Abstract
Tgf-β1-/- mice develop a progressive, lethal, inflammatory syndrome, but mechanisms leading to the spontaneous activation of Tgf-β1-/- T cells remain unclear. Here we show the disruption of CD28 gene expression accelerates disease in Tgf-β1-/- mice, and we link this increase in severity to a reduction in the number of CD4+CD25+ regulatory T cells. CD4+CD25+ T cells develop normally in Tgf-β1-/- mice and display characteristic expression of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), glucocorticoid-induced tumor necrosis factor receptor (GITR), αEβ7 integrin, and Foxp3. Adoptive transfer of Tgf-β1-/- splenocytes to Tgf-β1+/+/Rag2-/- mice induced an autoimmune inflammatory disease with features similar to those of the Tgf-β1-/- phenotype, and disease transfer was accelerated by the depletion of Tgf-β1-/- CD4+CD25+ T cells from donor splenocytes. Cotransfer of Tgf- β1-/- CD4+CD25+ T cells clearly attenuated disease in Rag2-/- recipients of CD25+-depleted Tgf-β1-/- spleen and lymph node cells, but suppression was incomplete when compared with Tgf-β1+/+ CD4+CD25+ T cells. These data demonstrate that CD4+CD25+ regulatory T cells develop in complete absence of endogenous transforming growth factor-β1 (TGF-β1) expression and that autocrine TGF-β1 expression is not essential for these cells to suppress inflammation in vivo. (Blood. 2004;103:4594-4601)
Introduction
Transforming growth factor-β1 (TGF-β1) plays a pivotal role in the regulation of immune cells.1,2 Mice deficient in Tgf-β1 gene expression (Tgf-β1-/-) exhibit severe multifocal inflammation, which begins in the neonatal period and results in early death at the age of 3 weeks.3-6 The aberrant activation of CD4+ and CD8+ T cells has been implicated in the pathogenesis of this syndrome by studies involving immune cell depletion either by intercrossing Tgf-β1-/- mice with lymphocyte-related gene-deficient mice or by in vivo lymphocyte depletion with T cell-specific antibodies.7-9 The involvement of T cells in this syndrome is also suggested by the observation that T cell-specific expression of a radically truncated dominant-negative TGF-β type II receptor results in autoimmune and lymphoproliferative disorders.10,11 Taken together, these results clearly demonstrate the importance of T-cell regulation by TGF-β1. However, precise mechanisms of spontaneous T-cell activation in the absence of TGF-β1 production by T cells remain unknown.
Complete activation of T lymphocytes requires at least 2 signals, one delivered by the T-cell receptor complex after antigen recognition and one provided on engagement of costimulatory receptors. It is widely accepted that the dysregulation of costimulation contributes to the initiation and maintenance of autoimmunity consequent to the activation of self-reactive T cells.12 CD28, which binds to B7-1 (CD80) and B7-2 (CD86) on antigen-presenting cells, is arguably the most effective costimulatory molecule. Disruption of CD28/B7 costimulation has been shown to attenuate the severity of inflammation in some autoimmune disease models. However, the disruption of CD28 has been reported to cause an exacerbation of disease manifestations in an experimental model of autoimmune diabetes in a manner attributed to the impaired development of CD4+CD25+ T-regulatory cells.13
CD4+CD25+ T cells are naturally arising regulatory T cells that play a key role in the maintenance of immunologic tolerance, and they suppress the activation and proliferation of other T cells through a mechanism that requires cell-cell contact.14,15 TGF-β1 has been implicated in the development,16 and as a mediator, of the suppressor function of these regulatory T cells.17,18 However, the in vivo significance of autocrine TGF-β1 in the development and function of CD4+CD25+ T cells is unclear and remains a point of great interest.19-22
In the present study, we show that the disruption of CD28 gene expression in Tgf-β1-/- mice accelerates the inflammatory process and shortens their survival. We link this disease exacerbation to the inherent decrease in the number of CD4+CD25+ regulatory T cells that associates with CD28 deficiency by demonstrating the capacity of Tgf-β1-/- CD4+CD25+ T cells to suppress inflammation induced by the adoptive transfer of Tgf-β1-/- spleen and lymph node cells into RAG2-/- recipients. These data suggest that the CD4+CD25+ regulatory T cells that develop in the complete absence of TGF-β1 gene expression retain the ability to suppress inflammation in vivo.
Materials and methods
Generation of Tgf-β1-/-/CD28-/- mice
Tgf-β1+/- mice used in this study were generated by gene targeting, as previously described.3 CD28-/- mice23 were obtained from The Jackson Laboratory (Bar Harbor, ME). These mice were crossed to generate founder (Tgf-β1+/-/CD28+/-) mice, which were then intercrossed to generate Tgf- β1 × CD28 double-null (Tgf-β1-/-/CD28-/-) mice. Mice were genotyped for Tgf-β1 and CD28 alleles by PCR, as described.3,23 Tgf-β1+/- mice were generated in the mixed genetic background of C57BL/6 and 129/SvJ strains, whereas CD28-/- mice were of C57BL/6 background. Littermates were used as controls for all experiments. All animal experiments were approved by the National Cancer Institute (NCI) Animal Care and Use Committee.
Pathology and immunohistochemical analysis
Tissues were harvested from moribund Tgf-β1-/-/CD28-/- mice and control Tgf-β1+/+/CD28-/- mice, all humanely killed by CO2 narcosis. Tissues were fixed in 10% buffered formalin (Sigma, St Louis, MO), embedded in paraffin, and sectioned for staining with either hematoxylin and eosin (H&E) or immunohistochemical staining with anti-CD3. For immunohistochemical staining with anti-CD4 and anti-CD8, tissues were frozen in optimum cutting temperature (OCT) compound (Miles Scientific, Naperville, IL). Goat biotinylated antimouse immunoglobulin G (IgG) and Vectastain Elite avidin-biotin complex (ABC) kits (Vector Laboratories, Burlingame, CA) were used for secondary reagents, as previously described.8
Flow cytometric analysis
Single-cell suspensions prepared from spleens, lymph nodes, and thymi were stained and analyzed on FACSCalibur (Becton Dickinson, Mountain View, CA) using CellQuest software. For direct staining to determine the phenotype of lymphocyte populations, the following conjugated antibodies were purchased from BD PharMingen (San Diego, CA): anti-CD69 fluorescein isothiocyanate (FITC), anti-CD25 FITC, phycoerythrin (PE), anti-CD62L PE, anti-CD103 PE, anti-CD44 FITC, anti-CD4 peridin chlorophyll protein (PerCP), allophycocyanin (APC), anti-CD8 PerCP, APC, anti-CD3 APC. Glucocorticoid-induced tumor necrosis factor receptor (GITR)-PE was purchased from R&D Systems (Minneapolis, MN). For intracellular cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), cells were stained with antibodies against surface antigens, then were fixed with Cytofix/Cytoperm (BD PharMingen) and stained with CTLA-4 PE. Before staining, Fc receptors were blocked with anti-CD16/32 antibody (2.4G2; BD PharMingen).
Purification of CD4+CD25+ and CD4+CD25- T cells
Single-cell suspensions of spleen and lymph nodes were prepared from either Tgf-β1-/- mice or control Tgf-β1+/+ mice at 10 to 14 days of life. CD4+ cells were purified by negative selection using CD4+ T-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Total effluent from the LD column was collected as CD4+ cells, which were then incubated with anti-CD25 FITC (clone 7D4) at 4°C for 20 minutes. Cells were washed with phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) and 2 mM EDTA (ethylenediaminetetraacetic acid) and were incubated with FITC magnetic microbeads (Miltenyi Biotec) and passed through the LS columns. The positive fraction captured in the column was flushed out and collected as CD4+CD25+ T cells, and the purity was more than 85%, confirmed by fluorescence-activated cell sorter (FACS) analysis with anti-CD25PE (clone PC61). The effluent from lymphocyte separation (LS) columns was applied on the lymphocyte depletion (LD) column and the elution was collected as CD4+CD25- T cells.
Real-time PCR
Total RNA was extracted from sorted CD4+CD25+ and CD4+CD25- cells using TRIZOL reagent (Invitrogen, Carlsbad, CA). RNA was reverse transcribed using Superscript II reverse transcriptase and oligo(dT)12-18 primer (Invitrogen). Polymerase chain reaction (PCR) amplicons were analyzed with SYBR green I with a LightCycler (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. PCR primer pairs were Foxp3 (5′-CAG CTG CCT ACA GTG CCC CTA G-3′ and 5′-CAT TTG CCA GCA GTG GGT AG-3′) and GAPDH (5′-CAT GTT CTT TAA CCC TGA GGA GTC G-3′ and 5′-CTC GAT CAA TCT TGT GCA G-3′).
Adoptive transfer of CD25+-depleted splenocytes into Rag-2-/- mice
Single-cell suspensions of splenocytes were prepared from either Tgf-β1-/-mice or control Tgf-β1+/+ mice at 8 to 18 days of life. Cells were incubated with anti-CD25 FITC (clone 7D4) at 4°C for 20 minutes in the presence of anti-CD16/32 antibody to block Fc receptors. After washing with PBS containing 0.5% BSA and 2 mM EDTA, cells were incubated with FITC magnetic microbeads and passed through the LD columns. Depletion efficiency was evaluated using FACS with anti-CD25PE (clone PC61). More than 99% of CD4+CD25+ T cells were removed from splenocytes by this procedure. Either CD25+ cell-depleted or unfractionated splenocytes (1 × 107 cells/mouse) from Tgf-β1-/- mice or Tgf-β1+/+ mice (n = 5 for each group) were injected intravenously into Sv129 Rag-2-/- mice (Taconic Farms, Germantown, NY). Four weeks after transfer, mice were treated with 5-bromodeoxyuridine (BrdU) for 16 hours before dissection.
Adoptive transfer of CD25+-depleted Tgf-β1-/- spleen and lymph node cells and CD4+CD25+ T cells into Rag-2-/- mice
The purification of CD4+CD25+ cells from either Tgf-β1-/- mice or Tgf-β1+/+mice and of CD25+-depleted Tgf-β1-/- spleen and lymph node cells was performed as described for methods for purification of CD4+CD25+ T cells. Tgf-β1-/- CD25- cells (1 × 107 cells/mouse) were injected intravenously into Sv129 Rag-2-/- mice (Taconic) alone, with Tgf-β1-/- CD4+CD25+ cells, or with Tgf-β1+/+ CD4+CD25+ cells (2.5 × 105 cells/mouse). Three weeks after transfer, mice were treated with BrdU for 1 hour before dissection (n = 5 for each group). For some experiments, CD4+CD25+ cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE), and recipients were analyzed 4 weeks later (n = 4 for Tgf-β1-/- CD25- cells alone; n = 3 for Tgf-β1-/- CD25- cells with Tgf-β1-/- CD4+CD25+ cells or Tgf-β1+/+ CD4+CD25+ cells).
In vivo labeling of mouse cells with BrdU
Mice were injected intraperitoneally with 1 mg BrdU solution. Mice were humanely killed after injection, as described previously, and spleen and lymph node cells were stained with fluorescent antibodies for cell-surface markers (anti-CD4 APC, anti-CD8 APC). BrdU staining was performed using a BrdU Flow kit (BD PharMingen) according to the manufacturer's instructions. Cells were fixed, permeabilized, and treated with DNase I (Sigma) for 1 hour at 37°C. After wash, cells were stained with anti-BrdU FITC and analyzed on FACSCalibur.
CFSE labeling
CD4+CD25+ cells purified from either Tgf-β1-/- or Tgf-β1+/+ mice were labeled with 0.3 μM CFSE (Molecular Probes, Eugene, OR) by incubation for 8 minutes at room temperature with agitation. Cell suspensions were incubated with equal volumes of fetal calf serum (FCS) for 1 minute and were washed 3 times with PBS.
Serum biochemistry profiles
Serum levels of alanine aminotransferase (ALT) and albumin were measured by staff in the Department of Laboratory Medicine, Clinical Center, National Institutes of Health (Bethesda, MD).
Data analysis
Data are summarized as mean ± SD. Results were statistically analyzed using the unpaired t test. Survival was analyzed using a log-rank nonparametric test and was expressed as Kaplan-Meier survival curves. P values less than .05 were considered significant.
Results
CD28 disruption exacerbates inflammation and decreases survival of Tgf-β1-/- mice
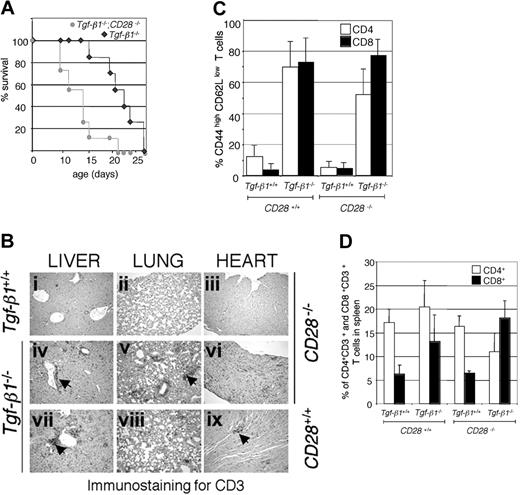
CTLA-4-deficient mice develop an autoimmune inflammatory syndrome that exhibits some similarities to that seen in Tgf-β1-/- mice. Blocking CD28/B7 costimulation by CTLA-4 immunoglobulin treatment is known to prevent lymphoproliferation and multiorgan inflammation in CTLA-4-/- mice.24,25 However, treating Tgf-β1-/- mice (4 to 6 days old) with the same regimen of CTLA-4 immunoglobulin (20 μg/mouse) neither attenuated the severity of inflammation nor improved survival (data not shown). To unequivocally disrupt CD28/B7 costimulation, we crossed Tgf-β1-/- mice with mice carrying a null mutation in the gene encoding CD28 (CD28-/-).23 Disruption of CD28 gene expression did not improve the survival of Tgf-β1-/- mice. On the contrary, we observed a statistically significant decrease in the survival of Tgf-β1-/- mice on the CD28-/- background (Figure 1A). Extensive infiltration of CD3+ T cells was observed in vital organs such as lung, heart, and liver in Tgf-β1-/-/CD28-/- mice, and inflammation in vital organs in them developed more rapidly than in Tgf-β1-/- mice (13 days old; age-matched images in Figure 1B). There was a predominance of peripheral T cells with a CD44highCD62Llow phenotype in Tgf-β1-/- and in Tgf-β1-/-/CD28-/- mice (Figure 1C). Expansion of CD8+ T cells, which is frequently observed in Tgf-β1-/- mice, was more prominent in the spleens of Tgf-β1-/-/CD28-/- mice (Figure 1D; percentages of CD4+CD3+ and CD8+CD3+ cells in total spleen are shown). These results show an increase in the severity of inflammation in Tgf-β1-/- mice in the absence of CD28 expression.
Disruption of CD28 resulted in shorter survival and accelerated infiltration of CD3+ T cells in vital organs in Tgf-β1-/- mice. (A) Survival curves of Tgf-β1-/-/CD28-/- mice and Tgf-β1-/- mice (n = 7). A significant reduction in survival (P = .004 with log-rank test) was observed by the cross of Tgf- β1-/- mice with CD28-/- mice. (B) CD3 immunostained sections from liver, lung, and heart from 13-day-old Tgf-β1+/+/CD28-/- mice (i-iii), Tgf-β1-/-/CD28-/- mice (iv-vi), and Tgf-β1-/-/CD28+/+ mice (vii-ix) are shown. Original magnification, × 400. Arrowheads indicate CD3+ T-cell infiltrates. (C) Expression of memory/activation markers on CD4+ and CD8+ T cells was not significantly altered by the disruption of CD28 in Tgf-β1-/- mice. The percentages of CD62LlowCD44high T cells are shown (n = 6 for Tgf-β1+/+/CD28+/+, Tgf-β1-/-/CD28+/+, Tgf-β1-/-/CD28-/-;n = 4 for Tgf-β1+/+/CD28-/-). (D) Disruption of CD28 resulted in CD8+ T-cell expansion in Tgf-β1-/- mice (n = 10 for Tgf-β1+/+/CD28+/+ and Tgf-β1-/-/CD28+/+ mice; n = 5 for Tgf-β1+/+/CD28-/- mice; and n = 7 for Tgf-β1-/-/CD28-/-). Percentages of CD3+CD4+ and CD3+CD8+ T cells in spleens are shown.
Disruption of CD28 resulted in shorter survival and accelerated infiltration of CD3+ T cells in vital organs in Tgf-β1-/- mice. (A) Survival curves of Tgf-β1-/-/CD28-/- mice and Tgf-β1-/- mice (n = 7). A significant reduction in survival (P = .004 with log-rank test) was observed by the cross of Tgf- β1-/- mice with CD28-/- mice. (B) CD3 immunostained sections from liver, lung, and heart from 13-day-old Tgf-β1+/+/CD28-/- mice (i-iii), Tgf-β1-/-/CD28-/- mice (iv-vi), and Tgf-β1-/-/CD28+/+ mice (vii-ix) are shown. Original magnification, × 400. Arrowheads indicate CD3+ T-cell infiltrates. (C) Expression of memory/activation markers on CD4+ and CD8+ T cells was not significantly altered by the disruption of CD28 in Tgf-β1-/- mice. The percentages of CD62LlowCD44high T cells are shown (n = 6 for Tgf-β1+/+/CD28+/+, Tgf-β1-/-/CD28+/+, Tgf-β1-/-/CD28-/-;n = 4 for Tgf-β1+/+/CD28-/-). (D) Disruption of CD28 resulted in CD8+ T-cell expansion in Tgf-β1-/- mice (n = 10 for Tgf-β1+/+/CD28+/+ and Tgf-β1-/-/CD28+/+ mice; n = 5 for Tgf-β1+/+/CD28-/- mice; and n = 7 for Tgf-β1-/-/CD28-/-). Percentages of CD3+CD4+ and CD3+CD8+ T cells in spleens are shown.
Characteristics of Tgf-β1-/- CD4+CD25+ regulatory T cells
It is known that the development and homeostatic regulation of CD4+CD25+ regulatory T cells is controlled by CD28/B7 costimulation13,26 and that their number is reduced in the absence of CD28 expression. Therefore, we hypothesized that the exacerbated inflammation in Tgf-β1-/-/CD28-/- mice is a direct result of the reduction of this regulatory population. If true, this would represent direct evidence that CD4+CD25+ T cells can mediate suppression in vivo in a manner independent of autocrine TGF-β1 expression, and this would be consistent with our evaluation of their function in vitro.19 CD4+CD25+ T cells existed at normal ratios in the spleens of Tgf-β1-/- mice (10 to 14 days old) but were significantly reduced in all Tgf-β1-/- mice on the CD28-/- background (Figure 2A-B). Expression of CTLA-4, GITR, and αEβ7 integrin (CD103) has been reported as characteristic of CD4+CD25+ regulatory T cells.18,27-29 Thus, we examined the expression patterns of these molecules in Tgf-β1-/- CD4+CD25+ regulatory T cells from thymus, lymph node, and spleen. Tgf-β1-/- CD4+CD25- T cells constitutively expressed higher levels of intracellular CTLA-4, and its expression level was even higher in the Tgf-β1-/- CD4+CD25+ subset compared with wild-type control (Figure 2C; data from spleen are shown). Expression of GITR was also higher on Tgf-β1-/- CD4+CD25+ T cells than on Tgf-β1+/+ CD4+CD25+ T cells (Figure 2C). However, the expression of αEβ7 integrin (CD103) was slightly lower on Tgf-β1-/- CD4+CD25+ T cells from spleen (Figure 2C) and thymus (data not shown), whereas lymph node CD4+CD25+ T cells expressed the same level with Tgf-β+/+ CD4+CD25+ T cells (data not shown). Because these molecules can be expressed on activated T cells, we next examined expression of the early activation marker CD69 on CD4+CD25+ T cells. The percentage of Tgf-β1-/- CD4+CD25+ T cells expressing CD69 was significantly higher than that in wild-type CD4+CD25+ T cells (Figure 2D; percentages of CD69+ cells in CD4+CD25+ T cells, Tgf-β+/+, 28.0% ± 3.6%; Tgf-β1-/-, 55.4% ± 11.0%), indicating that a proportion of the CD4+CD25+ population observed at this age in Tgf-β1-/- mice may be activated T cells. The forkhead transcription factor Foxp3 has been reported as a key regulatory gene in the development and function of CD4+CD25+ regulatory T cells.20-22 Foxp3 mRNA is expressed at similar levels in Tgf-β1-/- and Tgf-β1+/+ CD4+CD25+ T cells (Figure 2E). These data further characterize the CD4+CD25+ regulatory T cells that develop in the absence of TGF-β1 gene expression in vivo, and they are consistent with our hypothesis regarding the mechanisms responsible for exacerbating inflammation in Tgf-β1-/- mice on a CD28-deficient background.
CD4+CD25+ regulatory T cells are present in Tgf-β1-/- mice but are reduced in number in Tgf-β1-/-/CD28-/- mice. (A) Percentages of CD4+CD25+ T cells in spleens are shown. Each histogram is representative of independent results from 8 mice with either the Tgf-β1+/+/CD28+/+ or the Tgf-β1-/-/CD28+/+ genotype, 6 Tgf-β1-/-/CD28-/- mice, and 4 Tgf-β1+/+/CD28-/- mice. (B) Percentages of CD4+CD25+ T cells in total spleen cells are shown (numbers of mice per genotype same as those described for panel A). P < .005 between Tgf-β1-/-/CD28+/+ and Tgf-β1-/-/CD28-/- genotypes using Student t test. (C) Expression of intracellular CTLA-4, GITR, αE integrin in CD4+CD25- T cells and CD4+CD25+ T cells of spleen from Tgf-β1+/+ mice and Tgf-β1-/- mice (1 representative plot shown; 3 mice evaluated for each genotype). (D) Percentages of CD69+ cells in the CD4+CD25+ T-cell subset of splenocytes from Tgf-β1+/+ mice and Tgf-β1-/- mice. (E) Foxp3 mRNA expression in CD4+CD25- T cells and CD4+CD25+ T cells of Tgf-β1+/+ mice and Tgf-β1-/- mice obtained by real-time PCR. Error bars (B,D) indicate SD.
CD4+CD25+ regulatory T cells are present in Tgf-β1-/- mice but are reduced in number in Tgf-β1-/-/CD28-/- mice. (A) Percentages of CD4+CD25+ T cells in spleens are shown. Each histogram is representative of independent results from 8 mice with either the Tgf-β1+/+/CD28+/+ or the Tgf-β1-/-/CD28+/+ genotype, 6 Tgf-β1-/-/CD28-/- mice, and 4 Tgf-β1+/+/CD28-/- mice. (B) Percentages of CD4+CD25+ T cells in total spleen cells are shown (numbers of mice per genotype same as those described for panel A). P < .005 between Tgf-β1-/-/CD28+/+ and Tgf-β1-/-/CD28-/- genotypes using Student t test. (C) Expression of intracellular CTLA-4, GITR, αE integrin in CD4+CD25- T cells and CD4+CD25+ T cells of spleen from Tgf-β1+/+ mice and Tgf-β1-/- mice (1 representative plot shown; 3 mice evaluated for each genotype). (D) Percentages of CD69+ cells in the CD4+CD25+ T-cell subset of splenocytes from Tgf-β1+/+ mice and Tgf-β1-/- mice. (E) Foxp3 mRNA expression in CD4+CD25- T cells and CD4+CD25+ T cells of Tgf-β1+/+ mice and Tgf-β1-/- mice obtained by real-time PCR. Error bars (B,D) indicate SD.
Depletion of CD4+CD25+ T-regulatory cells accelerates adoptive transfer of the Tgf-β1-/- phenotype
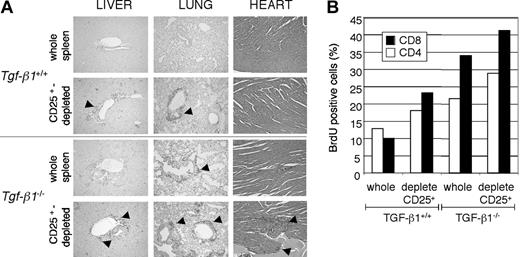
We have previously demonstrated that Tgf-β1-/- CD4+CD25+ regulatory T cells have the capacity to suppress T-cell proliferation in vitro.19 However, the capacity of Tgf-β1-/- CD4+CD25+ regulatory T cells to suppress inflammation caused by Tgf-β1-/- pathogenic cells in vivo has not been demonstrated. To address this question, we performed adoptive transfer using whole splenocytes or CD25+-depleted splenocytes from either Tgf- β1+/+ or Tgf-β1-/- donor mice for transfer into Tgf-β1+/+/Rag2-/- recipient mice. If these regulatory T cells had suppressor function, depleting CD25-expressing cells from the Tgf- β1-/- spleen would accelerate disease transfer in the Rag2-/- recipients. Transfer of the inflammatory Tgf-β1-/- syndrome to Rag2-/- recipients was assessed by several parameters, including histopathology and lymphocyte proliferation in vivo by BrdU uptake. In Rag2-/- recipients of wild-type splenocytes, pathologic evaluation by H&E stain revealed lymphocyte infiltration into vital organs (such as lung, heart, and liver) only when CD25-expressing cells were depleted before transfer, as originally reported14,15 (Figure 3A; 4 weeks after transfer). In contrast, infiltrates of T lymphocytes were readily detectable in soft tissues of Rag2-/- recipients of Tgf-β1-/- whole splenocytes 4 weeks after transfer (Figure 3A). However, recipients of CD25+-depleted Tgf-β1-/- splenocytes were notably more symptomatic and experienced increased severity of inflammation of the lung, liver, and heart (Figure 3A). Histology data correlated with proliferation profiles of lymphocytes in Rag2-/- recipients, with greater incorporation of BrdU by CD4+ and CD8+ T lymphocytes in Rag2-/- recipients of CD25+-depleted Tgf-β1-/- splenocytes compared with that observed in recipients of whole Tgf-β1-/- splenocytes (Figure 3B). Consistent with these data, we also observed more rapid onset of mortality in Rag2-/- recipients of CD25+-depleted Tgf-β1-/- splenocytes (4 weeks) than in Rag2-/- recipients of Tgf-β1-/- whole splenocytes (6 weeks) after cell transfer. There were no deaths in the groups receiving Tgf-β1+/+ whole splenocytes or CD25+-depleted Tgf-β1+/+ splenocytes during the period of evaluation. Exacerbation of disease transfer by depletion of CD4+CD25+ T cells from Tgf-β1-/- splenocytes suggested that this regulatory subset was functional in vivo despite the absence of autocrine TGF-β1 production. However, the fact that the endogenous CD4+CD25+ T cells are insufficient to prevent the onset and progression of inflammation in Tgf-β1-/- mice may suggest a requirement for autocrine TGF-β1 to attain maximal suppression in vivo.
Depletion of CD25-expressing cells from Tgf-β1-/- spleen cells accelerated lymphocyte expansion and inflammation in Rag2-/- recipients. Whole splenocytes or CD25-depleted splenocytes (1 × 107 cells/mouse) from Tgf-β1+/+ or Tgf-β1-/- donor mice were transferred into Rag2-/- recipient mice. (A) H&E-stained sections of heart and CD3 immunostained sections of liver and lung of Rag2-/- recipients of whole or CD25-depleted splenocytes are shown. Original magnification, × 100. (B) BrdU-positive CD4+ and CD8+ cells in spleens from the recipients are shown. One representative result from 5 separate analyses is shown.
Depletion of CD25-expressing cells from Tgf-β1-/- spleen cells accelerated lymphocyte expansion and inflammation in Rag2-/- recipients. Whole splenocytes or CD25-depleted splenocytes (1 × 107 cells/mouse) from Tgf-β1+/+ or Tgf-β1-/- donor mice were transferred into Rag2-/- recipient mice. (A) H&E-stained sections of heart and CD3 immunostained sections of liver and lung of Rag2-/- recipients of whole or CD25-depleted splenocytes are shown. Original magnification, × 100. (B) BrdU-positive CD4+ and CD8+ cells in spleens from the recipients are shown. One representative result from 5 separate analyses is shown.
Absence of autocrine TGF-β1 compromises suppression by CD4+CD25+ T cells in vivo
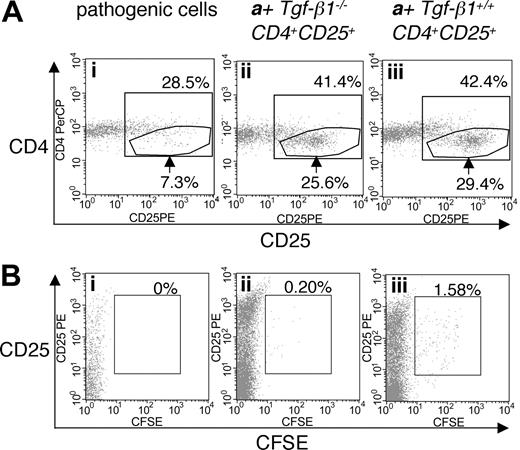
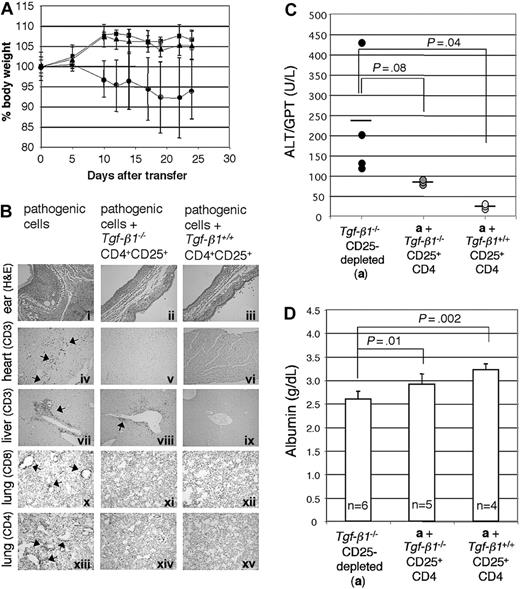
To address the efficacy of Tgf-β1-/- CD4+CD25+-mediated suppression of the inflammatory syndrome in Tgf-β1-/- mice, we used a similar adoptive transfer approach. Tgf-β1-/- spleen and lymph node cells depleted of CD25-expressing cells were transferred into Rag2-/- recipients (as pathogenic cells, 1 × 107 cells/mouse) in the presence or absence of either Tgf-β1-/- CD4+CD25+ T cells or Tgf-β1+/+ CD4+CD25+ T cells (2.5 × 105 cells/mouse), at an effector/suppressor ratio of 40:1. Mice were killed 4 weeks after transfer, when Rag2-/- recipients of CD25+-depleted Tgf-β1-/- splenocytes are overtly symptomatic (recipients with pathogenic cells alone, n = 4; recipients with pathogenic cells plus Tgf-β1-/- CD4+CD25+ T cells or plus Tgf-β1+/+ CD4+CD25+ T cells, n = 3). Cotransfer of CD4+CD25+ regulatory T cells was confirmed by gating the cell population with lower expression of CD4 (Figure 4A).30 CD4+CD25+ T cells were labeled with CFSE to trace their homeostatic proliferation26,30 in Rag2-/- recipients. In lymphopenic recipients, CD4+CD25+ T cells proliferated rapidly, diluting CFSE substantially by 4 weeks. Although a small number of slowly dividing, CFSE-positive Tgf-β1+/+ CD4+CD25+ T cells did persist, CFSE-positive cells were rarely observed in the Rag2-/- recipients of Tgf-β1-/- CD4+CD25+ T cells (Figure 4B). Recipients of pathogenic cells alone lost weight, showing obvious signs of systemic inflammation, but recipients of pathogenic cells plus either Tgf-β1-/- or Tgf-β1+/+ CD4+CD25+ T cells continued to thrive and gained weight (Figure 5A). Histopathology (routine H&E and immunohistochemical stain for T-cell markers) showed that inflammation in skin, lung, heart, liver, cecum, and pancreas was significantly attenuated by the cotransfer of Tgf-β1-/- CD4+CD25+ T cells and was completely suppressed by Tgf-β1+/+ CD4+CD25+ T cells (ears, heart, lung, and liver; shown in Figure 5B). Serum biochemistry data correlated well with histopathology findings and were evaluated to assess and quantify organ damage in the Rag2-/- recipients. Serum levels of ALT, which reflect the severity of liver damage, were high in Rag2-/- recipients of pathogenic cells alone and were completely suppressed to normal range by Tgf-β1+/+ CD4+CD25+ T cells. In contrast, Tgf-β1-/- CD4+CD25+ T cells showed partial suppression (Figure 5C). A similar correlation was observed for serum albumin (Figure 5D), which can reflect the effects of inflammation on liver or renal function or malabsorption caused by intestinal inflammation. These data suggest that Tgf- β1-/- CD4+CD25+ T cells are indeed functional as suppressors but that they have a reduced capacity to exert suppression in vivo when compared with Tgf-β1+/+ CD4+CD25+ T cells for their ability to suppress the inflammatory process associated with the Tgf-β1 gene deletion.
Cotransfer of CD4+CD25+ T cells into Rag2-/- recipient mice with CD25-depleted Tgf-β1-/- cells. (A) Expression of CD25 on CD4+CD3+ T cells isolated from Rag2-/- recipients of CD25-depleted Tgf-β1-/- cells (n = 4) with cotransfer of either Tgf-β1-/- CD4+CD25+ T cells (ii) or Tgf-β1+/+ CD4+CD25+ T cells (iii) (n = 3), or without cotransfer (i). (B) CFSE labeling of CD4+CD25+ T cells in the recipient mice described in panel A. Dot plots are shown for CD4+CD3+-gated populations, and are representative of the groups described above. Percentages over the inner box gate indicate total CD4+CD25+ T cells. Arrows indicate CD4+ T cells with high expression of CD25+. Percentages in panel B indicate percentage of CD4+CD25+ T cells positive for CFSE.
Cotransfer of CD4+CD25+ T cells into Rag2-/- recipient mice with CD25-depleted Tgf-β1-/- cells. (A) Expression of CD25 on CD4+CD3+ T cells isolated from Rag2-/- recipients of CD25-depleted Tgf-β1-/- cells (n = 4) with cotransfer of either Tgf-β1-/- CD4+CD25+ T cells (ii) or Tgf-β1+/+ CD4+CD25+ T cells (iii) (n = 3), or without cotransfer (i). (B) CFSE labeling of CD4+CD25+ T cells in the recipient mice described in panel A. Dot plots are shown for CD4+CD3+-gated populations, and are representative of the groups described above. Percentages over the inner box gate indicate total CD4+CD25+ T cells. Arrows indicate CD4+ T cells with high expression of CD25+. Percentages in panel B indicate percentage of CD4+CD25+ T cells positive for CFSE.
Tgf-β1-/- CD4+CD25+ T cells attenuate inflammation in Rag2-/- recipients of CD25-depleted Tgf-β1-/- cells. (A) The percentage body weight (relative to start weight) of Rag2-/- recipient mice is shown (Tgf-β1-/- CD25-depleted; •) and indicates suppression of disease transfer by Tgf-β1-/- (▪) and Tgf-β1+/+ (▴) CD4+CD25+ T cells. (B) H&E-stained sections of ears (i-iii) show diminished inflammation and swelling in both groups of Rag2-/- recipients receiving suppressor cells (ii-iii); immunohistochemical staining for CD3 in the heart (iv-vi) and liver (vii-ix) also show suppression of infiltration of T cells into these organs; immunohistochemical staining of lung sections for CD8 (x-xii) and of CD4 (xiii-xv) reveals tissue infiltrates of each subset and suppression by Tgf-β1-/- and Tgf-β1+/+ CD4+CD25+ T cells. Original magnifications: × 100 (i-iii); × 400 (iv-xv). (C) Serum ALT levels of Rag2-/- recipients are shown. Horizontal bars indicate average value. (D) Serum albumin levels of Rag2-/- recipients are shown. Error bars (A, C-D) indicate SD.
Tgf-β1-/- CD4+CD25+ T cells attenuate inflammation in Rag2-/- recipients of CD25-depleted Tgf-β1-/- cells. (A) The percentage body weight (relative to start weight) of Rag2-/- recipient mice is shown (Tgf-β1-/- CD25-depleted; •) and indicates suppression of disease transfer by Tgf-β1-/- (▪) and Tgf-β1+/+ (▴) CD4+CD25+ T cells. (B) H&E-stained sections of ears (i-iii) show diminished inflammation and swelling in both groups of Rag2-/- recipients receiving suppressor cells (ii-iii); immunohistochemical staining for CD3 in the heart (iv-vi) and liver (vii-ix) also show suppression of infiltration of T cells into these organs; immunohistochemical staining of lung sections for CD8 (x-xii) and of CD4 (xiii-xv) reveals tissue infiltrates of each subset and suppression by Tgf-β1-/- and Tgf-β1+/+ CD4+CD25+ T cells. Original magnifications: × 100 (i-iii); × 400 (iv-xv). (C) Serum ALT levels of Rag2-/- recipients are shown. Horizontal bars indicate average value. (D) Serum albumin levels of Rag2-/- recipients are shown. Error bars (A, C-D) indicate SD.
Splenomegaly was also observed only in Rag2-/- recipients of pathogenic cells alone (Figure 6A). To determine the effect of CD4+CD25+ T cells on T-cell proliferation in vivo, mice were humanely killed 3 weeks after transfer (n = 5 for each group). Spleen cell numbers and incorporation of BrdU by CD4+ and CD8+ cells in Rag2-/- recipients followed the same hierarchy, with proliferation suppressed most effectively by Tgf-β1+/+ CD4+CD25+ T cells (Figure 6B). Although the expression of CD69 on CD4+ T cells is not significantly different among the recipient groups, the percentage of CD69+CD8+ cells is significantly reduced by cotransfer of either Tgf-β1+/+ or Tgf-β1-/- CD4+CD25+ T cells (Figure 6C). The expression of CD25 on CD4+ and CD8+ T cells followed a pattern similar to that for CD69expression, with cotransfer of either Tgf-β1+/+ or Tgf- β1-/- CD4+CD25+ T cells leading to a significant reduction in the number of CD25+CD8+ cells (Figure 6D). CD8+ T-cell expansion in the recipients of pathogenic cells alone is similar to that observed in the Tgf-β1-/-/CD28-/- mice (Figure 1D) and was suppressed by cotransfer of either Tgf-β1-/- or Tgf-β1+/+ CD4+CD25+ T cells. Taken together, these data show the capacity of Tgf-β1-/- CD4+CD25+ T cells to suppress inflammation in vivo, but they suggest that there may be a requirement for autocrine TGF-β1 to attain maximal suppression.
Cotransfer of Tgf-β1-/- CD4+CD25+ T cells partially suppressed the activation and proliferation of T lymphocytes. (A) Spleens of Rag2-/- recipients are shown. (B) Lymphocyte proliferation in spleen of Rag2-/- recipient mice was determined by BrdU uptake. The numbers of BrdU-positive CD4+ and CD8+ cells in spleens of Rag2-/- recipient mice are shown. (C) The numbers of CD69+ CD4+CD3+ and CD8+CD3+ cells in spleens of Rag2-/- recipient mice are shown. (D) The numbers of CD25+ CD4+CD3+ and CD8+CD3+ cells in spleens of Rag2-/- recipient mice are shown. Error bars (B-D) indicate SD.
Cotransfer of Tgf-β1-/- CD4+CD25+ T cells partially suppressed the activation and proliferation of T lymphocytes. (A) Spleens of Rag2-/- recipients are shown. (B) Lymphocyte proliferation in spleen of Rag2-/- recipient mice was determined by BrdU uptake. The numbers of BrdU-positive CD4+ and CD8+ cells in spleens of Rag2-/- recipient mice are shown. (C) The numbers of CD69+ CD4+CD3+ and CD8+CD3+ cells in spleens of Rag2-/- recipient mice are shown. (D) The numbers of CD25+ CD4+CD3+ and CD8+CD3+ cells in spleens of Rag2-/- recipient mice are shown. Error bars (B-D) indicate SD.
Discussion
We have shown that the disruption of CD28 in Tgf-β1-/- mice neither prevents nor ameliorates the inflammatory syndrome of TGF-β1 deficiency but, instead, exacerbates the inflammatory process. This clearly contrasts with findings in CTLA-4-/- mice, in which T cell-dependent autoimmune disease and lymphoproliferation are totally prevented by interference with CD28/B7 costimulation,24,25 suggesting that the underlying mechanisms leading to spontaneous T-cell activation differ between Tgf-β1-/- mice and CTLA-4-/- mice.
It has been reported that costimulation with CD28/B7 plays a major role in eliminating autoreactive T cells.13,31 A reduction in the number of CD4+CD25+ regulatory T cells has been linked to disease exacerbation in an autoimmune diabetes model when bred onto a CD28-/- and B7-/- background.13 A significant decrease in CD4+CD25+ T cells also exists in Tgf-β1-/-/CD28-/- mice compared with Tgf-β1-/-/CD28+/+ mice (Figure 2A-B). The reduction in the number of CD4+CD25+ regulatory T cells may contribute to the in vivo expansion of CD8+ T cells in Tgf-β1-/- mice on the CD28-deficient background. Although this effect could also be attributed to a reduced threshold for activation, given the loss of CTLA-4 expression associated with CD28 disruption (data not shown and Gajewski et al32 ), the expansion of CD8+ T cells in Rag2-/- recipients of pathogenic cells alone (Figure 6) suggests the loss of regulatory cells is a critical event.33 More important, our adoptive transfer studies show an exacerbation of Tgf-β1-/- inflammatory disease by the depletion of Tgf-β1-/- CD4+CD25+ T cells, and they also demonstrate the ability of Tgf-β1-/- CD4+CD25+ T cells to attenuate inflammatory disease induced by Tgf-β1-/- pathogenic cells in vivo. These observations are consistent with our hypothesis that the significantly shorter survival and the more aggressive autoimmune inflammation of CD28-deficient Tgf-β1-/- mice are the consequences of decreased numbers of CD4+CD25+ regulatory T cells in this background.
TGF-β has recently been implicated in the development of and in mediating the suppressor function of CD4+CD25+ regulatory T cells.16-18 If TGF-β1 were absolutely essential for the development or function of these cells, one might attribute the autoimmune inflammatory syndrome in Tgf-β1-/- mice to a defect or a deficiency of function in this regulatory population. Here we report that CD4+CD25+ T cells were present at a normal ratio in Tgf-β1-/- mice, and we demonstrate the characteristic expression of CTLA-4, GITR, αE integrin, and Foxp3 in freshly isolated Tgf-β1-/- CD4+CD25+ cells. The authors of recent reports documenting Foxp3 as a key regulatory gene for the development and function of CD4+CD25+ regulatory T cells failed to find a link between TGF-β1 and the suppressor function of CD4+CD25+ regulatory T cells in vitro.20,22 Although we have found that Tgf-β1-/- CD4+CD25+ cells are comparable in phenotype to wild-type CD4+CD25+ regulatory T cells, there appears to be a difference in their capacity to mediate suppression in vitro and in vivo. These data can be interpreted in several ways. One might conclude that CD4+CD25+ regulatory T cells require autocrine TGF-β1 for full suppressor function in vivo. Given the ability of Tgf-β1-/- CD4+CD25+ cells to exert suppression in vitro, the differences observed here may reflect a requirement for TGF-β1 to modulate or maintain expression of receptors or cell adhesion molecules that are important for trafficking the regulatory cell to sites of inflammation, a question currently under investigation. It is also possible that the CD4+CD25+ T-cell pool is a heterogeneous population in Tgf-β1-/- mice and that it includes the CD25+ regulatory cells and the CD25+ activated effector cells, as suggested by the more abundant CD69 expression in Tgf-β1-/- CD4+CD25+ T cells. Although this question might best be approached through analysis of Tgf-β1-/- mice during the early postnatal period, before the onset of symptoms, the embryonic lethality and the rapid onset of inflammation associated with Tgf-β1 gene deletion make such analysis difficult to pursue. Ultimately, strategies that conditionally disrupt Tgf-β1 gene expression in this regulatory T-cell subset may prove the best approach.
In summary, we report the first clear demonstration that CD4+CD25+ regulatory T cells can mediate suppression in vivo, independent of the expression of autocrine TGF-β1. These data support our hypothesis that disruption of CD28 gene expression exacerbates inflammation in Tgf-β1-/- mice because of the reduction of CD4+CD25+ regulatory T cells, and they improve our understanding of the mechanisms responsible for the spontaneous T-cell activation associated with disruption of the TGF-β1 gene in mice.
Prepublished online as Blood First Edition Paper, March 11, 2004; DOI 10.1182/blood-2003-08-2897.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Hidehiro Yamane, Anita Roberts, and Frank Ruscetti for critical reading of the manuscript and for helpful discussions.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal