Abstract
In vivo evidence suggests that interleukin-18 (IL-18) shapes the development of adaptive immunity toward T-helper cell type 1 (Th1) responses. Monocyte-derived dendritic cells 1 (DC1s) preferentially induce a Th1 response, while plasmacytoid DC-derived DC2s have been linked to a Th2 response. We analyzed the role of IL-18 during the initiation phase of a Th response in vitro to elucidate the basis of these in vivo observations. IL-18 was constitutively released from DC1s, but not DC2s. Neutralization of IL-18 in coculture experiments of DC1s with allogeneic naive T lymphocytes did not alter the Th1/Th2 phenotype, while anti–IL-12 efficiently down-regulated the Th1 response. Unexpectedly, IL-18 receptor (IL-18R) α and β chains were expressed on DC2 lineage. IL-18R expression was functional, as IL-18 induced chemotaxis in plasmacytoid DCs (pre-DC2s) and enhanced the allostimulatory capacity of IL-3–differentiated DC2s. Pre-DC2s exposed to IL-18 skewed the development of Th cells toward Th1 in coculture experiments of DC2s and allogeneic naive T cells, which was inhibited by IL-12 p70 neutralization. IL-18 might have a profound role during the initiation phase of an immune response by recruiting pre-DC2s and modulating the function of DC2s.
Introduction
Interleukin-18 (IL-18) is a polypeptide cytokine identified by its ability to induce interferon-γ (IFN-γ), therefore formerly called IFN-γ–inducing factor (IGIF).1,2 From a structural point of view, IL-18 is related to the IL-1β family of cytokines and shares caspase-1 (IL-1β–converting enzyme [ICE]) for processing pro–IL-18 to bioactive IL-18.3 Furthermore, one of the IL-18 receptor chains (IL-18Rα) is the IL-1 receptor–related protein (IL-1Rrp), a member of the IL-1 receptor family.4 Recently IL-18–binding protein (IL-18BP), a soluble antagonist of IL-18, has been identified.5 IL-18BP shares some homology with the IL-1 type II receptor.5
From a functional point of view, IL-18 might be more related to IL-12.6,7 IL-12 and IL-18 synergize in inducing IFN-γ production in CD4+ T lymphocytes.1,8 IL-18 acts as a costimulant for T-helper type 1 (Th1) cells to augment IL-2, granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-2Rα production, and it induces cell proliferation, whereas IL-18 has no effect on Th2 clones.7 Furthermore, IL-18Rα is selectively expressed on the surface of Th1 cells but not of Th2 cells.9 While IL-12 is well established as a major determinant of development of naive T cells into Th1 cells,10-12 the role of IL-18 in the commitment of naive Th cells to Th1 lineage is less clear. However, data primarily derived from knock-out experiments indicate that IL-18 might indeed favor a Th1 response in vivo, although the cellular/molecular pathway waits to be established.13-20
Induction of primary immune responses and consequently differentiation of naive Th cells into Th1 and Th2 cells depend on the interaction between dendritic cells (DCs) as antigen-presenting cells and naive T cells.21 Two distinct types of DC precursors have been identified in humans: myeloid monocytes (pre-DC1s) and plasmacytoid DC precursors (pre-DC2s).12,22 Pre-DC1's differentiate into DC1's by IL-4 and GM-CSF,23 while pre-DC2's require IL-3 for their development into DC2's.24 Activated myeloid DC1's produce large amounts of IL-12 and preferentially induce Th1 development, while activated lymphoid DC2's preferentially induce Th2 development.12,22,25-28 As outlined above, IL-18 might preferentially support the development of a Th1 response in vivo, but how this is achieved is currently not understood. Therefore, we designed the current study to elucidate the role of IL-18 during the initiation phase of an immune response.
Materials and methods
Reagents
The culture medium used in the present study was RPMI 1640 (Schoeller Pharma, Vienna, Austria) supplemented with 10% heat-inactivated (30 minutes, 56°C) fetal calf serum (FCS) (Gibco, Life Technologies, Vienna, Austria), 100 U/mL penicillin G, and 100 μg/mL streptomycin (both Schoeller Pharma). Recombinant human (rhu) IL-4 was generously supplied by Schering-Plough Research Institute (Kenilworth, NJ); rhuGM-CSF was purchased from Schering-Plough (AESCA, Traiskirchen, Austria); rhuIL-3 and rhuIL-18 were from Peprotech (London, United Kingdom); and stromal-derived factor–1 (SDF-1) was from R&D Systems (Minneapolis, MN). The following antibodies were used: phycoerythrin (PE)–conjugated anti–IL-18Rα monoclonal antibody (mAb) (R&D Systems); IL-3Rα (CD123) mAb (Santa Cruz Biotechnology, Heidelberg, Germany); anti-CD3 mAb and anti-CD28 mAb (Pharmingen, Hamburg, Germany); fluorescein isothiocyanate (FITC)–conjugated IFN-γ mAb and PE-conjugated IL-4 mAb (Pharmingen); FITC-conjugated anti-CD80 mAb, PE-conjugated anti-CD86 mAb, PE-conjugated anti–IL-12Rβ1, and PE-conjugated anti–IL-12Rβ2 (Pharmingen); FITC-conjugated human leukocyte antigen–DR (HLA-DR) mAb (Serotec, Raleigh, NC); CD40 mAb (Pharmingen); CD83 mAb (Immunotech, Instrumentation Laboratories, Vienna, Austria); FITC-conjugated antimouse immunoglobulin G (IgG) (Sigma, Vienna, Austria); and PE-conjugated mouse IgG1κ and PE-conjugated rat IgG2a (Pharmingen). Lipopolysaccharide (LPS) (Escherichia coli 055:B5) was obtained from Sigma.
Dendritic cells
Peripheral blood mononuclear cells (PBMCs) were obtained by density gradient centrifugation (Histopaque 1077; Sigma) of buffy coats obtained from the local blood bank. DC1 precursors (eg, monocytes) were obtained by immunomagnetic sorting by means of CD14-coated magnetic activated cell sorting (MACS) microbeads (purity exceeding 98%) according to manufacturer's protocol (Miltenyi Biotec, Bergisch-Gladbach, Germany). DC1s were generated as described.23,29 In brief, monocytes were cultured at a density of 1 × 106/mL in culture medium supplemented with 1 × 103 IU/mL IL-4 and 1 × 103 IU/mL GM-CSF. Culture medium, IL-4, and GM-CSF were replenished on day +2 and day +5. DC1s were used at day +6 of culture. DC2 precursors (pre-DC2s; eg, plasmacytoid dendritic cells)12,24 have been recently described to selectively express a novel type II C-type lectin, which is recognized by mAbs termed BDCA2 and BDCA4.30,31 Pre-DC2s were isolated from PBMCs by immunomagnetic sorting of BDCA4+ cells (purity exceeding 94%; typical recovery, 0.8 × 106 to 2.5 × 106 per 500 mL whole blood) as recently described.30 DC2s were cultured for 3 days at a density of 1 × 106/mL RPMI/10% FCS with addition of rhuIL-3 at 10 ng/mL as described.12,24,32,33 RhuIL-18 was added at the onset of DC2 culture as indicated at 100 ng/mL. In selected experiments, DC1s or DC2s were activated/matured12 by irradiated (30 Gy) murine myeloma cells transfected with human CD154/CD40 ligand (P3 × TBA7 cells; one P3 × TBA7 cell per 2 DCs),34 kindly provided by R. A. Kroczek (Robert Koch Institute, Berlin, Germany) and N. Romani (Department of Dermatology, University of Innsbruck, Austria).
DC T-cell cocultures
Allogeneic CD4+CD45RA+ naive T lymphocytes were isolated from PBMCs by immunomagnetic purification. In brief, untouched CD4+ T lymphocytes were obtained through MACS CD4+ T-cell isolation kit according to the manufacturer's instructions (Miltenyi Biotec). In the next step, naive CD45RA+ T lymphocytes were positively isolated by CD45RA-coated immunomagnetic microbeads (Miltenyi Biotec).12,35 Naive T lymphocytes were cocultured with extensively washed DC1s and DC2s, respectively, for 6 days at a ratio of 5:1 in culture medium.12,35 Where indicated, DC1s, DC2s, and IL-18–DC2s were activated through CD40 ligand (CD40L)–expressing irradiated P3 × TBA7 cells as described in the previous paragraph. Anti–IL-18 and anti–IL-12 mAbs (10 μg/mL each) were added at the onset of coculture of DC1 and T cells as indicated. On day 6, cells were restimulated at a density of 1 × 106/mL with plate-bound anti-CD3 (5 μg/mL in phosphate buffered saline [PBS], overnight, 4°C) and anti-CD28 (1 μg/mL) for 5 hours (for intracellular cytokine staining) and 24 hours (for quantification of cytokines in the supernatant), respectively.12,35 Figure 1 shows an outline of the experiments performed.
Flow cytometry analysis
For intracellular 2-color flow cytometry, T cells were restimulated as described in the preceding paragraph. Brefeldin A (1 μg/mL; Sigma) was added into the cultures for 2 hours before the staining to prevent cytokine secretion. Cells were washed and fixed with paraformaldehyde, permeabilized with 1% saponin (Sigma), and incubated with PE–IL-4 and FITC–IFN-γ. After washing, cells were immediately analyzed on a FACSCalibur (Becton Dickinson, Vienna, Austria).
For surface flow cytometry of DCs, DCs were incubated with indicated FITC- or PE-labeled mAbs for 30 minutes, followed by washing and immediate analysis, as recently described.29 For CD40 and CD83 staining, unlabeled primary mAbs were used with FITC-antimouse IgG secondary reagent (1:40 dilution) as described previously.29 Data evaluation was performed by Cellquest software (Becton Dickinson).
Apoptosis assessment
Apoptosis was determined by fluorescent-activated cell sorter (FACS) analysis via propidium iodine (Sigma) exclusion test or terminal deoxynucleotidyl transferase-mediated deoxyuridine nick-end labeling (TUNEL) staining (Roche Applied Science, Mannheim, Germany) according to the manufacturers' protocols.
Cytokine determination in the supernatant
Commercially available matched antibody pairs and recombinant protein standards were used according to manufacturers' instructions for enzyme-linked immunosorbent assays (ELISAs) for the following cytokines: IFN-γ, IL-4, IL-12 p70, IL-10 (Pharmingen), and IL-18 and IFN-α (Bender MedSystems, Vienna, Austria).
Polymerase chain reaction
IL-18Rα and IL-18Rβ chain, IL-12 p35 and p40, IL-23 p19, and IL-27 p28 and EBI3 chain cDNAs were obtained by reverse transcription–polymerase chain reaction (RT-PCR). Total RNA was extracted from indicated cell types (Th1-polarized cells, obtained by coculture of allogeneic naive Th cells with DC1s for 6 days, which have been shown by FACS analysis to express IL-18Rα abundantly [data not shown], served as a positive control for IL-18Rα and IL-18Rβ chain amplification). Reverse transcription was performed with Omniscript reverse transcriptase (Qiagen, Hilden, Germany) with the use of a random hexanucleotide mix (Roche, Basel, Switzerland). Complementary DNA PCR was performed with Hot Star Taq Polymerase (Qiagen) with the use of 10 mM deoxynucleoside triphosphates (dNTPs) (Amresco, Solon, OH) and with 15 pmol of each sense and antisense primer in 30 cycles (IL-18Rα, IL-18Rβ, β-actin) or 33 cycles (all others) of 95°C for 1 minute, at annealing temperatures for 1 minute (IL-18Rα, 57°C; IL-18Rβ, 59°C; IL-12 p35, 58.4°C; IL-12 p40, 59.4°C; IL-23 p19, 59.4°C; IL-27 p28, 63.4°C; EBI3, 63.4°C; β-actin, 59°C), and at 72°C for 1 minute in a total volume of 50 μL. Primers were as follows: IL-18Rα chain, 5′-TGA CTC CAG AAG GCAAAT GGC-3′ (position 833) and 5′-AAA GAG ATT TAT CGG CCT TCC-3′ (position 1523); IL-18Rβ chain, 5′-GCA TCC TGT GAG TAT TCC GCA TC-3′ (position 940) and 5′-CAG CAC GGC CAC CAG GGT CC-3′ (position 1589); IL-12 p35, 5′-ACC TGC CGC GGC CAC AGG TC-3′ (position 140) and 5′-CTG TTG AAA TTC AGG GCC TGC-3′ (position 704); IL-12 p40, 5′-GGT ATC ACC TGG ACC TTG GAC C-3′ (position 211) and 5′-TGC TGG CAT TTT TGC GGC AG-3′ (position 939); IL-23 p19, 5′-CAG CAG CCC TGC CTG GAC TC-3′ (position 225) and 5′-AAT TTT CAA CAT ATG CAG GTC CC-3′ (position 833); IL-27 p28, 5′-CGC CAG GAA GCT GCT CTC CG-3′ (position 159) and 5′-CCC TGT AAG GCG CTG CCC AG-3′ (position 553); EBI3, 5′-TCG GTA CCC GAT CGC CGT GG-3′ (position 127) and 5′-AGG TTG CCC GGC AGC TCA GC-3′ (position 823); β-actin, 5′-GTG ACG AGG CCC AGA GCA AGA G-3′ and 5′-AGG GGC CGG ACT CAT CGT ACT C-3′.
Proliferation assays
Proliferation was assessed in triplicate by [3H]methylthymidine (NEN; Perkin Elmer, Freiburg, Germany) incorporation after 3 days of culture. Cells were pulsed with 1 μCi (0.037 MBq) per well for 19 hours in 96-well plates.
Chemotaxis
Migration of cells into nitrocellulose to gradients of soluble attractants was measured with the use of a 48-well Boyden microchemotaxis chamber (Neuroprobe, Bethesda, MD) in which a 5 μm-pore filter (Sartorius, Göttingen, Germany) separated the upper from the lower chamber.36
As indicated, cells were incubated with antibodies to IL-18 receptor for 20 minutes prior to migration experiments. To exclude nonspecific migra-tion, IL-18 was coincubated with antibodies to IL-18 in the lower chamber as indicated. Migration time into the filters was 180 minutes; subsequently filters were dehydrated, fixed, and stained with hematoxylin-eosin.29 Migration depth of cells into the filter was quantified by microscopy, measuring the distance (in micrometers) from the surface of the filter to the leading front of cells, before any cells had reached the lower surface (leading-front assay). Data are expressed as the chemotaxis index: the ratio of the distance of stimulated and random migration of leukocytes into the nitrocellulose filters.
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Statistical evaluation was performed by Student t test and for chemotaxis experiments with Kruskal-Wallis analysis of variance (ANOVA) and post-hoc Mann-Whitney U test by means of the SPSS 9.0 software package (SPSS, Chicago, IL); P < .05 was considered significant.
Results
IL-18 secreted by DC1 cells does not contribute to Th1 development
As expected from previously published data,37,38 experiments studying the effect of maturation of DC1s revealed that the activation process results in substantial up-regulation of IL-18 mRNA (data not shown). In contrast, IL-18 protein secretion is not up-regulated by maturational stimuli but rather is constitutive in DC1 cells (data not shown), which might be related to down-regulation of the enzyme processing IL-18, namely caspase-1 (data not shown). This is in apparent contrast to IL-12 p70, which is markedly up-regulated during maturation (data not shown). Furthermore, it should be noted that far less IL-18 (sometimes below the detection limit of our assay) than IL-12 p70 was released from DC1s (data not shown). In contrast to DC1s, DC2s did not release detectable amounts of IL-18 and IL-12 p70 (data not shown).
To identify the role of IL-18 during the induction phase of an immune response driven by DC1s, anti–IL-18 mAb was added to coculture experiments with allogeneic naive Th cells. After 6 days of DC1-Th coculture, Th cells were harvested and restimulated with anti-CD3 and anti-CD28 to determine the Th phenotype. The outline of these experiments is shown in Figure 1 and is virtually identical to previously reported experimental approaches.12,28,35 As expected, DC1 preferentially induced a Th1 phenotype, releasing high amounts of IFN-γ and low amounts of IL-4 (Figure 2A-B).12 Intracellular FACS staining revealed a predominance of IFN-γ–secreting Th1 cells and few IL-4–secreting Th2 cells (Figure 2C). As shown in Figure 2, neutralization of IL-18 with anti–IL-18 mAb during the coculture did not significantly change the Th1 phenotype, while neutralization of IL-12 significantly reduced the amount of IFN-γ released and the number of IFN-γ+ Th1 cells. Interestingly, the precentage of IFN-γ–producing cells could not be reduced below 20% to 40%, suggesting that Th1-driving factors other than IL-12 might be present.39
Effect of IL-18 secreted from DC1s on Th1 response. IL-18 secreted from DC1 does not drive a Th1 response. (A-B) Th1 (IFN-γ) and Th2 (IL-4) cytokine release from polarized Th cells. As outlined in Figure 1, allogeneic naive Th lymphocytes were cocultured with DC1s (either immature [DC1 w/o CD40L] or matured by a further 24-hour culture with CD40L-expressing P3 × TBA7 cells [DC1 + CD40L]) or DC2s as indicated for 6 days. During this period, neutralizing antibodies (anti–IL-12, anti–IL-18, and isotype-matched control immunoglobulin) were present as indicated. On day 6, Th cells were harvested, counted, and restimulated for 24 hours with anti-CD3 and anti-CD28. IFN-γ and IL-4 released into the supernatant were detected by ELISA. Neutralization of IL-12 resulted in a substantial decrease of IFN-γ release, whereas anti–IL-18 mAb had no significant effect. Th cells obtained through coculture with DC2 secreted substantially more IL-4 than those from DC1 cocultures. One of 2 experiments is shown. (C) Th phenotype determined by intracellular FACS. Th cells generated through coculture of naive allogeneic Th cells with DC1, as described, were restimulated for 5 hours, and intracellular accumulation of IFN-γ and IL-4 was analyzed. The percentages of positive cells are given in the respective quadrants. Anti–IL-12 mAb during coculture significantly decreased the number of IFN-γ+IL-4– cells. One experiment of 2 is shown.
Effect of IL-18 secreted from DC1s on Th1 response. IL-18 secreted from DC1 does not drive a Th1 response. (A-B) Th1 (IFN-γ) and Th2 (IL-4) cytokine release from polarized Th cells. As outlined in Figure 1, allogeneic naive Th lymphocytes were cocultured with DC1s (either immature [DC1 w/o CD40L] or matured by a further 24-hour culture with CD40L-expressing P3 × TBA7 cells [DC1 + CD40L]) or DC2s as indicated for 6 days. During this period, neutralizing antibodies (anti–IL-12, anti–IL-18, and isotype-matched control immunoglobulin) were present as indicated. On day 6, Th cells were harvested, counted, and restimulated for 24 hours with anti-CD3 and anti-CD28. IFN-γ and IL-4 released into the supernatant were detected by ELISA. Neutralization of IL-12 resulted in a substantial decrease of IFN-γ release, whereas anti–IL-18 mAb had no significant effect. Th cells obtained through coculture with DC2 secreted substantially more IL-4 than those from DC1 cocultures. One of 2 experiments is shown. (C) Th phenotype determined by intracellular FACS. Th cells generated through coculture of naive allogeneic Th cells with DC1, as described, were restimulated for 5 hours, and intracellular accumulation of IFN-γ and IL-4 was analyzed. The percentages of positive cells are given in the respective quadrants. Anti–IL-12 mAb during coculture significantly decreased the number of IFN-γ+IL-4– cells. One experiment of 2 is shown.
DC2-lineage but not DC1-lineage cells express IL-18R
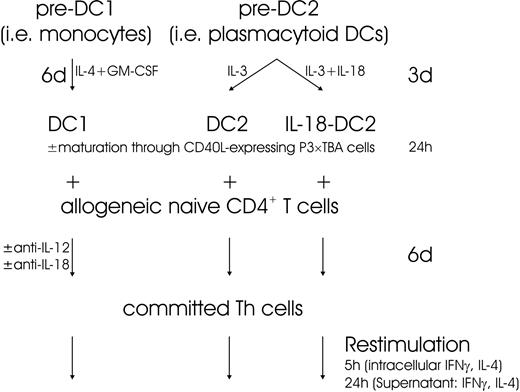
Based on these data, we asked whether IL-18 might act one step earlier (eg, at the DC level). IL-18 signals through IL-18 receptor, consisting of the ligand-binding α chain (formerly called IL-1 receptor–related protein [IL-1Rrp])4,40 and the signal-transducing β chain (formerly called IL-1 receptor accessory protein–like protein [IL-1RAcPL]).41 Cytofluorometric analysis revealed abundant IL-18Rα expression on pre-DC2s (Figure 3A), which were isolated by immunomagnetic sorting of BDCA4+ cells. Identity and purity of pre-DC2s was determined by CD123 (IL-3Rα) (Figure 3B) and HLA-DR (Figure 3C) expression and the absence of lineage markers (data not shown).12,24 Pre-DC2s faintly expressed IL-12Rβ1, while IL-12Rβ2 was absent (Figure 3D-E). DC2s also expressed IL-18Rα as shown in Figure 3F. In contrast to DC2 lineage, DC1-lineage cells do not express IL-18Rα chain, as demonstrated on pre-DC1s (eg, monocytes) (Figure 3G) and DC1s (Figure 3H). Accordingly, DC1s do not express IL-18Rα mRNA at any time during their differentiation (Figure 3I), whereas IL-18Rα mRNA was readily detected in pre-DC2s, DC2s, and CD40L-matured DC2s (Figure 3I). A similar mRNA expression pattern was detected for the signal-transducing IL-18Rβ chain, except that IL-18Rβ mRNA was also expressed in pre-DC1s (Figure 3I). All together, this suggested that IL-18R is present and could be functional in DC2 lineage.
IL-18R expression in DC2 lineage. (A-H) FACS surface analysis. IL-18Rα surface expression on pre-DC2s (eg, plasmacytoid DCs) (panel A). Identity and purity of pre-DC2s as determined by IL-3Rα (CD123) (panel B) and HLA-DR (panel C) expression. Pre-DC2s were negative for lineage markers (not shown). IL-12Rβ1 (panel D) and IL-12Rβ2 (panel E) expression on pre-DC2s. IL-18Rα surface expression on DC2s (panel F), pre-DC1s (panel G), and DC1s (panel H). Solid lines indicate the specific antibodies, and dotted lines indicate isotype-matched immunoglobulin controls. (I) PCR analysis of IL-18Rα and IL-18Rβ chain on DC1 and DC2 lineage, and Th1-polarized cells (positive control). An IL-18Rα fragment spanning nucleotides 833 to 1523 was amplified in DC2 lineage as a 690-bp band. Please note that in pre-DC2s, in addition to the expected band, another smaller band was reproducibly coamplified. Nucleotides 940 to 1589 of IL-18Rβ chain were amplified in DC2 lineage and in pre-DC1s, resulting in a band at 649 bp. As a positive control for IL-18Rα and IL-18Rβ, mRNA was obtained on day 6 of coculture of naive Th cells with DC1s, at which time Th cells abundantly express surface IL-18Rα as determined by FACS analysis (data not shown). Control amplification was performed for β-actin.
IL-18R expression in DC2 lineage. (A-H) FACS surface analysis. IL-18Rα surface expression on pre-DC2s (eg, plasmacytoid DCs) (panel A). Identity and purity of pre-DC2s as determined by IL-3Rα (CD123) (panel B) and HLA-DR (panel C) expression. Pre-DC2s were negative for lineage markers (not shown). IL-12Rβ1 (panel D) and IL-12Rβ2 (panel E) expression on pre-DC2s. IL-18Rα surface expression on DC2s (panel F), pre-DC1s (panel G), and DC1s (panel H). Solid lines indicate the specific antibodies, and dotted lines indicate isotype-matched immunoglobulin controls. (I) PCR analysis of IL-18Rα and IL-18Rβ chain on DC1 and DC2 lineage, and Th1-polarized cells (positive control). An IL-18Rα fragment spanning nucleotides 833 to 1523 was amplified in DC2 lineage as a 690-bp band. Please note that in pre-DC2s, in addition to the expected band, another smaller band was reproducibly coamplified. Nucleotides 940 to 1589 of IL-18Rβ chain were amplified in DC2 lineage and in pre-DC1s, resulting in a band at 649 bp. As a positive control for IL-18Rα and IL-18Rβ, mRNA was obtained on day 6 of coculture of naive Th cells with DC1s, at which time Th cells abundantly express surface IL-18Rα as determined by FACS analysis (data not shown). Control amplification was performed for β-actin.
IL-18 down-regulates proliferation of pre-DC2s
As reported previously, IL-3 induces the proliferation of pre-DC2s.12,24 IL-18 down-regulated the proliferative response of pre-DC2s cultured with IL-3 as demonstrated in Figure 4A. Interestingly, this did not result in a decrease in the number of DC2s recovered on day 3, as shown in Figure 4B. Since the number of DCs decreases similarly over time in the IL-3 and IL-3 plus IL-18 group, a lower proliferation index in the IL-3 plus IL-18 group might be explained by increased survival in this group compared with the IL-3 group. However, there is no difference in the apoptosis rate 4 and 24 hours after culture of pre-DC2s with IL-3 versus IL-3 plus IL-18 as determined by propidium iodine exclusion and TUNEL staining (data not shown).
Effect of IL-18 on DC2 differentiation. (A) Proliferation. Pre-DC2s were cultured with indicated agents for 3 days and subsequently pulsed with 3H-thymidine for 19 hours. IL-18 significantly inhibited proliferation of pre-DC2s expanded with IL-3 (n = 3). (B) Cell count. Pre-DC2s (initially 250 000 cells) were cultured with indicated cytokines and counted at the given time points (n = 2). Error bars indicated SEM.
Effect of IL-18 on DC2 differentiation. (A) Proliferation. Pre-DC2s were cultured with indicated agents for 3 days and subsequently pulsed with 3H-thymidine for 19 hours. IL-18 significantly inhibited proliferation of pre-DC2s expanded with IL-3 (n = 3). (B) Cell count. Pre-DC2s (initially 250 000 cells) were cultured with indicated cytokines and counted at the given time points (n = 2). Error bars indicated SEM.
IL-18 induces chemotaxis in pre-DC2s
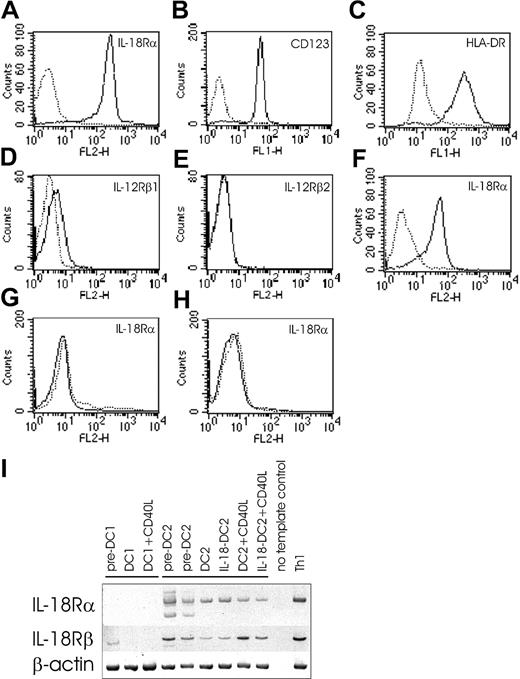
As depicted in Figure 5A, IL-18 dose-dependently induced a migratory response in Boyden chamber microchemotaxis experiments, which exceeded the response induced by (CXCL12) used as positive control. Receptor blockade by anti–IL-18Rα mAb (Figure 5B), as well as blocking of IL-18 by anti–IL-18 mAb (Figure 5C), dose-dependently abrogated migration, demonstrating the specificity of IL-18–induced chemotaxis of pre-DC2s.
Effect of IL-18 on pre-DC2 chemotaxis. IL-18 induces chemotaxis in pre-DC2s. (A) Direct chemotaxis of cells toward IL-18. Pre-DC2s were allowed to migrate into nitrocellulose toward various concentrations of IL-18 present in the lower wells of a Boyden microchemotaxis chamber. SDF (1μg/mL) served as positive control. (B) IL-18 receptor antibodies abrogate pre-DC2 migration toward IL-18. Pre-DC2s were preincubated with anti–IL-18Rα mAb at indicated concentrations and allowed to migrate toward a concentration gradient of IL-18 (10 ng/mL). (C) Anti–IL-18 antibodies abrogate pre-DC2 migration toward IL-18. Anti–IL-18 mAb was added to the lower wells of a microchemotaxis chamber containing IL-18 (10 ng/mL), and pre-DC2s were allowed to migrate into nitrocellulose. Migration time periods for all experiments were 180 minutes. Migration depth was quantified microscopically by the leading front assay. Data are expressed as the chemotaxis index: the ratio of the distance of stimulated and random migration of leukocytes into nitrocellulose filters (n = 6). *P < .05 in the Mann-Whitney U test after Kruskal-Wallis ANOVA. Error bars indicate SEM.
Effect of IL-18 on pre-DC2 chemotaxis. IL-18 induces chemotaxis in pre-DC2s. (A) Direct chemotaxis of cells toward IL-18. Pre-DC2s were allowed to migrate into nitrocellulose toward various concentrations of IL-18 present in the lower wells of a Boyden microchemotaxis chamber. SDF (1μg/mL) served as positive control. (B) IL-18 receptor antibodies abrogate pre-DC2 migration toward IL-18. Pre-DC2s were preincubated with anti–IL-18Rα mAb at indicated concentrations and allowed to migrate toward a concentration gradient of IL-18 (10 ng/mL). (C) Anti–IL-18 antibodies abrogate pre-DC2 migration toward IL-18. Anti–IL-18 mAb was added to the lower wells of a microchemotaxis chamber containing IL-18 (10 ng/mL), and pre-DC2s were allowed to migrate into nitrocellulose. Migration time periods for all experiments were 180 minutes. Migration depth was quantified microscopically by the leading front assay. Data are expressed as the chemotaxis index: the ratio of the distance of stimulated and random migration of leukocytes into nitrocellulose filters (n = 6). *P < .05 in the Mann-Whitney U test after Kruskal-Wallis ANOVA. Error bars indicate SEM.
IL-18 increases the allostimulatory capacity of DC2s
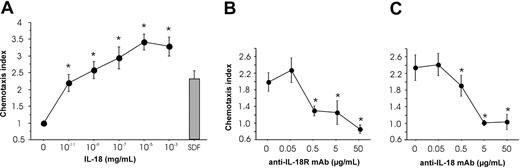
To further characterize the functional consequences of IL-18 exposure of pre-DC2s, we compared DC2s (differentiated from pre-DC2s for 3 days with IL-3) and IL-18–DC2s (differentiated with IL-3 plus IL-18) for their allostimulatory capacity. DC2s and IL-18–DC2s were washed, subsequently activated/matured through CD40L (transfected in P3 × TBA7 cells) as indicated in Figure 6, and cocultured with allogeneic naive Th cells for another 3 days. As shown in Figure 6, IL-18–stimulation of pre-DC2s increased the proliferative capacity of mature DC2s as assessed by 3H-thymidine incorporation into Th cells. FACS analysis ruled out the possibility that increased HLA-DR expression on CD40L-activated IL-18–DC2s versus CD40L-activated DC2s might account for the difference in allostimulatory capacity (data not shown).
Allogeneic proliferation of naive Th cells. DC2s were obtained by culture of pre-DC2s with IL-3 (DC2) or IL-3 plus IL-18 (IL-18–DC2). After washing, DC2s and IL-18–DC2s were either immediately cocultured with allogeneic naive Th cells for 3 days (w/o CD40L) or, alternatively, matured by addition of CD40L-expressing irradiated P3 × TBA7 cells (+ CD40L), followed after 24 hours by addition of Th cells. Proliferation of Th cells was determined on day 3 of Th/DC coculture by addition of 3H-thymidine for 19 hours (n = 4). Error bars indicate SEM.
Allogeneic proliferation of naive Th cells. DC2s were obtained by culture of pre-DC2s with IL-3 (DC2) or IL-3 plus IL-18 (IL-18–DC2). After washing, DC2s and IL-18–DC2s were either immediately cocultured with allogeneic naive Th cells for 3 days (w/o CD40L) or, alternatively, matured by addition of CD40L-expressing irradiated P3 × TBA7 cells (+ CD40L), followed after 24 hours by addition of Th cells. Proliferation of Th cells was determined on day 3 of Th/DC coculture by addition of 3H-thymidine for 19 hours (n = 4). Error bars indicate SEM.
IL-18 skews DC2s toward induction of a Th1 phenotype
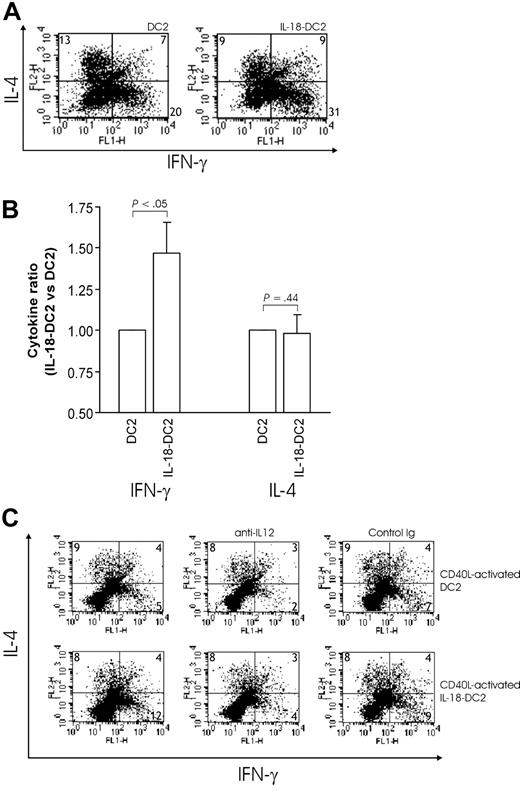
On the basis of the results presented in the previous paragraphs, we asked whether IL-18 might influence Th1/Th2 differentiation via modulation of the functional properties of DC2s. As described above for DC1s, we cocultured DC2s (either differentiated from pre-DC2s with IL-3 [DC2] or IL-3 plus IL-18 [IL-18–DC2]) and allogeneic naive Th cells for 6 days; then we recovered Th cells and restimulated them with anti-CD3 and anti-CD28, revealing their Th phenotype (Figure 1) (see also Rissoan et al,12 Zou et al,28 and Soumelis et al35 ). In our hands, DC2s induced a Th phenotype releasing substantially more IL-4 than Th cells differentiated through DC1 cells (Figure 2B), although DC2s still induced both IFN-γ–secreting Th1 cells and IL-4–secreting Th2 cells at varying ratios (Figures 2A-B and 7A). Interestingly, CD40 ligation of DC2s with CD40L-transfected P3 × TBA7 cells did not significantly alter the induced Th1/Th2 ratio as compared with DC2s applied without CD40 ligation (Figure 7C). Notably, IL-18–DC2s induced a higher proportion of IFN-γ–secreting Th1 cells than DC2s (13.74% ± 2.25% versus 20.65% ± 3.68%; 56% relative increase; P < .05), while the number of IL-4–secreting Th2 cells showed a tendency to decrease (Figure 7A), although this did not reach statistical significance (6.3% ± 1.82% versus 5.09% ± 1.18%; 12% relative decrease; P = .09). Accordingly, restimulated Th cells released significantly more IFN-γ, while IL-4 release was unaffected (Figure 7B). Comparable results were obtained with CD40L-matured DC2: IL-18–DC2s induced 24% more IFN-γ–secreting Th1 cells (P < .05; n = 3), while a statistically nonsignificant 10% decrease in IL-4–producing Th2 cells was noted (Figure 7C).
Induction of Th phenotype by IL-18–DC2's. (A) Th phenotype determined by intracellular FACS. DC2s were obtained by culture of pre-DC2s with IL-3 (DC2) or IL-3 plus IL-18 (IL-18–DC2). After washing, DC2/IL-18–DC2s were cocultured with allogeneic naive Th cells for 6 days. Subsequently, Th cells were harvested and restimulated with anti-CD3 and anti-CD28 for 5 hours. Intracellular FACS staining was performed as described in “Materials and methods.” The percentage of IFN-γ+ and IL-4+ cells is given for each quadrant. The experiment shown is representative of 5 performed. (B) IFN-γ and IL-4 secretion by restimulated Th cells. Experiments were performed as described in the legend for panel A, with restimulation of Th cells for 24 hours instead of 5 hours, and cytokine secretion determined by ELISA. IFN-γ and IL-4 secretion induced in Th cells by DC2 was set at 1 in each case for further analysis owing to the large interindividual variation (medium baseline IFN-γ, 23 ± 9 ng/mL; baseline IL-4, 661 ± 324 pg/mL). The y-axis shows the ratio of Th cytokine secretion induced by IL-18–DC2 per DC2. Differentiation of allogeneic naive Th cells with IL-18–DC2 significantly increased IFN-γ release upon restimulation as compared with DC2 (n = 6). (C) Th phenotype and effect of IL-12 neutralization after differentiation with CD40L-matured DC2. Experiments were performed as described for panel A, except that CD40L-activated DC2 and IL-18–DC2 were added for 24 hours before the onset of coculture with allogeneic naive Th cells. Anti–IL-12 mAb or isotype-matched control immunoglobulin was present during the coculture as indicated. The percentage of IFN-γ+ and IL-4+ cells is given for each quadrant. The experiment shown is representative of 3 performed with respect to CD40L-matured DC2/IL-18–DC2, and is representative of an additional 2 with respect to the effect of anti–IL-12. Error bars indicate SEM.
Induction of Th phenotype by IL-18–DC2's. (A) Th phenotype determined by intracellular FACS. DC2s were obtained by culture of pre-DC2s with IL-3 (DC2) or IL-3 plus IL-18 (IL-18–DC2). After washing, DC2/IL-18–DC2s were cocultured with allogeneic naive Th cells for 6 days. Subsequently, Th cells were harvested and restimulated with anti-CD3 and anti-CD28 for 5 hours. Intracellular FACS staining was performed as described in “Materials and methods.” The percentage of IFN-γ+ and IL-4+ cells is given for each quadrant. The experiment shown is representative of 5 performed. (B) IFN-γ and IL-4 secretion by restimulated Th cells. Experiments were performed as described in the legend for panel A, with restimulation of Th cells for 24 hours instead of 5 hours, and cytokine secretion determined by ELISA. IFN-γ and IL-4 secretion induced in Th cells by DC2 was set at 1 in each case for further analysis owing to the large interindividual variation (medium baseline IFN-γ, 23 ± 9 ng/mL; baseline IL-4, 661 ± 324 pg/mL). The y-axis shows the ratio of Th cytokine secretion induced by IL-18–DC2 per DC2. Differentiation of allogeneic naive Th cells with IL-18–DC2 significantly increased IFN-γ release upon restimulation as compared with DC2 (n = 6). (C) Th phenotype and effect of IL-12 neutralization after differentiation with CD40L-matured DC2. Experiments were performed as described for panel A, except that CD40L-activated DC2 and IL-18–DC2 were added for 24 hours before the onset of coculture with allogeneic naive Th cells. Anti–IL-12 mAb or isotype-matched control immunoglobulin was present during the coculture as indicated. The percentage of IFN-γ+ and IL-4+ cells is given for each quadrant. The experiment shown is representative of 3 performed with respect to CD40L-matured DC2/IL-18–DC2, and is representative of an additional 2 with respect to the effect of anti–IL-12. Error bars indicate SEM.
IL-18 might skew DC2s toward Th1 induction via IL-12 and other Th1-promoting cytokines
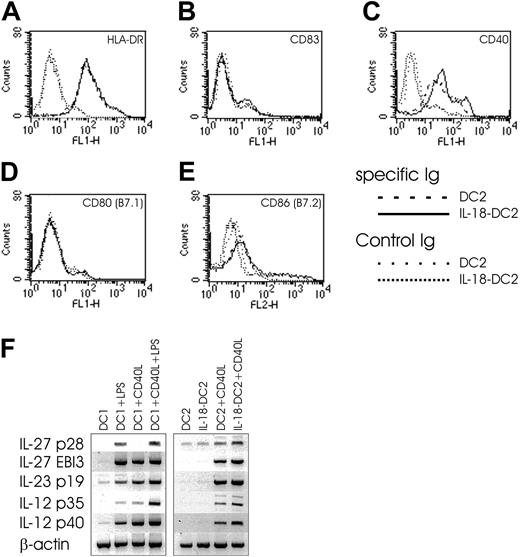
To gain some clues as to the understanding of this Th1-skewing property of IL-18–DC2s, we evaluated the surface phenotype and cytokine secretion pattern of DC2s and IL-18–DC2s (Figure 8). While FACS analysis revealed no differences between DC2s and IL-18–DC2s in surface staining of HLA-DR, CD83, CD80 (B7.1), and CD86 (B7.2), the IL-18–DC2s showed increased surface expression of CD40 compared with DC2s (Figure 8A-E). While IFN-γ secretion was absent in 5 of 6 DC2 supernatants tested, IL-18–DC2 commonly secreted faint amounts of IFN-γ (data not shown). Little IL-4 detected in some culture supernatants of DC2s was nonsignificantly further reduced in IL-18–DC2s (data not shown). While IL-12 p70 was not detected in DC or IL-18–DC2 supernatant (data not shown), both populations equally released small amounts of IL-10 and IFN-α (data not shown). In contrast to IL-12 p70 protein, CD40L-stimulated DC2s express IL-12 p35 and p40 mRNA, which are both up-regulated by IL-18 in CD40L-stimulated IL-18–DC2s (Figure 8F). In coculture experiments of DC2s and allogeneic Th cells, minute IL-12 p70 levels just at the detection limit of our ELISA were observed (data not shown). Notably, addition of a neutralizing anti–IL-12 p70 mAb to the cocultures described in the previous paragraph reduced both baseline Th1 induction in CD40L-stimulated DC2s as well as the Th1-promoting capacity of CD40L-stimulated IL-18–DC2s (Figure 7C), suggesting that trace amounts of IL-12 p70 released during the coculture might be sufficient to induce a Th1 phenotype. Recently, 2 novel IL-12–like Th1-promoting cytokines have been described: IL-23 (consisting of IL-12 p40 and the p19 chain) primarily acting on memory Th cells,42 and IL-27, composed of EBI3 (Epstein-Barr virus-induced gene 3, an IL-12 p40 homolog)43 and p28 (a newly discovered IL-12 p35-related polypeptide).44 IL-27 has been proposed to drive rapid clonal expansion and Th1 induction of naive but not memory Th cells,44 although data derived from EBI3–/– mice called this into question.45 Notably, CD40L-stimulated DC2s express mRNAs for all of the aforementioned polypeptides, and their expression is up-regulated in IL-18–DC2s stimulated with CD40L (Figure 8F).
Effect of IL-18 on DC2 phenotype. (A-E) FACS surface analysis of DC2s and IL-18–DC2s. DC2s were obtained by culture of pre-DC2s with IL-3 (DC2) or IL-3 plus IL-18 (IL-18–DC2) for 3 days, and stained with indicated antibodies. Surface expression on DC2s is compared with IL-18–DC2s. (F) IL-27, IL-23, and IL-12 mRNA expression in DC1s and DC2s. DC1s were obtained by culture of monocytes for 6 days in the presence of IL-4 and GM-CSF (DC1) and further stimulated with LPS (1 μg/mL) and CD40L-expressing irradiated P3 × TBA7 cells (+CD40L) as indicated. DC2s were derived from plasmacytoid DCs by culture with IL-3 (DC2), or IL-3 plus IL-18 (IL-18–DC2) for 3 days, and further matured as indicated by addition of CD40L-expressing irradiated P3 × TBA7 cells (+ CD40L). Messenger RNA expression of IL-27 (consisting of p28 and EBI3 chains), IL-23 (p19 and IL-12 p40), and IL-12 (p35 and p40) was analyzed by PCR as outlined in “Materials and methods.” Control amplification was performed for β-actin. This figure is representative of 4 independent experiments.
Effect of IL-18 on DC2 phenotype. (A-E) FACS surface analysis of DC2s and IL-18–DC2s. DC2s were obtained by culture of pre-DC2s with IL-3 (DC2) or IL-3 plus IL-18 (IL-18–DC2) for 3 days, and stained with indicated antibodies. Surface expression on DC2s is compared with IL-18–DC2s. (F) IL-27, IL-23, and IL-12 mRNA expression in DC1s and DC2s. DC1s were obtained by culture of monocytes for 6 days in the presence of IL-4 and GM-CSF (DC1) and further stimulated with LPS (1 μg/mL) and CD40L-expressing irradiated P3 × TBA7 cells (+CD40L) as indicated. DC2s were derived from plasmacytoid DCs by culture with IL-3 (DC2), or IL-3 plus IL-18 (IL-18–DC2) for 3 days, and further matured as indicated by addition of CD40L-expressing irradiated P3 × TBA7 cells (+ CD40L). Messenger RNA expression of IL-27 (consisting of p28 and EBI3 chains), IL-23 (p19 and IL-12 p40), and IL-12 (p35 and p40) was analyzed by PCR as outlined in “Materials and methods.” Control amplification was performed for β-actin. This figure is representative of 4 independent experiments.
Discussion
In our effort to identify the role of IL-18 during the initiation phase of an immune response, we first analyzed release and function of IL-18 derived from DC1s. These data essentially are in accordance with Stoll et al37 and de Jong et al,39 demonstrating that IL-18 released from DC1s and acting on naive Th cells might not have a role in the cells' commitment to Th1 lineage37,39 . However, there is increasing in vivo evidence that IL-18 indeed influences the induction phase of an immune response and seems to tip the balance toward Th113-17,19,20 . Therefore, we asked whether IL-18 might act one step earlier, namely by instructing DCs. Indeed, we identified expression of the IL-18 receptor on DC2 lineage cells, whereas IL-18R was absent from DC1 lineage cells. The expression of the receptor of the “Th-1 cytokine” IL-18 on DC2 but not DC1 is somewhat unexpected and raised the question of whether IL-18 might play a role in the regulation of DC2 differentiation. Rissoan et al12 demonstrated that the Th2 cytokine IL-4 enhances DC1 differentiation but kills pre-DC2s, an effect that is blocked by the Th1 cytokine IFN-γ. Because IL-18 is not a product of the “final pathway” of Th immunity (eg, not released from Th1 cells), but by contrast is primarily secreted from activated macrophages (and DC1s), one would expect IL-18 to down-regulate the development of DC2 lineage cells. Indeed, we could demonstrate a reduced proliferative capacity of IL-18–stimulated pre-DC2s cultured with IL-3, although this did not result in a decreased cell count on day 3. In conclusion, a consistent regulatory “feedback loop” might not be functional, although IL-18 obviously interacts with the differentiation pathway of DC2s.
Whereas myeloid DCs are recruited by a variety of inflammatory chemokines, the same chemokine receptors expressed on plasmacytoid DCs are largely nonfunctional.46 Among a wide variety of chemokines tested for their chemotactic properties on pre-DC2s, only the lymph node homing chemokine SDF-1 consistently induced migration.28,46-48 The mode of recruitment of pre-DC2s toward sites of foreign antigen uptake (eg, inflammatory sites) is currently unclear. We identified IL-18 as a potent chemotactic factor for plasmacytoid DCs. These data unambiguously demonstrate the functionality of IL-18 receptor on pre-DC2s and suggest an unexpected role for IL-18 in the recruitment of pre-DC2s to sites of inflammation. A chemotactic function of IL-18 on T lymphocytes has recently been described by Komai-Koma et al.49 Of note is the fact that activated plasmacytoid DC2s themselves secrete chemokines preferentially attracting Th1 cells (CCR1/CCR5 ligands).46
Selective induction of Th2 cells from naive Th cells has been ascribed to DC2 cells,12,22,28 although the extent of Th2 over Th1 induction might vary.50 Furthermore, it should be noted that factors other than DC lineage might also contribute to Th1/Th2 balance.51-54 We could demonstrate that IL-18 increases the allostimulatory capacity of mature DC2s. More importantly, compared with DC2s, IL-18–DC2s increased the proportion of IFN-γ+ Th cells as well as IFN-γ release upon restimulation of in vitro–differentiated Th cells. All together, these results might be interpreted to state that IL-18 shifts the Th1/Th2 differentiation pathway toward Th1 through modulation of DC2 function. This pathway of IL-18 action might explain how IL-18 promotes an initial Th1 response in vivo in the absence of IL-18R on naive Th cells. As to the underlying mechanism of this Th1 shift, we present evidence that this might be dependent on IL-12 p70. The up-regulation of IL-23 and IL-27 mRNA in CD40L-stimulated IL-18–DC2s compared with CD40L-stimulated DC2s might suggest an adjunctive role for these heterodimeric cytokines, although the formal proof awaits the availability of neutralizing antibodies or respective knockout mice.55
In the context of the initiation phase of an immune response, what is the cellular source that releases IL-18, which might recruit plasmacytoid pre-DC2s and alter the properties of DC2s? Many cell types have been reported to produce IL-18, including macrophages and DCs,37 Kupffer cells,56 astrocytes and microglia,57 intestinal and airway epithelial cells,58 keratinocytes,59 and osteoblasts.60 The factors leading to release of IL-18 have not been extensively studied, but IL-18 is found after bacterial and viral infection and in many other infectious diseases.61 Therefore, it might be anticipated that foreign antigen binding to respective pattern recognition receptors on cells other than DCs might recruit pre-DC2s and shape the properties of DC2 via release of IL-18. Our data are another indication of the importance of the cytokine microenvironment for the steering of DCs and subsequent Th1/Th2 induction; for example, Soumelis et al35 recently showed that epithelial-derived thymic stromal lymphopoietin (TSLP) potently activates CD11c+ DC and induces Th2 development, which might have a prominent role in allergic inflammation.
Prepublished online as Blood First Edition Paper, September 22, 2003; DOI 10.1182/blood-2002-07-2322.
Supported by grants 14641 and 15783 of the Austrian Science Fund (H.T.); grant P8833 of the Jubiläumsfonds of the Austrian National Bank (A.K.); and grant 62 of the Medizinischer Forschungsfonds (MFF) Tyrol (A.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We gratefully acknowledge Drs R. A. Kroczek (Robert Koch Institute, Berlin, Germany) and N. Romani (Department of Dermatology, University of Innsbruck, Austria) for providing CD40L-transfected P3 × TBA7 cells. This study was supported by the Verein zur Förderung der Forschung in Gastroenterologie und Hepatologie an der Universität Innsbruck.

![Figure 2. Effect of IL-18 secreted from DC1s on Th1 response. IL-18 secreted from DC1 does not drive a Th1 response. (A-B) Th1 (IFN-γ) and Th2 (IL-4) cytokine release from polarized Th cells. As outlined in Figure 1, allogeneic naive Th lymphocytes were cocultured with DC1s (either immature [DC1 w/o CD40L] or matured by a further 24-hour culture with CD40L-expressing P3 × TBA7 cells [DC1 + CD40L]) or DC2s as indicated for 6 days. During this period, neutralizing antibodies (anti–IL-12, anti–IL-18, and isotype-matched control immunoglobulin) were present as indicated. On day 6, Th cells were harvested, counted, and restimulated for 24 hours with anti-CD3 and anti-CD28. IFN-γ and IL-4 released into the supernatant were detected by ELISA. Neutralization of IL-12 resulted in a substantial decrease of IFN-γ release, whereas anti–IL-18 mAb had no significant effect. Th cells obtained through coculture with DC2 secreted substantially more IL-4 than those from DC1 cocultures. One of 2 experiments is shown. (C) Th phenotype determined by intracellular FACS. Th cells generated through coculture of naive allogeneic Th cells with DC1, as described, were restimulated for 5 hours, and intracellular accumulation of IFN-γ and IL-4 was analyzed. The percentages of positive cells are given in the respective quadrants. Anti–IL-12 mAb during coculture significantly decreased the number of IFN-γ+IL-4– cells. One experiment of 2 is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/2/10.1182_blood-2002-07-2322/6/m_h80245543002.jpeg?Expires=1769095687&Signature=JnSP9P7Z86lbsnhRiBzYTxpHEA3DLehfizTCFLyj5EXXh17yc2JdnXk7oSPFVaDcMyW67cWz5Ox6TZszAheyOI~lfX-bHhuU-oLwCny5s98S8dMm6xUoJ8HNt3OTKmxy3NfKnM1kIg5uKfdZzwaX6PFyIRLApXuLyPalBfF89QvGQpiAZbhahY77piU6FSqQ3WzzhXn9GsVH-gQUavBjCUH29SlGn4VnwkX3Qoigrw7KG-DQzkIukC0wXQ0cg0nqobpRqpZ3KECCGJlNTkorGQfBkbQTI0kbak6E08GKpDdT46ZqQUMVsaVbgy7tLyGB~SRWw-LSmnIFywhC-P2B5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal