Abstract
Marrow stromal cells (MSCs) inhibit allogeneic T-cell responses, yet the molecular mechanism mediating this immunosuppressive effect of MSCs remains controversial. Recently, expression of indoleamine 2,3-dioxygenase (IDO), which is induced by interferon-γ (IFN-γ) and catalyzes the conversion from tryptophan to kynurenine, has been identified as a T-cell inhibitory effector pathway in professional antigen-presenting cells. Here we show that human MSCs express IDO protein and exhibit functional IDO activity upon stimulation with IFN-γ. MSCs inhibit allogeneic T-cell responses in mixed lymphocyte reactions (MLRs). Concomitantly, IDO activity resulting in tryptophan depletion and kynurenine production is detected in MSC/MLR coculture supernatants. Addition of tryptophan significantly restores allogeneic T-cell proliferation, thus identifying IDO-mediated tryptophan catabolism as a novel T-cell inhibitory effector mechanism in human MSCs. As IDO-mediated T-cell inhibition depends on MSC activation, modulation of IDO activity might alter the immunosuppressive properties of MSCs in different therapeutic applications. (Blood. 2004;103:4619-4621)
Introduction
Human bone marrow stromal cells (MSCs), more recently referred to as mesenchymal stem cells, are capable of differentiating along multiple mesenchymal lineages in addition to supporting hematopoiesis.1,2 Due to their potential for differentiation into osteocytes, chondrocytes, myocytes, and adipocytes, MSCs have emerged as a promising tool for clinical applications such as tissue engineering and cell and gene therapy.3,4 MSCs are of inherently low immunogenicity and, more importantly, are capable of inhibiting allogeneic T-cell responses.5-8 These intriguing observations have prompted clinical studies to investigate cotransplantation of MSCs in allogeneic hematopoietic stem cell transplantation (HSCT) in order to promote hematopoietic engraftment by preventing host-versus-graft reactivity and to suppress graft-versus-host reactions.9,10 As of yet, the molecular mechanisms responsible for the immunosuppressive effects of MSCs have not been unequivocally identified. The reports describing a potential role of transforming growth factor-β1 and hepatocyte growth factor as mediators of T-cell inhibition remain controversial, but most studies agree that soluble factors are involved.6-8,11 In professional antigen-presenting cells (APCs), expression of indoleamine 2,3-dioxygenase (IDO) induced by interferon-γ (IFN-γ) and other proinflammatory cytokines catalyzes conversion from tryptophan to kynurenine and has recently been identified as a major immunosuppressive effector pathway that inhibits T-cell responses to autoantigens and fetal alloantigens in vivo.12-16 Based on these findings, we investigated whether MSCs exhibit IFN-γ-inducible IDO activity and whether this mechanism contributes to T-cell inhibition mediated by MSCs.
Study design
Culture of human bone marrow-derived MSCs
Bone marrow aspirates were harvested from volunteer donors who had provided informed consent; the study was approved by the institutional review board of the University Clinic, Düsseldorf, Germany. Primary human MSCs were generated as previously described17 except that culture medium was supplemented with 3 ng/mL basic fibroblast growth factor (R&D Systems, Minneapolis, MN).
Mixed lymphocyte reactions (MLRs)
Standard 5-day MLR cultures were set up with 5 × 104 mitomycin C-treated human peripheral blood mononuclear cells (PBMCs) as stimulators and 2 × 105 human T cells purified using sheep red blood cell rosetting as responder cells.5,11 In MSC/MLR coculture experiments, MLRs were performed on a layer of either 5 × 103 or 2 × 104 MSCs seeded one day before. IFN-γ concentration was determined in MSC/MLR coculture supernatants using a commercially available enzyme-linked immunosorbent assay (ELISA; R&D Systems) according to manufacturer's instructions.
Detection of IDO expression and activity
MSCs were stimulated with IFN-γ (R&D Systems) and assayed for IDO expression and function. Standard Western blot analysis for IDO protein expression was performed.18 IDO enzyme activity following IFN-γ stimulation of MSCs was measured by tryptophan-to-kynurenine conversion with photometric determination of kynurenine concentration in the supernatant as the readout.19 In addition, IDO activity was quantified in MSC/MLR cocultures. In MLR experiments, tryptophan and kynurenine concentrations were determined in cell culture supernatant by high-performance liquid chromatography (HPLC; Alliance Separations Module 2690; Waters, Milford, MA). For separation, reversed-phase C18 columns (Supelcosil; Supelco, Bellefonte, PA) were used. Tryptophan was detected by fluorescence at 285-nm excitation and 365-nm emission wavelengths, kynurenine was detected by UV absorption at 360 nm.
Results and discussion
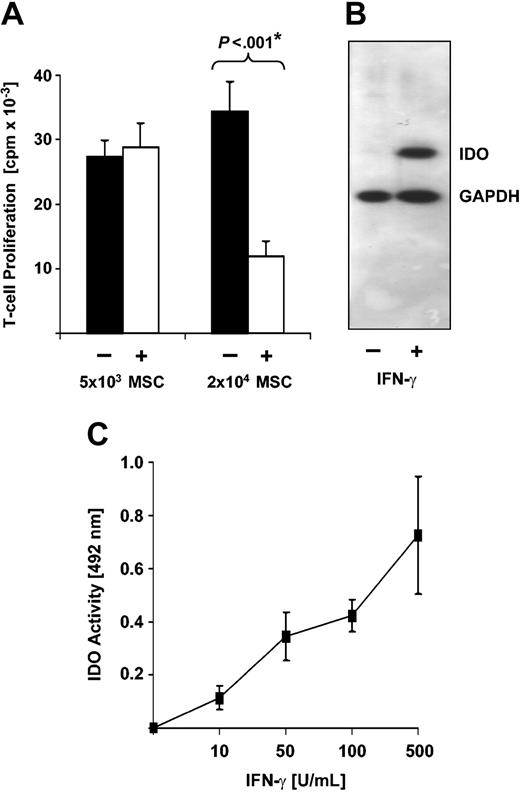
Primary human MSC lines were generated from adherent fraction of bone marrow-derived mononuclear cells. After 2 to 3 passages, MSC cultures consist of a single spindle-shaped cell type devoid of contaminating hematopoietic cells and exhibit an immunophenotype characteristic for human MSCs (CD14-/CD34-/CD45-; CD29+/CD44+/CD90+/CD105+/SH-2+).2 Under appropriate culture conditions, MSCs differentiated along osteogenic and adipogenic lineages as verified by alkaline phosphatase staining and accumulation of lipid-containing droplets, respectively.2 To initially assess whether these MSCs inhibit allogeneic T-cell responses, MLRs were performed on a layer of MSCs. As shown in Figure 1A, the presence of 5 × 103 MSCs had no impact on allogeneic T-cell responses, while 2 × 104 MSCs significantly impaired T-cell proliferation in 7 of 8 experiments to 33% ± 4% of the T-cell response in the absence of MSCs (P < .001). This confirms previous reports describing dose-dependent inhibition of allogeneic T-cell responses by MSCs.5,6,8
Human MSCs inhibit allogeneic T-cell responses and express IDO. (A) Human MSCs inhibit allogeneic T-cell responses in a dose-dependent manner. Responding T cells (1 × 105) were incubated for 5 days with 5 × 104 allogeneic PBMCs as stimulator cells in the presence (+) or absence (-) of 5 × 103 or 2 × 104 human MSCs. Each experiment was performed in triplicate. T-cell proliferation is expressed as averaged means of triplicates ± SEM obtained from a series of 11 experiments with 5 × 103 MSCs from 7 different donors and 7 experiments with 2 × 104 MSCs from 6 different donors. *P value of difference as calculated by Student t test. (B) Human MSCs express IDO protein upon stimulation with IFN-γ. MSCs were cultured in the absence (-) or presence (+) of 500 U/mL IFN-γ. After 3 days, cells were harvested and subjected to Western blot analysis using an IDO-specific monoclonal antibody. As a control, expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was analyzed in parallel. (C) Human MSCs express functional IDO activity upon stimulation with IFN-γ. MSCs (2 × 104) were stimulated with increasing amounts of IFN-γ. After 3 days of culture, supernatant was harvested and IDO activity was measured by determining kynurenine concentration as absorbance (OD) at 492 nm. Background OD (approximately 0.15) was subtracted. Data obtained from 4 independent experiments each using MSCs from different individual donors are presented as mean OD ± SEM.
Human MSCs inhibit allogeneic T-cell responses and express IDO. (A) Human MSCs inhibit allogeneic T-cell responses in a dose-dependent manner. Responding T cells (1 × 105) were incubated for 5 days with 5 × 104 allogeneic PBMCs as stimulator cells in the presence (+) or absence (-) of 5 × 103 or 2 × 104 human MSCs. Each experiment was performed in triplicate. T-cell proliferation is expressed as averaged means of triplicates ± SEM obtained from a series of 11 experiments with 5 × 103 MSCs from 7 different donors and 7 experiments with 2 × 104 MSCs from 6 different donors. *P value of difference as calculated by Student t test. (B) Human MSCs express IDO protein upon stimulation with IFN-γ. MSCs were cultured in the absence (-) or presence (+) of 500 U/mL IFN-γ. After 3 days, cells were harvested and subjected to Western blot analysis using an IDO-specific monoclonal antibody. As a control, expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was analyzed in parallel. (C) Human MSCs express functional IDO activity upon stimulation with IFN-γ. MSCs (2 × 104) were stimulated with increasing amounts of IFN-γ. After 3 days of culture, supernatant was harvested and IDO activity was measured by determining kynurenine concentration as absorbance (OD) at 492 nm. Background OD (approximately 0.15) was subtracted. Data obtained from 4 independent experiments each using MSCs from different individual donors are presented as mean OD ± SEM.
As induction of IDO activity has recently been described as a major T-cell inhibitory mechanism in APC/T-cell interactions,13,14,16 we next investigated whether IDO is also expressed in MSCs. Western blot analysis demonstrates that human MSCs do not constitutively express IDO, but a substantial amount of IDO protein is induced by IFN-γ (Figure 1B). Moreover, in MSCs, IFN-γ stimulates IDO enzyme activity in a dose-dependent manner (Figure 1C). Of note, IDO activity is observed at IFN-γ concentrations as low as 10 to 50 U/mL. This level is readily generated in allogeneic T-cell reactions even in the presence of MSCs, as verified in our experimental setting by measurement of IFN-γ concentration in supernatants of MSC/MLR cocultures with 23.3 ± 7.3 U/mL (mean ± SEM; n = 3).
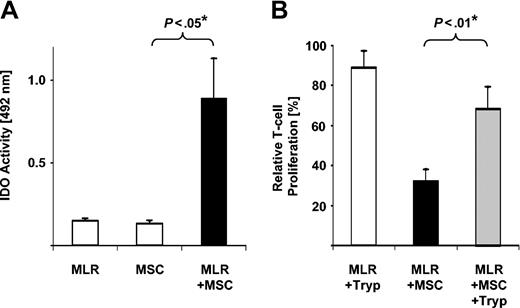
Next, MSC/MLR cocultures set up in parallel to those documenting MSC-mediated T-cell inhibition (Figure 1A) were assessed for IDO activity by measuring tryptophan-to-kynurenine conversion. Significant IDO activity was detected in MSC/MLR cocultures in comparison to background levels observed in MLRs and MSCs alone (Figure 2A). Taken together these data provide evidence that MSCs are the primary source of IDO activity in MSC/MLR cocultures. MSC activation, provided in our study by allogeneic responder T cells, is required for induction of functional IDO activity.
IDO-mediated tryptophan degradation can act as T-cell inhibitory effector pathway in human MSCs. (A) Cocultures of MSCs and MLRs exhibit IDO activity. Supernatants from 4-day cultures of MSCs alone, MLRs alone, or MLR/MSC coculture were harvested and set up for analysis of IDO activity in triplicates. Background OD was not subtracted to allow for comparison of IDO activity of MLRs and MSCs alone. ODs are shown as averaged means of triplicates ± SEM obtained from a total of 5 experiments using MSCs from 4 different donors. *P value of difference as calculated by Student t test. (B) Addition of tryptophan restores T-cell proliferation in MLR/MSC cocultures. MLR cultures were set up in presence (+MSC) or absence of 2 × 104 MSCs. Proliferation is depicted relative to the proliferation of MLR cultures in the absence of MSCs (100%). In parallel experiments, 100 μg/mL tryptophan was added (+Tryp) at the time of initiation and at day 3 of MLR culture or was left out. Relative proliferation is depicted as averaged means of triplicates ± SEM from a series of 5 experiments using MSCs from 4 individual donors. *P value of difference as calculated by Student t test.
IDO-mediated tryptophan degradation can act as T-cell inhibitory effector pathway in human MSCs. (A) Cocultures of MSCs and MLRs exhibit IDO activity. Supernatants from 4-day cultures of MSCs alone, MLRs alone, or MLR/MSC coculture were harvested and set up for analysis of IDO activity in triplicates. Background OD was not subtracted to allow for comparison of IDO activity of MLRs and MSCs alone. ODs are shown as averaged means of triplicates ± SEM obtained from a total of 5 experiments using MSCs from 4 different donors. *P value of difference as calculated by Student t test. (B) Addition of tryptophan restores T-cell proliferation in MLR/MSC cocultures. MLR cultures were set up in presence (+MSC) or absence of 2 × 104 MSCs. Proliferation is depicted relative to the proliferation of MLR cultures in the absence of MSCs (100%). In parallel experiments, 100 μg/mL tryptophan was added (+Tryp) at the time of initiation and at day 3 of MLR culture or was left out. Relative proliferation is depicted as averaged means of triplicates ± SEM from a series of 5 experiments using MSCs from 4 individual donors. *P value of difference as calculated by Student t test.
In previous reports, IDO-mediated tryptophan depletion from culture medium to levels less than 1 μM has been shown to inhibit allogeneic T-cell responses. Consequently, T-cell proliferation can partially be restored by addition of tryptophan.13,14 Therefore, to unequivocally demonstrate IDO-mediated tryptophan degradation in our experimental setup, we performed HPLC analysis in supernatants directly obtained from MSC/MLR cocultures exhibiting MSC-mediated T-cell inhibition. Tryptophan was depleted to less than 0.3 μM from culture medium initially containing 20.2 ± 0.3 μM (mean ± SEM; n = 3). Concomitantly, a significant increase of the tryptophan metabolite kynurenine from 0.4 ± 0.1 to 17.7 ± 4.9 μM was observed in these supernatants. To finally confirm that IDO-mediated tryptophan depletion functions as a T-cell inhibitory effector mechanism in MSC/MLR cocultures, tryptophan repletion experiments were performed. Addition of tryptophan to MSC/MLR cocultures significantly restored T-cell proliferation without any stimulatory capacity of tryptophan on T-cell proliferation in the absence of MSCs (Figure 2B). Restoration of T-cell function was consistently demonstrated in a series of 5 individual experiments using MSCs generated from a total of 4 different donors, even though the degree of restoration was variable. These results demonstrate that IDO-mediated tryptophan depletion can act as a T-cell inhibitory effector mechanism in human MSCs.
Recent data suggest that IDO-facilitated conversion of tryptophan to kynurenine not only depletes tryptophan from the cellular environment but also results in production of kynurenine downstream metabolites that mediate inhibition of T-cell proliferation in their own right.20,21 As a consequence, inhibition of T-cell proliferation mediated by such kynurenine breakdown products is not reversible by tryptophan replacement. Thus, variable reversibility of T-cell proliferation by tryptophan replacement might reflect variable amounts of T-cell inhibitory kynurenine metabolites produced in individual MSC/MLR cocultures. As more than one T-cell inhibitory mechanism seems to play a role in IDO-mediated tryptophan breakdown,20,21 the recently reported failure to achieve significant restoration of T-cell proliferation via tryptophan addition in one MSC line might be attributed to the production of T-cell inhibitory kynurenine metabolites in this experiment.8
IDO expression by tumor cells has recently been shown to prevent T-cell-mediated tumor rejection in vivo.22 In addition, coadministration of MSCs and tumor cells at a distant site resulted in accelerated tumor outgrowth in a murine melanoma model.11 Our finding that MSCs can limit T-cell responses via IDO-mediated tryptophan degradation links these observations. Thus, careful evaluation of potential beneficial and undesirable effects of MSC-mediated inhibition of allogeneic T-cell responses with regard to modulation of graft-versus-host and graft-versus-tumor reactions is warranted. Yet, the identification of IDO as a T-cell inhibitory effector pathway that is not constitutively expressed in MSCs but depends on their activation might allow for modulation of this effector mechanism, when MSCs are used in different therapeutic applications.
Prepublished online as Blood First Edition Paper, March 4, 2004; DOI 10.1182/blood-2003-11-3909.
Supported by German Federal Ministry of Education and Research grant no. 01GN0130 (D.D.), German Research Council grant no. 221/3-1 (W.D.), and “Elterninitiative Kinderkrebsklinik Düsseldorf e.V.”
R.M. and A.Z. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors want to thank D. Klostermann for technical assistance. Bone marrow samples were partially provided by J. Fischer (Institute for Transplantation Diagnostics and Cell Therapy, Düsseldorf University Hospital, Germany), and IDO-specific mouse monoclonal antibody was provided by O. Takikawa (Department of Chemistry and Australian Cataract Research Foundation, University of Wollongton, Australia).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal