weakly detected in total fetal liver from wild-type embryos and was up-regulated in Pik3r1 fetal liver (Figure 4D). Together these results suggest that transformation of Pik3r1 null cells is possible because other regulatory isoforms are expressed in ABL-transformed pro/pre-B cells. These alternative isoforms appear to be sufficient to stabilize p110 catalytic isoforms and maintain PI3K signaling capacity.
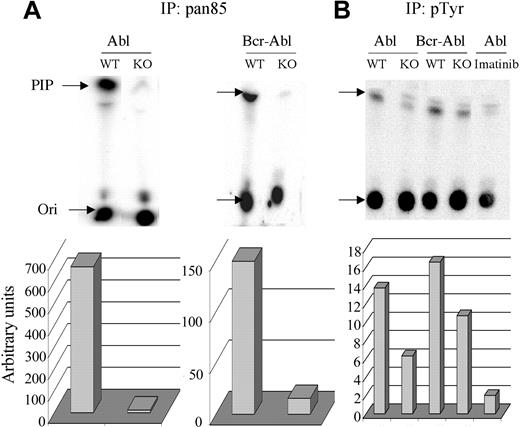
To test this possibility further, the amount of total class IA PI3K activity was measured in anti-pan-p85 immunoprecipitates and compared with the activity associated with signaling complexes, as measured in antiphosphotyrosine immunoprecipitates (Figure 5). As seen previously in other tissues,28 anti-pan-p85 precipitated much less PI3K activity from Pik3r1 null cells compared with wild-type, both in the v-ABL and BCR-ABL cell lines (Figure 5). The remaining activity in Pik3r1 null cells is presumably associated with p85β and p55γ. Despite the dramatic reduction in total class IA PI3K activity in the cells, PI3K activity associated with phosphotyrosine-containing signaling complexes was only modestly reduced (Figure 5). Treatment with imatinib mesylate greatly reduced the PI3K activity in phosphotyrosine immunoprecipitates from v-ABL wild-type cells (Figure 5), demonstrating that association of PI3K with phosphotyrosine-containing signaling complexes was Abl-dependent.
Measurement of total and phosphotyrosine-associated PI3K activity. Lysates from 1 × 106 cells from WT and KO cells were immunoprecipitated with pan85 antibody (A) or lysates from 5 × 106 cells with pTyr antibody (B). Imatinib was included for 15 minutes prior to cell lysis in the indicated sample (v-ABL WT). Immune complexes were subjected to an in vitro PI3K assay using phosphatidylinositol as substrate and the products resolved by thin-layer chromatography (Ori indicates origin). Radioactivity in the phosphatidylinositol-3-phosphate product (PIP, indicated by arrow) was quantitated by phosphoimager and graphed below. Similar results were obtained in 3 independent experiments of 2 different cell lines per genotype. The specificity of the immune complex kinase assays was verified in control experiments showing that kinase activity was completely blocked by in vitro treatment with wortmannin (50 nM), a selective PI3K inhibitor (not shown).
Measurement of total and phosphotyrosine-associated PI3K activity. Lysates from 1 × 106 cells from WT and KO cells were immunoprecipitated with pan85 antibody (A) or lysates from 5 × 106 cells with pTyr antibody (B). Imatinib was included for 15 minutes prior to cell lysis in the indicated sample (v-ABL WT). Immune complexes were subjected to an in vitro PI3K assay using phosphatidylinositol as substrate and the products resolved by thin-layer chromatography (Ori indicates origin). Radioactivity in the phosphatidylinositol-3-phosphate product (PIP, indicated by arrow) was quantitated by phosphoimager and graphed below. Similar results were obtained in 3 independent experiments of 2 different cell lines per genotype. The specificity of the immune complex kinase assays was verified in control experiments showing that kinase activity was completely blocked by in vitro treatment with wortmannin (50 nM), a selective PI3K inhibitor (not shown).
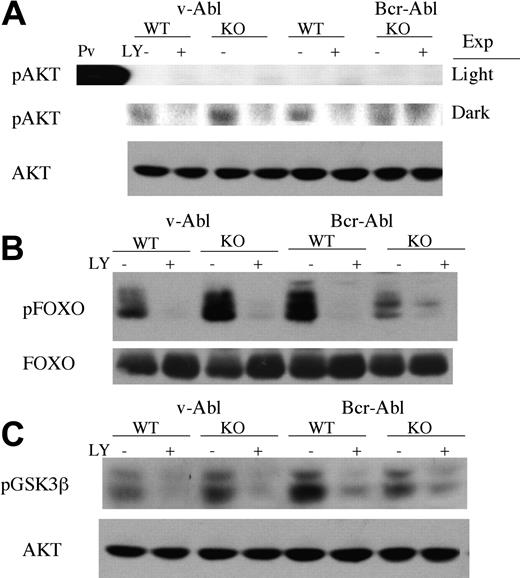
To study PI3K signaling further in pro/pre-B cells transformed by v-ABL or BCR-ABL, we assessed the phosphorylation state of proteins in the PI3K signaling pathway. Akt has been shown to be an important downstream target of PI3K,13,34 and its phosphorylation is often used as an indirect measure of PI3K activation. Phosphorylation of Akt was detected in v-ABL and BCR-ABL cell lines, and could be blocked by short pretreatment with LY294002 (Figure 6A). Pik3r1 null cell lines showed comparable levels of Akt phosphorylation, consistent with the model that PI3K signaling is functionally intact. Surprisingly, the stoichiometry of Akt phosphorylation was very low in all cell lines (Figure 6A and data not shown), and only visible when blots were exposed much longer than the time required to visualize phosphoAkt in cells treated with the phosphatase inhibitor pervanadate (15 minutes vs 1 second).
Comparable expression and phosphorylation of downstream targets of PI3K signaling. Cell lines were incubated with LY294002 or diluent alone (0.1% EtOH) for 15 minutes before lysis. Per sample, 40 μg protein was resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antibodies to phosphorylated forms of Akt (A), FOXO (B), or GSK3β (C). Total Akt or total FOXO1 was used as a loading control. Pv indicates pervanadate; Exp, exposure to film: light (upper panel) and dark (lower panel). Data represent at least 3 different experiments and at least 2 different cell lines for each genotype.
Comparable expression and phosphorylation of downstream targets of PI3K signaling. Cell lines were incubated with LY294002 or diluent alone (0.1% EtOH) for 15 minutes before lysis. Per sample, 40 μg protein was resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antibodies to phosphorylated forms of Akt (A), FOXO (B), or GSK3β (C). Total Akt or total FOXO1 was used as a loading control. Pv indicates pervanadate; Exp, exposure to film: light (upper panel) and dark (lower panel). Data represent at least 3 different experiments and at least 2 different cell lines for each genotype.
To ascertain if the small fraction of phosphorylated Akt was functionally active in cells, we measured the phosphorylation state of known Akt substrates. One important group of Akt substrates is the FOXO subfamily of Forkhead transcription factors.35,36 FOXO factors promote cell cycle arrest, and these functions are inhibited by Akt phosphorylation. It was recently reported that FOXO proteins are constitutively phosphorylated in several CML cell lines.37 Likewise, we found that FOXO1 was expressed and phosphorylated on consensus Akt sites, in both v-ABL and BCRABL pro/pre-B cell lines (Figure 6B). Although there was some variability in levels of phospho-FOXO1 in different cell lines, there was no consistent trend when 3 independent clones of wild-type and Pik3r1 null cells were compared (data not shown). Phospho-FOXO1 levels could be reduced by a short treatment with either LY294002 or imatinib mesylate (Figure 6 and data not shown). Another well-established Akt substrate, GSK-3β, was also phosphorylated in a PI3K-dependent manner in v-ABL and BCR-ABL cell lines (Figure 6C). These observations indicate that PI3K signaling through Akt does occur in pro/pre-B cells transformed by ABL oncogenes, but it is not demonstrably affected by loss of Pik3r1 gene products.
In summary, the biochemical data indicate that PI3K-dependent (LY294002-sensitive) phosphorylation of certain Akt substrates occurs in these cells, even though Akt appears only marginally activated. Regulatory isoforms other than p85α are expressed and are sufficient to maintain PI3K signaling output as measured by expression of catalytic subunits, association with tyrosine-phosphorylated signaling complexes, and phosphorylation of downstream targets.
Functional importance of FOXO inactivation
Expression of a PI3K-independent form of FOXO3a that lacks the Akt phosphorylation sites (FOXO3a(A3)) was reported to induce cell cycle arrest and apoptosis in human CML cell lines or IL-3-dependent BaF3 cells.37,38 To determine if FOXO phosphorylation also plays a critical role in proliferation or survival of pro/pre-B cells transformed by v-ABL or p190-BCR-ABL, cells were infected with retroviruses expressing FOXO3a wild-type or FOXO3a(A3). Cell death was not increased at early time points (24 hours) following FOXO3a expression (data not shown). However, by 48 hours there was a marked increase in apoptosis in cells transduced with FOXO3a(A3) (Figure 7). Expression of wild-type FOXO3a caused an intermediate increase in the fraction of dying cells. Similar results were seen in v-ABL and BCR-ABL cells regardless of Pik3r1 genotype (Figure 7), although the knock-out v-ABL cell line shown was somewhat less sensitive to the effects of FOXO3a and FOXO3a(A3). The increased death with FOXO3a(A3) relative to wild-type FOXO3a supports the model that PI3K/Akt signaling is sufficiently active in these cells to partially inactivate the overexpressed wild-type FOXO3a protein.
Overexpression of FOXO protein promotes apoptosis. Transformed pro/pre-B cells were retrovirally infected with pMIT, FOXO3a, or FOXO3a(A3) and analyzed by FACS 48 hours after infection. The extent of apoptosis in cells with different levels of Thy1.1 expression was determined by Annexin V staining. The numbers in the upper-right quadrant represent the fraction of Thy1.1-positive cells that is Annexin V-positive. Data are representative of 3 experiments and 1 to 2 cell lines per genotype.
Overexpression of FOXO protein promotes apoptosis. Transformed pro/pre-B cells were retrovirally infected with pMIT, FOXO3a, or FOXO3a(A3) and analyzed by FACS 48 hours after infection. The extent of apoptosis in cells with different levels of Thy1.1 expression was determined by Annexin V staining. The numbers in the upper-right quadrant represent the fraction of Thy1.1-positive cells that is Annexin V-positive. Data are representative of 3 experiments and 1 to 2 cell lines per genotype.
Discussion
Several reports have indicated that PI3K is required for BCR-ABL-mediated myeloid transformation, or conversion of model cell lines to growth factor-independence.18,19 It has been reported that PI3K and Akt are active in primary murine B lymphoblasts transformed with BCRABL20,22 ; however, the role of PI3K signaling in the transformed phenotype was not investigated directly. We have shown that a PI3K inhibitor blocks initial transformation of pro/pre-B cells by v-ABL or p190-BCR-ABL, and inhibits proliferation and survival of established cell lines. In addition, we have used a dominant-negative p85 construct to establish a specific role for class IA PI3K function in survival of these cells. We have shown that FOXO proteins are phosphorylated in ABL-transformed pro/pre-B cells and that expression of the PI3K-independent FOXO3a(A3) causes a marked increase in apoptosis. As the wild-type FOXO3a had only a partial effect, these findings support a role for continued PI3K signaling to FOXO inactivation as a mechanism for survival in B-lineage cells transformed by ABL oncogenes.
The ubiquitous expression and function of PI3K will likely prevent the use of global inhibitors for chronic treatment. Determining if specific PI3K isoforms are required in leukemic cells may make it possible to develop more selective inhibitors for treatment of Abl-dependent disease. A previous study demonstrated that antisense inhibition of expression of p85α decreased proliferation and colony formation of primary human CML cells and several model cell lines expressing p210-BCR-ABL.18 The data presented here demonstrate that p85α (and/or p55α, p50α) also contributes to optimal transformation of primary murine B-lineage cells by p190-BCR-ABL. However, Pik3r1 gene products are dispensable for v-ABL transformation. The apparently lesser role of p85α and its variants in murine B-lineage transformation relative to human CML cells might be the result of several factors. One variable is that v-Abl and the p190 isoform of Bcr-Abl are more active kinases with greater oncogenic potential relative to p210,4 so compensatory signaling pathways may be more strongly triggered. There may also be differences in expression of alternative regulatory isoforms in the different cell lineages or species studied. We do not believe that the p85β isoform is more critical for ABL transformation in this system, as no defects were observed in transformation of bone marrow cells from Pik3r2 (p85β)-deficient mice (data not shown). Our future studies will investigate whether combined deletion of Pik3r1 and Pik3r2 impairs transformation. However, this might not fully impair class IA PI3K signaling as the p55γ isoform (Pik3r3) is expressed in ABL-transformed pro/pre-B cells regardless of Pik3r1 genotype (Figure 4).
Class IA regulatory subunits are required for stabilization of p110 catalytic subunits, association of the enzyme with phosphotyrosine-containing signaling complexes, and allosteric activation.11 Consistent with the likely redundant functions of p85β and/or p55γ in cells lacking p85α/p55α/p50α, class IA PI3K catalytic (p110) subunit expression was relatively unaltered in the transformed cell lines when compared with the fetal liver cells taken prior to transduction. Furthermore, PI3K activity associated with phosphotyrosine-containing signaling complexes was only modestly diminished in the Pik3r1 null cells. In addition, we found no consistent differences between wild-type and Pik3r1 null cells in the phosphorylation of Akt, S6K (data not shown), or the Akt substrates GSK3β and FOXO1. Thus, PI3K signaling appeared to be intact in the absence of p85α. It should be noted that class IB PI3K (the p110γ isoform) might contribute to PI3K signaling output in this system, as it can be activated by ligands present in serum, whose presence was found to be required for survival of these cells (Figure 3D).
Stimulation of Akt phosphorylation is considered a hallmark of PI3K activation and has been demonstrated in a great number of cellular contexts.12,13,34 However, despite the requirement for continued PI3K signaling in our system, we detected very low levels of phosphorylated Akt in transformed cell lines. Of note, Akt phosphorylation was somewhat more prominent in bone marrow cells isolated from mice with leukemic disease (data not shown). In studies of ABL oncogenes, Akt activation has been reported in a mast cell line bearing a temperature-sensitive v-ABL variant, and in BaF3 cells and primary myeloid or B-lymphoblastoid cells transduced with p210-BCR-ABL.17,22 In contrast, we recently showed that in hematopoietic precursors differentiated from embryonic stem cells, inducible expression of p210-BCR-ABL did not lead to appreciable increases in phosphorylated Akt.24 Thus, the maintenance of high-level Akt activation appears dependent on cell context. Regardless, the wild-type or Pik3r1 null cells showed abundant LY294002-sensitive phosphorylation of 2 known Akt substrates, GSK3β and FOXO1. It is possible that these targets may be phosphorylated by kinases of the Sgk family, which are also PI3K regulated and whose substrate selectivity overlaps with Akt.39
Although we have shown that p85α, p55α, and p50α regulatory isoforms are dispensable for transformation by v-ABL, Pik3r1 gene products do appear to contribute quantitatively to colony formation and leukemogenesis by p190-BCR-ABL. It is worth testing whether other PI3K components have nonredundant functions. In particular, the catalytic isoforms p110γ and p110δ are specifically expressed in leukocytes and may be attractive drug targets.40
Prepublished online as Blood First Edition Paper, February 19, 2004; DOI 10.1182/blood-2003-07-2193.
Supported by National Institutes of Health grants (AI50831 to D.A.F., CA76204 to O.N.W, and CA24220 to N.R) and a New Investigator Award from the Leukemia Research Foundation (D.A.F.). O.N.W. is an Investigator of the Howard Hughes Medical Institute.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to Lewis Cantley for initial support of this project. We thank Kay Lee-Fruman, Robert Hawley, Philippa Marrack, Boudewijn Burgering, and Tetsuo Shioi for kind gifts of reagents; Stefan Kaluz for assistance with PI3K assays; and James Johnson, Amber Donahue, Pratibha Sareen, and Travis Moore for genotyping help and mouse work.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal