Abstract
Antithrombin, a key serpin family regulator of blood coagulation proteases, is transformed into a potent antiangiogenic factor by limited proteolysis or mild heating. Here, we show by cDNA microarray, semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR), Northern blotting, and immunoblotting analyses that the expression of the proangiogenic heparan sulfate proteoglycan (HSPG), perlecan, but not other HSPGs, is dramatically down-regulated in human umbilical vein endothelial cells (HUVECs) treated with antiangiogenic cleaved and latent forms of antithrombin but not with the native form. Down-regulation of perlecan expression by cleaved and latent antithrombins was observed in both basic fibroblast growth factor (bFGF)–stimulated and unstimulated cells, whereas the antiangiogenic antithrombins inhibited the proliferation of only bFGF-stimulated HUVECs by arresting cells at the G1 cell cycle phase. The importance of perlecan expression levels in mediating the antiproliferative effect of the antiangiogenic antithrombins was suggested by the finding that transforming growth factor-β1, a potent stimulator of perlecan expression in endothelial cells, blocked the down-regulation of perlecan expression and antiproliferative activity of cleaved antithrombin on endothelial cells. The previously established key role of perlecan in mediating bFGF stimulation of endothelial cell proliferation and angiogenesis suggests that a primary mechanism by which antiangiogenic antithrombins exert their effects is through the down-regulation of perlecan expression.
Introduction
Antithrombin, a member of the serpin superfamily of proteins, is an essential anticoagulant regulator of blood coagulation.1,2 This regulation is achieved principally through the inhibition of the clotting cascade proteases, factor IXa, factor Xa, and thrombin, and requires the glycosaminoglycan, heparin, as a cofactor. Heparin binds antithrombin with high affinity and thereby activates the serpin to inhibit clotting proteases at a physiologically significant rate.3,4 In addition to its role in hemostasis, anithrombin has more recently been shown to possess a variety of other biologic activities that are anti-inflammatory, antiviral, and antiangiogenic.5-7 The antiangiogenic activity is produced only by conformationally altered forms of antithrombin produced by proteolytic cleavage or transition to a latent or prelatent form and is evidenced by the ability of these antithrombin forms to inhibit blood vessel growth in the chick embryo, to inhibit the proliferation of endothelial cells in culture, and to induce tumor regression in mice.7-9 The physiologic relevance of this activity is suggested by the finding that primary human pancreatic cancer cells can inhibit secondary tumor growth in mice due to their ability to convert endogenous native antithrombin into the antiangiogenic cleaved and latent forms of the serpin.10
As a member of the serpin superfamily, antithrombin shares a common tertiary structure with other serpins and functions as a protease inhibitor like other inhibitory members of the family.2 The conformational changes in antithrombin that result in the cleaved and latent antiangiogenic forms are similar, both involving the insertion of an exposed reactive loop of the serpin into the major β-sheet of the protein core, the A sheet.11,12 In the case of the latent form, the insertion of the loop is induced by mild heating without proteolysis,13 whereas for the cleaved form limited proteolysis in the loop is sufficient to cause the insertion.14,15 A similar cleavage-induced insertion of the loop into sheet A occurs when the serpin forms a stable complex with a target protease, and this is associated with a large-scale movement and deformation of the protease.16,17 However, reactive loop insertion does not appear to be a requirement for the induction of antiangiogenic activity in antithrombin because a conformationally altered form in which this insertion had not occurred was found to produce antiangiogenic activity comparable to that of the cleaved and latent antithrombins.9 Both cleaved and latent forms of antithrombin are produced under normal physiologic conditions,18,19 suggesting that their antiangiogenic activity may be physiologically relevant.
While the antiangiogenic activity of conformationally altered forms of antithombin appears to be well established, the molecular mechanisms underlying the serpin's antiangiogenic activity remain to be elucidated. Because angiogenesis is a complex physiological process thought to be controlled by multiple genes,20 we expected that antiangiogenic forms of antithrombin would induce their antiproliferative effect on endothelial cells by altering the profile of expressed genes. In the present study, the effects of cleaved and latent antiangiogenic forms of antithrombin and of native antithrombin on the expression of genes in primary human umbilical vein endothelial cells (HUVECs) were analyzed by the microarray technique.21 A striking finding was that the antiangiogenic antithrombins but not the native serpin produced a dramatic down-regulation of the gene for the proangiogenic heparan sulfate proteoglycan, perlecan.22,23 This down-regulation was confirmed at the mRNA level by Northern blotting and semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) and at the protein level by immunoblotting and was correlated with the inhibition of basic fibroblast growth factor (bFGF)–stimulated endothelial cell proliferation and the blocking of the G1-S phase transition in cultured HUVECs by the antiangiogenic antithrombins. Up-regulation of perlecan gene expression in HUVECs by transforming growth factor-β1 (TGF-β1) overcame the inhibition of HUVEC proliferation produced by the antiangiogenic antithrombins. Collectively, our results demonstrate a strong correlation between anithrombin antiangiogenic activity and the down-regulation of perlecan gene expression.
Materials and methods
Materials
Oligonucleotides for PCR analysis were synthesized by Sigma-Aldrich (St Louis, MO). TGF-β1 was purchased from Oncogene (San Diego, CA), and bFGF was from Invitrogen (Carlsbad, CA). Human umbilical vein endothelial cells (HUVECs) were obtained either from the American Type Culture Collection (Manassas, VA) or isolated from freshly harvested umbilical cords from the Stanford University Medical Center as described in “Isolation of primary HUVECs.” A mouse monoclonal antibody to human perlecan and antimouse immunoglobulin G (IgG) labeled with horseradish peroxidase were purchased from ZYMED (San Francisco, CA). Neutrophil elastase was purchased from Athens Research and Technology (Athens, GA), and human thrombin was a gift from John Fenton (New York Department of Public Health, Albany).
Preparation and purification of native and conformationally altered forms of human plasma antithrombin
Antithrombin was purified from human plasma as described previously.24 The cleaved form of antithrombin was generated either by human thrombin cleavage in the presence of heparin in a low ionic strength buffer25 or by neutrophil elastase cleavage as described.15 The latent form of antithrombin was prepared by incubating the serpin at 50°C to 60°C in the presence of sodium citrate as described.13 Cleaved and latent forms of antithrombin were purified by Hi-Trap Heparin and Mono-Q chromatography (Amersham Biosciences, Piscataway, NJ) as in previous studies.8,26 Sodium dodecyl sulfate (SDS) and native polyacrylamide gel electrophoresis (PAGE) confirmed that the cleaved and latent forms had the expected changes in electrophoretic mobility when compared with the native serpin.8,19,24 Both forms lacked detectable inhibitor activity against thrombin.
Isolation of primary HUVECs
Fresh umbilical cords were obtained 2 to 6 hours after normal-term deliveries from Dr Maurice L. Druzin of the Stanford University Medical Center, Department of Gynecology and Obstetrics. Human umbilical vein endothelial cells were isolated as previously published with slight modification.27 Briefly, the umbilical vein was cannulated with a luer adapter and perfused with collagenase (1000 U/mL, Type 2; Worthington Biochemical, Lakewood, NJ) at 37°C for 10 minutes. Cells liberated by the collagenase treatment were collected in EGM-2-MV EC medium (Cambrex Bioscience, Walkersville, MD) and cultured in a 0.2% gelatin-coated Falcon T25 flask (Fisher Scientific, Pittsburgh, PA). For those HUVECs containing significant contaminating red blood cells, the cells were centrifuged in a 35% Percoll gradient (Amersham Biosciences) to remove the red cells.28
Microarray analysis
Confluent passage-2 HUVECs in Falcon T12.5 flasks (Fisher Scientific) grown in the presence or absence of vascular endothelial growth factor-165 (VEGF-165) (10 ng/mL) (Sigma-Aldrich) were treated with native, cleaved, or latent antithrombins (20 μg/mL) for 24 hours in 3 mL M-199 with 2% heat-inactivated fetal bovine serum (FBS) and antibiotics. Following this treatment, total RNA was extracted from the cells by the Trizol method (Invitrogen). The total RNA was subjected to one cycle of linear RNA amplification using the MessageAmp antisense RNA kit according to the manufacturer's instructions (Ambion, Austin, TX), which routinely generated about 7 to 10 μg antisense RNA for every microgram of total RNA extracted from confluent HUVECs cultured in a T12.5 flask. The quality of the antisense RNA was verified on a denaturing agarose gel. Only samples showing a distribution of sizes from 250 to 5500 nt with a peak centered at 1000 to 1500 nt were used to generate the “test” cDNA samples for the subsequent array experiments. DNA labeling and hybridizations were performed essentially as described,21 with slight modifications. Briefly, the “test” endothelial cell antisense RNA samples together with Universal Human Reference RNA (Stratagene, Cedar Creek, TX) were used to generate Cy3/Cy5 (Amersham Biosciences)–labeled cDNA for array hybridization on the Stanford 43K human cDNA microarray (www.microarray.org/sfgf/jsp/servicesFrame.jsp#productionArrays). The Stanford microarray used in this study consists of 18 416 named genes with UniGene symbol, 4145 expressed sequence tags (ESTs) with known function, 19 365 ESTs with unknown function, and about 1000 repeated spots as internal controls. Hybridized arrays were scanned on a GenePix 4000B scanner (Axon Instruments, Union City, CA) and fluorescence ratios (test-reference) were calculated using the Stanford Microarray Database software (available at http://genome-www.stanford.edu/microarray).29 Fluorescence ratios were normalized for each array by setting the average log fluorescence ratio for all array elements equal to 0. Genes whose expression was at least 3-fold induced or 3-fold repressed by growth factor or antithrombin treatment in at least one experiment with regression correlation more than 0.6 were considered significant. For UniGene clusters represented by multiple arrayed elements, mean fluorescence ratios (for all elements representing the same UniGene cluster) are reported. The entire data set described here can be accessed at the Stanford Microarray Database.
Cell culture and cell proliferation assays
Commercially procured HUVECs were routinely maintained in F12-K medium with 10% FBS, 100 μg/mL heparin, 30 to 50 μg/mL endothelial cell growth supplement, and 1% penicillin and streptomycin (Invitrogen). To determine the growth rates of cells exposed to various treatments, only the cells between passages 5 and 10 were used. HUVECs, at 5000 to 6000 per well, were seeded into 96-well plates in triplicate. After attachment, cells were made quiescent by incubation with F12-K medium containing 0.2% FBS plus 1% penicillin and streptomycin. After 24 hours of incubation, fresh medium containing various combinations of additional agents including native, cleaved, or latent forms of antithrombin (10 to 20 μg/mL), TGF-β1 (5 ng/mL), or bFGF (10 ng/mL) was added and the cells were incubated for another 48 hours. The number of viable cells was quantitated using the nonradioactive colorimetric CellTiter 96 AQ Cell Proliferation Assay according to the manufacturer's instructions (Promega, Madison, WI).
Cell cycle analysis
The effects of bFGF and antithrombin treatments on the cell cycle distribution of cultured HUVECs was analyzed according to Hanai et al.30 HUVECs were growth arrested by contact inhibition for 48 hours. The cells (0.1 × 106 cells per well) were harvested and plated onto a T25 flask in duplicate in endothelial cell growth medium containing 2% FBS and either native, cleaved, or latent forms of antithrombin (20 μg/mL) in the presence and absence of recombinant bFGF (10 ng/mL). The cells were harvested after 72 hours and then fixed in ice-cold 70% ethanol. Fixed cells were incubated at 4°C for 30 minutes in phosphate-buffered saline containing 2% FBS and 0.1% Tween 20 and then centrifuged and resuspended in 0.5 mL of the same buffer. RNase digestion (5 μg/mL) was carried out at 37°C for 1 hour followed by staining with propidium iodide (5 μg/mL). The cells in G1, S, and G2 phases were quantitated using a FACScan (BD PharMingen, San Diego, CA) flow cytometer and Multicycle AV software (Phoenix Flow Systems, San Diego, CA).
Isolation of RNA and RT-PCR analysis
Total mRNA from HUVECs was isolated using the RT-PCR Miniprep kit (Stratgene) according to the manufacturer's instructions. Semiquantitative RT-PCR assays were performed by synthesizing a first-strand cDNA from 200 ng total mRNA with Stratascript RT (Stratagene). PCR amplification of perlecan cDNA was then performed using primers from perlecan domain III,31 5′-ACAGTGCAACAAGTGCAAGG-3′ and 5′-CTGAAGTGACCAGGCTCCTC-3′, which were expected to amplify a 500-bp perlecan gene fragment (nt +2450 to +2950). Simultaneous amplification of human cytoplasmic β-actin25 as a reference used the primers 5′-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3′ and 5′-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3′, which were expected to amplify a 336-bp cDNA fragment. Semiquantitative PCR reactions were performed using the QIAGEN PCR Kit (Qiagen Sciences, Germantown, MD) and included 0.1 μM each of the 2 β-actin primers, 0.16 μM each of the perlecan gene-specific primers, and about 5 ng cDNA template. Twenty-six PCR cycles were found to yield amounts of perlecan and β-actin products that were in the linear range of the logarithm of the product versus number of PCR cycles. Reactions containing all PCR components except for the cDNA template were also amplified as a negative control to check for the presence of contaminating DNA. The products of PCR reactions were separated by electrophoresis on 1.5% agarose gels and detected by staining with SYBR Green dye (Molecular Probes, Eugene OR). The intensity of PCR product bands was quantitated with a Kodak Image Station 440CF (Eastman Kodak, Rochester, NY).
Northern blot analysis
Isolation of total RNA from HUVECs was performed as in the previous paragraph. The RNA was formaldehyde denatured, fractionated on 1.2% agarose gels, transferred by capillary blotting onto Hybond N membranes (Amersham Buchler, Braunschweig, Germany), and fixed by UV cross-linking. The Northern blots were probed with a P32 -labeled 500-bp perlecan cDNA synthesized by RT-PCR as described in the previous paragraph and with a labeled glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA as a loading control. Blots were exposed to X-ray film for detection of bound probe and band intensities were quantitated with the Kodak Image Station.
Slot immunoblotting
For immunoblotting, 106 cells were seeded in 6-well dishes and incubated in serum-free medium. After 12 hours of incubation, cells were replaced with fresh serum-free medium and various treatment agents added, and the cells were then incubated for 72 hours. Serial amounts of the medium were applied to nitrocellulose filters (Schleicher & Schuell, Keene, NH) and air dried. The membranes were blocked with 5% Carnation nonfat dry milk in Tris (tris(hydroxymethyl)aminomethane)–borate buffer containing 0.1% Nonidet P-40 (NP-40)32 and incubated with a monoclonal antibody directed against domain III of perlecan for 1 hour at room temperature. After 3 washes with blocking buffer, the membranes were incubated with an antimouse IgG labeled with horseradish peroxidase for another hour at room temperature, washed with buffer, and then bound antibody was detected by incubation with SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) followed by autoradiography for 10 to 60 seconds. Band intensities were quantified with the Kodak Image Station.
Results
Antiproliferative effects of antithrombin forms
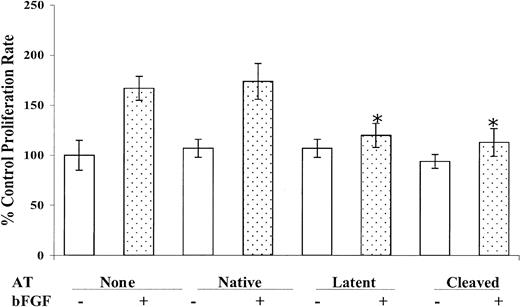
The effects of native, cleaved, and latent antithrombin forms on the proliferation of cultured HUVECs were analyzed in the presence and absence of bFGF (Figure 1). bFGF stimulated the growth of resting HUVECs up to 180%, and latent and cleaved antithrombins significantly inhibited this stimulation, whereas native antithrombin had no effect. In contrast, the basal growth rate of HUVECs in the absence of bFGF was not significantly influenced by treatment with any of the 3 antithrombin forms. Varying the dosage of the cleaved and latent antithrombins used to treat bFGF-stimulated cells showed that the concentration employed in the experiments of Figure 1 (10 μg/mL) was sufficient for maximal inhibition of cell growth (not shown). No significant differences were found between cleaved antithrombins prepared by thrombin or neutrophil elastase cleavage in this assay. Similar differential effects of the antithrombins on the growth of primary HUVECs were observed when VEGF was used to stimulate the cells (not shown). These results confirm the antiproliferative effects of the latent and cleaved forms of antithrombin and inability of native antithrombin to produce such effects as has been established in previous studies.7,8
Inhibition of bFGF-induced endothelial cell proliferation by cleaved and latent antithrombins. Resting HUVEC cells were cultured in the absence or presence of 10 μg/mL each of native, cleaved, or latent forms of antithrombin both in the absence and presence of 10 ng/mL bFGF as indicated. The number of viable cells was determined after 72 hours of incubation as described in “Materials and methods.” Mean values ± SD (bars) derived from 5 independent determinations are shown. *A statistically significant difference from the control (P < .001) based on a Student t test.
Inhibition of bFGF-induced endothelial cell proliferation by cleaved and latent antithrombins. Resting HUVEC cells were cultured in the absence or presence of 10 μg/mL each of native, cleaved, or latent forms of antithrombin both in the absence and presence of 10 ng/mL bFGF as indicated. The number of viable cells was determined after 72 hours of incubation as described in “Materials and methods.” Mean values ± SD (bars) derived from 5 independent determinations are shown. *A statistically significant difference from the control (P < .001) based on a Student t test.
Effects on G1-S phase transition
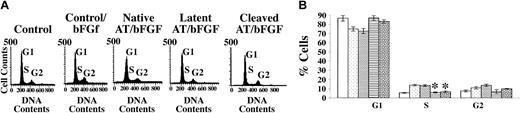
Because growth factors such as bFGF induce cells to exit from a quiescent phase (G0/G1) into an active dividing phase (S phase)30 and cleaved and latent forms of antithrombin inhibit growth factor–induced cell proliferation, it was of interest to determine the effects of the 3 forms of antithrombin on the transition from the G1 to the S phase in HUVECs stimulated by bFGF. Flow cytometric analyses based on the DNA content of HUVECs treated with the 3 forms of antithrombin in the presence and absence of bFGF showed the distribution of cells at each phase of the cell cycle for each of the conditions tested (Figure 2). bFGF increased the fraction of cells making the transition from the resting phase (G1) to the DNA synthesis phase (S) by about 3-fold (from 5.5% to 14%), and this enhancement was unaffected by treatment with native antithrombin. However, treatment of the bFGF-stimulated cells with latent or cleaved antithrombin abolished the enhancement in the fraction of cells in S phase. No effects on the fraction of cells in S phase were observed in those HUVECs treated with any of the 3 forms of antithrombin in the absence of bFGF (data not shown). These results showed that cleaved and latent forms of antithrombin inhibit the G1-S phase cell cycle transition in growth factor–stimulated HUVECs but not in unstimulated quiescent cells.
Effects of antithrombin forms on endothelial cell cycle transitions. Synchronized HUVEC cells (2 × 106) were cultured with different antithrombin forms (20 μg/mL) in the presence or absence of bFGF (10 ng/mL) for 48 hours. Cells were fixed and stained with propidium iodide for detection of DNA and then analyzed by flow cytometry as detailed in “Materials and methods.” (A) Plots of the distribution of cells among the G1, S, and G2 phases of the cell cycle as reflected by their DNA content under the indicated culture conditions. (B) The percentage of cells in each cell cycle phase measured from the data in panel A and other experiments as mean values ± SD (bars) from 3 independent determinations. The different bar patterns represent from left to right the same sequence of conditions given in panel A. *A statistically significant difference from the control (P < .001).
Effects of antithrombin forms on endothelial cell cycle transitions. Synchronized HUVEC cells (2 × 106) were cultured with different antithrombin forms (20 μg/mL) in the presence or absence of bFGF (10 ng/mL) for 48 hours. Cells were fixed and stained with propidium iodide for detection of DNA and then analyzed by flow cytometry as detailed in “Materials and methods.” (A) Plots of the distribution of cells among the G1, S, and G2 phases of the cell cycle as reflected by their DNA content under the indicated culture conditions. (B) The percentage of cells in each cell cycle phase measured from the data in panel A and other experiments as mean values ± SD (bars) from 3 independent determinations. The different bar patterns represent from left to right the same sequence of conditions given in panel A. *A statistically significant difference from the control (P < .001).
cDNA microarray profiling of changes in endothelial cell gene expression
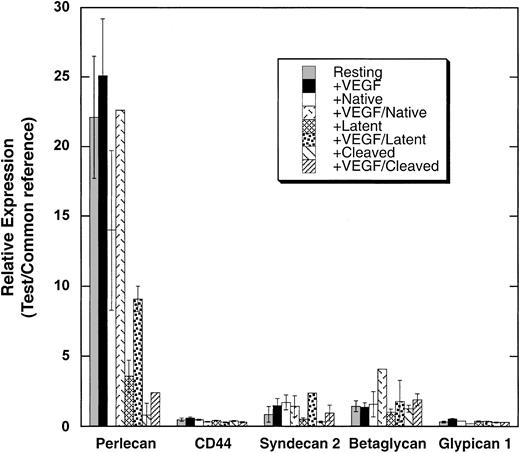
To investigate the mechanism by which latent and cleaved forms of antithrombin inhibit the bFGF-stimulated proliferation of endothelial cells, we analyzed the transcript profile of primary HUVECs untreated and treated with the 3 forms of antithrombin in the presence and absence of vascular endothelial growth factor (VEGF) using the Stanford 43K human cDNA microarray. The gene expression patterns of HUVECs incubated for 24 hours with the 3 forms of antithrombin in the presence and absence of VEGF were compared with untreated controls. Of particular note was the effect of the antithrombins on the expression of the gene for the heparan sulfate proteoglycan, perlecan (Figure 3). Perlecan gene expression was significantly suppressed from 3-fold to 6-fold in cells treated with latent antithrombin and more than 10-fold in cells treated with cleaved antithrombin relative to its expression in untreated cells or cells treated with native anithrombin, and this suppressive effect was observed whether cells were cultured with or without VEGF. By contrast, the expression of other heparan sulfate proteoglycans including syndecan, glypican, betaglycan, and CD44 was 10-fold to 20-fold less than that of perlecan with or without VEGF and not greatly affected by the antithrombin forms (Figure 3).
cDNA microarry analysis of the effects of antithrombin forms on the expression of select heparan sulfate proteoglycans in primary HUVECs. HUVECs were cultured with or without different antithrombin forms (20 μg/mL) in the presence and absence of VEGF (10 ng/mL) as indicated. Total mRNA was isolated from the cells, amplified, and analyzed by cDNA microarray as detailed in “Materials and methods.” The expression of the indicated heparan sulfate proteoglycan mRNAs relative to a universal RNA reference is shown for the different treatment conditions ± SEM.
cDNA microarry analysis of the effects of antithrombin forms on the expression of select heparan sulfate proteoglycans in primary HUVECs. HUVECs were cultured with or without different antithrombin forms (20 μg/mL) in the presence and absence of VEGF (10 ng/mL) as indicated. Total mRNA was isolated from the cells, amplified, and analyzed by cDNA microarray as detailed in “Materials and methods.” The expression of the indicated heparan sulfate proteoglycan mRNAs relative to a universal RNA reference is shown for the different treatment conditions ± SEM.
RT-PCR, Northern blotting, and immunoblotting analyses of perlecan gene expression
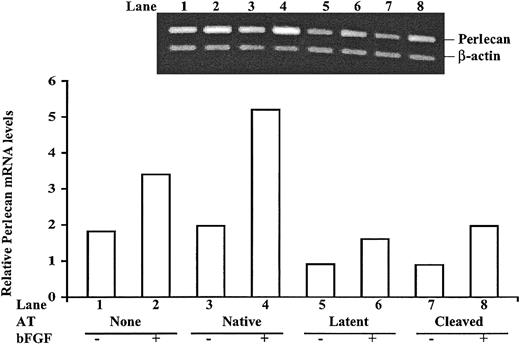
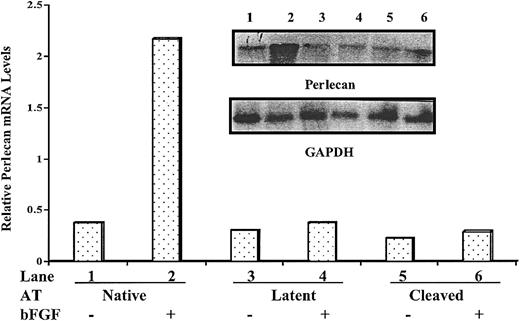
Because of the key role of perlecan in angiogenesis, tissue remodeling, and cell transformation,33 we wished to confirm that perlecan gene expression was suppressed in HUVECs treated with antiangiogenic forms of antithrombin. Relative perlecan mRNA levels were therefore analyzed by semiquantitative RT-PCR in cultured HUVECs treated with the different forms of antithrombin with or without bFGF after establishing the linear range of the assay and normalizing the results to an internal β-actin control (Figure 4). HUVECs treated with bFGF contained 1.8 times more perlecan mRNA than untreated cells, and when native antithrombin was present bFGF caused perlecan mRNA to increase an even greater 2.7-fold in the cells. In contrast, perlecan mRNA levels in cells treated with latent or cleaved forms of antithrombin were all reduced from the untreated cells by 2-fold to 3-fold whether cells were cultured in the presence or absence of bFGF. These results confirmed the findings of the cDNA microarray analysis—that is, that antiangiogenic forms of antithrombin not only abolished bFGF-enhanced expression of the perlecan gene in HUVECs but also suppressed the basal level of perlecan gene expression.
Semiquantiative RT-PCR analysis of perlecan mRNA expression in antithrombin-treated HUVECs. HUVECs were cultured with or without 10 μg/mL of the different antithrombin forms in the presence and absence of 10 ng/mL bFGF as indicated for 72 hours. Total mRNA was isolated, and the content of perlecan mRNA relative to β-actin mRNA was analyzed by semiquantitative RT-PCR. The inset shows the relative intensities of 500 bp perlecan and 336 bp β-actin cDNA fragments amplified from reverse-transcribed mRNA with specific primers under the different experimental conditions, as described in “Materials and methods.” Band intensities from the representative experiment shown were quantified and are presented as the ratio of perlecan to β-actin bands in the bar graph. Similar results were obtained in replicate experiments.
Semiquantiative RT-PCR analysis of perlecan mRNA expression in antithrombin-treated HUVECs. HUVECs were cultured with or without 10 μg/mL of the different antithrombin forms in the presence and absence of 10 ng/mL bFGF as indicated for 72 hours. Total mRNA was isolated, and the content of perlecan mRNA relative to β-actin mRNA was analyzed by semiquantitative RT-PCR. The inset shows the relative intensities of 500 bp perlecan and 336 bp β-actin cDNA fragments amplified from reverse-transcribed mRNA with specific primers under the different experimental conditions, as described in “Materials and methods.” Band intensities from the representative experiment shown were quantified and are presented as the ratio of perlecan to β-actin bands in the bar graph. Similar results were obtained in replicate experiments.
To further confirm the down-regulation of perlecan gene expression in endothelial cells treated with antiangiogenic forms of antithrombin, total RNAs from HUVECs were isolated and perlecan mRNA levels were quantitated by Northern blot analysis after normalization of results to an internal control (Figure 5). Perlecan mRNA levels were increased about 4-fold in cells cultured in the presence of bFGF relative to the levels in cells cultured without bFGF when native antithrombin was present. However, the bFGF-mediated enhancements of perlecan mRNA levels were completely inhibited in HUVECs cultured in the presence of latent or cleaved forms of antithrombin. Although the levels of perlecan mRNA in unstimulated cells also appeared to be lower in cells treated with the antiangiogenic antithrombins than with native antithrombin, the differences could not be reliably determined at the low expression levels observed. These results nevertheless confirmed the marked down-regulation of perlecan transcript in bFGF-stimulated cells treated with the antiangiogenic antithrombins.
Northern blotting analysis of perlecan mRNA expression in antithrombin-treated HUVECs. Total mRNA was isolated from HUVECs cultured under the conditions of Figure 4 and perlecan mRNA expression was analyzed by Northern blotting using a perlecan domain III cDNA probe as described in “Materials and methods.” The same blot was probed with GAPDH cDNA as a loading control. Normalized band intensities are shown.
Northern blotting analysis of perlecan mRNA expression in antithrombin-treated HUVECs. Total mRNA was isolated from HUVECs cultured under the conditions of Figure 4 and perlecan mRNA expression was analyzed by Northern blotting using a perlecan domain III cDNA probe as described in “Materials and methods.” The same blot was probed with GAPDH cDNA as a loading control. Normalized band intensities are shown.
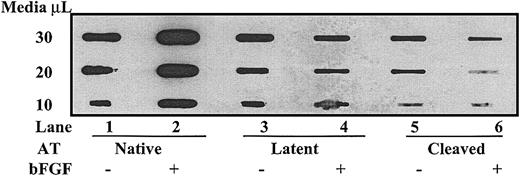
To determine if the down-regulation of perlecan mRNA levels by antiangiogenic forms of antithrombin was paralleled by a decrease in perlecan protein levels, we analyzed perlecan protein in the medium of cultured cells by immunoblotting using a perlecan domain III–specific antibody (Figure 6). Perlecan protein levels were significantly increased about 2-fold to 3-fold in cells cultured in the presence of bFGF plus native antithrombin as compared with cells cultured in the absence of growth factor. The bFGF enhancement of perlecan protein levels was completely abolished in cells treated with latent or cleaved forms of antithrombin, and perlecan levels were in fact reduced from the level found in unstimulated cells. Treatment of HUVECs cultured in the absence of bFGF with latent and cleaved forms of antithrombin also appeared to reduce the levels of perlecan protein from those observed in cells treated with native antithrombin. Together, these results established that antiangiogenic forms of antithrombin significantly down-regulate perlecan gene expression in growth factor–stimulated and unstimulated HUVECs.
Cleaved and latent forms of antithrombin down-regulate perlecan protein expression. HUVECs were cultured in serum-free media with or without 10 μg/mL of the different forms of antithrombin in the presence and absence of 10 ng/mL bFGF as indicated for 72 hours. The conditioned media were applied to a nitrocellulose membrane in the indicated amounts, and perlecan protein was detected by enhanced chemiluminescence using a primary antibody to domain III of perlecan followed by a secondary enzyme-conjugated antibody as detailed in “Materials and methods.”
Cleaved and latent forms of antithrombin down-regulate perlecan protein expression. HUVECs were cultured in serum-free media with or without 10 μg/mL of the different forms of antithrombin in the presence and absence of 10 ng/mL bFGF as indicated for 72 hours. The conditioned media were applied to a nitrocellulose membrane in the indicated amounts, and perlecan protein was detected by enhanced chemiluminescence using a primary antibody to domain III of perlecan followed by a secondary enzyme-conjugated antibody as detailed in “Materials and methods.”
Modulation of antithrombin antiproliferative activity by TGF-β1
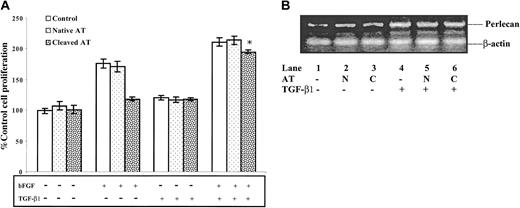
Because bFGF and antiangiogenic forms of antithrombin exert opposite effects on cell proliferation and perlecan gene expression in HUVECs, we evaluated how the antiangiogenic activity of latent and cleaved antithrombins would be affected by another known stimulator of perlecan gene expression, TGF-β134 (Figure 7). Control experiments verified that bFGF stimulated HUVEC proliferation and this stimulation was not affected by treatment with native antithrombin but was nearly abolished in HUVECs treated with cleaved antithrombin. TGF-β1 did not significantly affect the growth of quiescent HUVECs in the absence or presence of native antithrombin. However, TGF-β1 amplified the stimulation by bFGF of HUVEC proliferation, and this enhanced proliferation was only slightly inhibited by cleaved antithrombin (Figure 7A). Relative perlecan mRNA levels measured by semiquantitative RT-PCR were significantly increased in bFGF-stimulated HUVECs treated with TGF-β1 when compared with nontreated cells, and additional treatment with cleaved antithrombin did not significantly affect the mRNA levels (Figure 7B). These results indicate that overexpression of the perlecan gene overcomes the antiproliferative effect of cleaved and latent antithrombins on HUVECs.
Reversal of the antiproliferative effect of cleaved antithrombin on bFGF-stimulated HUVECs by TGF-β1–induced overexpression of perlecan. HUVECs were cultured with or without 10 μg/mL native (N) or cleaved (C) forms of antithrombin in the absence or presence of 10 ng/mL bFGF and/or 5 ng/mL TGF-β1 as indicated for 48 hours, and then the numbers of viable cells were counted as described in “Materials and methods.” (A) The number of viable cells expressed relative to the control as mean ± SD (bars) from 3 independent determinations. *A statistically significant difference from the control in the absence of TGF-β1 (P < .001). (B) The relative perlecan and β-actin control mRNA levels measured in bFGF-stimulated HUVECs cultured in the absence (lanes 1 to 3) or in the presence of TGF-β1 (lanes 4 to 6) by semiquantitative RT-PCR as in Figure 4.
Reversal of the antiproliferative effect of cleaved antithrombin on bFGF-stimulated HUVECs by TGF-β1–induced overexpression of perlecan. HUVECs were cultured with or without 10 μg/mL native (N) or cleaved (C) forms of antithrombin in the absence or presence of 10 ng/mL bFGF and/or 5 ng/mL TGF-β1 as indicated for 48 hours, and then the numbers of viable cells were counted as described in “Materials and methods.” (A) The number of viable cells expressed relative to the control as mean ± SD (bars) from 3 independent determinations. *A statistically significant difference from the control in the absence of TGF-β1 (P < .001). (B) The relative perlecan and β-actin control mRNA levels measured in bFGF-stimulated HUVECs cultured in the absence (lanes 1 to 3) or in the presence of TGF-β1 (lanes 4 to 6) by semiquantitative RT-PCR as in Figure 4.
Discussion
The results of the present study have shown that the antiproliferative effects of the cleaved and latent conformations of antithrombin on human umbilical vein endothelial cells, a component of the more complex antiangiogenic biologic activity of these antithrombin forms,7,8 is correlated with the down-regulation of a key proangiogenic factor, the extracellular matrix heparan sulfate proteoglycan, perlecan.22,23,33,35 Down-regulation of perlecan expression was shown to occur both at the mRNA and protein levels, to be induced by the antiangiogenic cleaved and latent conformations of antithrombin and not the native serpin conformation, and to be dramatic—that is, up to about 6-fold to 10-fold by microarray analysis of primary low-passage HUVEC cultures and about 3-fold to 4-fold by semiquantitative RT-PCR and Northern blot analyses of somewhat higher passage HUVEC cultures. Perlecan down-regulation was observed in both resting and growth factor–stimulated endothelial cells. The expression of other endothelial cell heparan sulfate proteoglycans was not greatly affected by any of the antithrombin forms, indicating that the antiangiogenic antithrombins specifically affected perlecan-type heparan sulfate proteoglycan expression in HUVECs.
We confirmed the observations of past studies that antiangiogenic forms of antithrombin principally inhibit the proliferation of growth factor–stimulated endothelial cells and minimally affect the growth of unstimulated cells, although our studies used HUVECs instead of the porcine aortic or bovine capillary endothelial cells used in past studies.7,8 The inhibition of bFGF-stimulated HUVEC proliferation by cleaved and latent antithrombins was shown to arise through the suppression of the growth factor–enhanced transition of cells from the G1 to the S phase of the cell cycle. The antiangiogenic collagen XVIII fragment, endostatin, produces an antiproliferative effect on endothelial cells by a similar mechanism.30 That perlecan levels critically mediate the growth factor–dependent proliferation of endothelial cells was shown by the finding that up-regulation of perlecan expression by TGF-β134 overcame the antiproliferative effects of the antiangiogenic antithrombins on HUVECs. The similar growth factor–dependent antiproliferative activity of cleaved and latent antithrombins on aortic, capillary, and umbilical vein endothelial cells observed in the present and past studies7,8 suggests that perlecan down-regulation may be a common mediator of this activity, although further studies will be required to validate this possibility.
The dramatic effects of antiangiogenic antithrombins on perlecan expression provide a readily understandable mechanism by which these antithrombins may block growth factor–stimulated endothelial cell poliferation as well as inhibit other reported growth factor–dependent activities associated with angiogenesis. Perlecan is a major heparan sulfate proteoglycan secreted by endothelial cells and highly expressed in several tumor cell lines.23,33,35 It functions as an essential coreceptor for bFGF and VEGF family growth factors, enabling them to bind and activate their receptors and to thereby promote cell growth and differentiation.33 In particular, perlecan-bFGF complexes mediate the proangiogenic activity of bFGF in which endothelial cells are stimulated to form new blood vessels. The coreceptor function of perlecan results from bFGF binding to the heparan sulfate chains of the proteoglycan. Suppression of perlecan expression by antisense approaches blocks bFGF activity and proliferation in 3T3 fibroblasts and several tumor cell lines23,35 as well as tumor cell–induced angiogenesis,10,36 and these activities are restored by addition of exogenous perlecan. A mouse knock-out of the perlecan gene causes aberrant cartilage development thought to reflect defective growth factor signaling.37,38 The well-established inhibition of bFGF-stimulated endothelial cell growth and angiogenesis as well as angiogenesis-dependent tumor growth by cleaved and latent forms of antithrombin could thus be due to the decreased levels of perlecan heparan sulfate chains and consequent inability of the growth factors to bind and activate their receptors. Other growth factor–dependent effects involved in angiogenesis and inhibited by antiangiogenic antithrombins include focal adhesion kinase activation, focal adhesion contact formation, and actin reorganization.21 All of these effects may similarly be accounted for by the blocking of growth factor stimulation of cells by antiangiogenic antithrombins through the down-regulation of perlecan expression.
Our finding that perlecan expression is down-regulated also in unstimulated endothelial cells without any significant effects on the growth or distribution of cells in G1 and S cell cycle phases suggests that perlecan levels are not critical for the growth of resting cells. Similarly, in 3T3 fibroblast and melanoma cells, suppression of perlecan gene expression by an antisense cDNA affected only the response of cells to bFGF-stimulated growth and not the basal growth rate.23 Antiangiogenic forms of antithrombin have also been shown to enhance apoptosis in both resting and growth factor–stimulated endothelial cells.21 The observation of both growth factor–independent and –dependent effects of cleaved and latent but not native forms of antithrombin on endothelial cells suggests that the antiangiogenic antithrombins produce global effects on endothelial cells independent of growth factor binding presumably mediated by a specific endothelial cell receptor interaction. Binding of the antiangiogenic antithrombins to this putative receptor presumably blocks growth factor–dependent angiogenesis activity by down-regulating perlecan expression and stimulating apoptosis.
Endostatin acts as an inhibitor of bFGF-dependent angiogenesis, but the antiangiogenic activity appears to be due in this case to endostatin binding to heparan sulfate proteoglycan coreceptors, which thereby blocks growth factor binding and activation of endothelial cells.39 This mechanism is suggested by the demonstrated requirement of the heparin binding site of endostatin for this activity and identification of specific heparan sulfate binding domains that mediate the activity.40 While our results suggest that antiangiogenic forms of antithrombin bind to an endothelial cell receptor to produce their effects, it is possible that these antithrombins may also require a heparan sulfate coreceptor to mediate this binding as is the case with bFGF and VEGF binding to their receptors. Cleaved and latent antiangiogenic forms of antithrombin bind heparin but have greatly reduced affinity for the polysaccharide when compared with the nonantiangiogenic native antithrombin conformation.13,14 The higher affinity of native antithrombin for heparin is due to the recognition of a specific anticoagulant sequence present in a small fraction of the chains.41 Binding of antiangiogenic antithrombins to nonanticoagulant heparan sulfate sequences could thus play a role in mediating the antiangiogenic effects of the serpin. This would be in keeping with the observation that anticoagulant heparan sulfate sequences associated with endothelial cells are localized in membrane-associated glypican-type heparan sulfate proteoglycans, whereas nonanticoagulant chains that bind antithrombin exist on matrix-associated perlecan.42 Future studies will need to address the importance of the heparin binding site of antithrombin for its antiangiogenic activity.
In summary, our results have shown that the antiproliferative effects of cleaved and latent forms of antithrombin on endothelial cells and presumably also the antiangiogenic effects may be explained by these antithrombins inducing a down-regulation of perlecan expression. Because perlecan is an essential coreceptor for bFGF-induced angiogenesis,33 the down-regulation of this coreceptor in endothelial cells by the antiangiogenic antithrombins can explain why bFGF-dependent angiogenesis is inhibited. This proposal is supported by the similar inhibition of bFGF-dependent proliferation and angiogenesis in tumor cells produced by down-regulation of perlecan expression using antisense methods.23,35,36 Moreover, the inhibition of bFGF-dependent endothelial cell proliferation by antiangiogenic antithrombins can be overcome through the up-regulation of perlecan expression by TGF-β1.34 Perlecan expression thus appears to be a key means of regulating the activities of both proangiogenic and antiangiogenic factors in endothelial cells and possibly in tumor cells as well.
Prepublished online as Blood First Edition Paper, October 16, 2003; DOI 10.1182/blood-2003-08-2920.
Supported by National Institutes of Health (NIH) grant R01-HL-39888.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Chiayeng Wang for help with the Northern blotting experiments and Peter Gettins for critiquing the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal