Abstract
The chemokine receptor, CXCR4, serves as the primary coreceptor for entry of T-cell tropic human immunodeficiency virus (HIV). Binding of either the CXC-chemokine, stromal-derived factor 1α (SDF-1α), or a CXCR4 antagonist, AMD3100, to CXCR4 inhibits infection of CD4+ T cells by T-tropic HIV-1, although only SDF-1α triggers T-cell signaling cascades. We have previously demonstrated that ligation of CD4 by T-cell tropic HIV-1 NL4-3 induces metalloproteinase-dependent L-selectin (CD62L) shedding on resting CD4+ T cells. However, the role of CXCR4 in HIV-induced L-selectin shedding is unclear. Here, we show that L-selectin shedding induced by HIV-1 NL4-3 is completely reversed by AMD3100, but not SDF-1α, although SDF-1α alone does not induce L-selectin shedding. These results indicate that engagement of both CD4 and CXCR4 is required for HIV-induced shedding of L-selectin on primary resting CD4+ T cells.
Introduction
Lymphocyte homing requires a sequence of critical adhesion events to allow naive lymphocytes to rapidly recirculate from the blood into lymphoid organs and is an important immune surveillance function in vivo. L-selectin (CD62L), which is constitutively expressed at high levels on naive T lymphocytes, mediates rolling and tethering of naive T lymphocytes along endothelial surfaces.1-3 After L-selectin binds to its ligand on high endothelial venules, G protein–linked receptors activate integrins that mediate the arrest and diapedesis of naive T lymphocytes into the peripheral lymph nodes (PLNs).4 In experiments using neutralizing anti–L-selectin monoclonal antibodies (mAbs) or mice that are genetically deficient in L-selectin, naive T lymphocytes do not home to PLNs, and primary T-cell responses to antigen are impaired.1,5,6 L-selectin is shed from the cell surface by a proteolytic mechanism upon crosslinking by mAbs7 or upon T-cell activation.8,9 The signal transduction mechanisms leading to L-selectin cleavage are not yet defined.
To date, little is known about homing and recirculation of lymphocytes in HIV disease. It has been shown that HIV-1 and simian immunodeficiency virus (SIV) induce preferential homing of T cells to the intestine in engrafted severe combined immunodeficiency mice.10 A study by Zhang et al showed a significant depletion of naive CD45RA+CD4+ T cells, out of proportion to total CD4+ T cells, in peripheral lymphoid tissue from HIV-1–infected individuals.11 Additionally, individuals infected with HIV-1 have a 3-fold increase of soluble L-selectin in serum,12 and recent studies show that L-selectin on lymphocytes is significantly reduced in HIV-1–infected children, compared with uninfected controls.13 We have previously reported that ligation of CD4 by mAb or by HIV-1 NL4-3 results in the metalloproteinase-dependent shedding of L-selectin from primary resting CD4+ T cells.14 We have also shown that this L-selectin shedding results in inhibition of T-cell homing to the lymph nodes.14 In this report, using the CXC chemokine receptor 4 (CXCR4) antagonist, AMD3100, and the CXCR4 natural ligand, stromal-derived factor 1α (SDF-1α), we show that engagement of CXCR4, in addition to CD4, is required for the L-selectin shedding induced by the glycoprotein (gp)120-CD4 interaction.
Study design
Isolation of human CD4+ T cells
Human peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (Amersham Pharmacia, Piscataway, NJ) density-gradient centrifugation of heparinized venous blood obtained from healthy donors. CD4+ T cells were purified by negative selection with a cocktail of antibodies directed against CD8 (OKT8; American Type Culture Collection [ATCC], Rockville, MD), CD14, CD16, and CD19 (PharMingen, San Diego, CA).14 More than 99% of cells were in the G0/G1 phase of the cell cycle, and 90% to 95% of cells were CD4+.
Reagents and cell lines
CD62L–phycoerythrin (PE) (Leu-8) and CD4-PE (Leu3a) were purchased from Becton Dickinson (San Jose, CA). CXCR4-PE (12G5) and CD69-PE (FN50) were purchased from PharMingen. AMD3100 was generously provided by J. Strizki (Schering-Plough, Kennilworth, NJ). SDF-1α was purchased from Peprotech (Rocky Hill, NJ). Jurkat T cells were obtained from the ATCC.
Coculture of resting CD4+ T cells with HIV-1–infected cells
Jurkat T cells (2 × 106) were incubated with HIV-1 NL4-3 at a multiplicity of infection (MOI) of 0.2 or mock infected at 37°C for 2 hours. Before coculture, CD4+ T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and treated with medium, 0.5 to 20μg/mL SDF-1α, 1 μg/mL AMD3100, or 10 μg/mL bovine serum albumin (BSA) at 37°C for one hour. For coculture, 1 to 2 × 106 CFSE-labeled resting CD4+ T cells were mixed with 1 to 2 × 106 mock- or HIV-1–infected cells, and cultured for 16 hours with or without SDF-1α, AMD3100, or BSA in the culture medium. The expression of L-selectin, CD69, CXCR4, and CD4 on CFSE+ cells was determined by flow cytometry. A total of 10 000 events was analyzed for each sample. After coculture, the cell viability and number of resting CD4+ T cells were examined by trypan blue. No increased cytopathicity or cell-cell fusion events were observed in the HIV-1 cocultures compared with the mock cocultures.
Results and discussion
HIV-1 entry into target cells is mediated by an interaction between CD4 and members of the chemokine receptor family of proteins.15,16 In this process, envelope gp120 first binds to the CD4 receptor with high affinity, resulting in the exposure of coreceptor binding sites in gp120. Coreceptor binding then triggers the fusion and entry of the viral genome into host cells. The major HIV-1 coreceptors are CCR5 and CXCR4, required for infection by M-tropic and T-tropic HIV-1 viruses, respectively. SDF-1α inhibits HIV-1 infection by T-tropic viral isolates.17,18 Ligation of CXCR4 by SDF-1α also triggers signaling cascades, including calcium mobilization, phosphorylation of Pyk2, and activation of mitogen-activated protein kinase (MAPK)/extracellular signal-related kinase (ERK) pathways.19,20 AMD3100, a small molecule inhibitor of CXCR4, also blocks HIV-1 entry and fusion by binding to the extracellular domain 2 of CXCR4, although AMD3100 itself does not induce signaling.21 In previous studies14 and in Figure 1, we showed that soluble CD4 (sCD4) inhibits L-selectin shedding on resting CD4+ T cells induced by T-tropic HIV-1 NL4-3, suggesting that this shedding is due to the binding of HIV gp120 to the CD4 receptor. These studies did not, however, address whether the chemokine receptors also play a role in L-selectin shedding.
Soluble CD4 (sCD4) inhibits HIV-induced L-selectin shedding on resting CD4+ T cells. HIV-1 NL4-3–infected (thick lines) or mock-infected (thin lines) Jurkat cells (2 × 106) were treated with medium, 10 μg/mL soluble CD4, or 10 μg/mL BSA at 37°C for one hour, and cocultured with 2 × 106 CFSE-labeled human CD4+ T cells at 37°C for 16 to 20 hours. The expression of L-selectin and CD69 on CFSE+ cells was determined by flow cytometry.
Soluble CD4 (sCD4) inhibits HIV-induced L-selectin shedding on resting CD4+ T cells. HIV-1 NL4-3–infected (thick lines) or mock-infected (thin lines) Jurkat cells (2 × 106) were treated with medium, 10 μg/mL soluble CD4, or 10 μg/mL BSA at 37°C for one hour, and cocultured with 2 × 106 CFSE-labeled human CD4+ T cells at 37°C for 16 to 20 hours. The expression of L-selectin and CD69 on CFSE+ cells was determined by flow cytometry.
Is binding of the chemokine receptor necessary for HIV-1–induced L-selectin shedding? The observed inhibition of HIV-1–induced L-selectin shedding by sCD4 is inconclusive, since, as discussed above, most strains of HIV-1 must bind CD4 prior to engagement of CCR5 or CXCR4. Our first indication that the chemokine receptor might play a critical role in L-selectin shedding came from analyses of T cells from human CD4 transgenic mice. Although activation with phorbol myristate acetate (PMA) or ligation of the antigen receptor induced L-selectin shedding in this transgenic mouse, HIV-1–infected Jurkat cells did not (data not shown). These data suggested that ligation of CD4 by HIV-1 is not sufficient for L-selectin shedding. In addition, soluble gp120 (sgp120) does not induce L-selectin shedding on human T cells (data not shown). One possible explanation for the triggering of L-selectin shedding by HIV-1, but not by sgp120, is that the stoichiometry of CD4 binding is different for monomeric sgp120 versus the gp120/gp41 oligomers on the virion surface.22 Alternatively, since sgp120 monomers reportedly have high affinity for CD4, but low affinity for the chemokine receptor,23 we reasoned that ineffective signaling through the chemokine receptor might fail to trigger shedding.
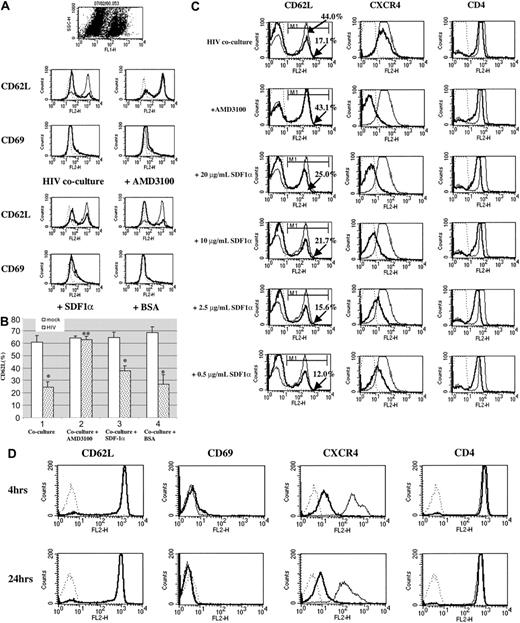
To directly test the role of the chemokine receptor in HIV-1–induced L-selectin shedding, we pretreated resting CD4+ T cells with AMD3100 or SDF-1α before coculturing the cells with NL4-3–infected Jurkat cells, and assessed the expression of L-selectin after 16 to 20 hours. AMD3100 completely inhibited the L-selectin shedding induced by HIV-1, as shown in Figure 2A-B. In contrast, SDF-1α only partially inhibited L-selectin shedding. In order to address the possibility that SDF-1α levels were insufficient to saturate CXCR4, increasing concentrations of SDF-1α (0.5-20 μg/mL) were used to pretreat CD4+ T cells. As shown in Figure 2C, a concentration of SDF-1α (20 μg/mL) yielding almost complete blocking of CXCR4, equivalent to that seen with AMD3100, still did not completely inhibit L-selectin shedding. Partial inhibition was evidenced by the titration effect of SDF-1α on L-selectin shedding (Figure 2C). The failure of SDF-1α to completely block L-selectin shedding has a number of possible explanations, including less potent inhibition of HIV-1 infection, relative to AMD3100, instability of bound SDF-1α, or internalization/degradation of CXCR4.24,25 Together, these data show that AMD3100 eliminates the down-regulation of L-selectin induced by HIV-1 on resting CD4+ T cells, suggesting that the chemokine receptor, CXCR4, in addition to CD4, regulates L-selectin shedding.
AMD3100, but not SDF-1α, completely inhibits HIV-induced L-selectin shedding on resting CD4+ T cells. CFSE-labeled human CD4+ T cells (2 × 106) were treated with medium, 1 μg/mL AMD3100, 10 μg/mL SDF-1α,or10 μg/mL BSA at 37°C for one hour, and cocultured with 2 × 106 HIV-1 NL4-3–infected or mock-infected Jurkat cells at 37°C for 16 to 20 hours. (A) The expression of L-selectin and CD69 on CFSE+ cells was determined by flow cytometry. Results shown are representative of 3 experiments. (B) As in Figure 1A, showing the average ± SEM of 3 independent experiments. *P < .05; **P > .05. (C) Dose response of SDF-1α in induction of L-selectin shedding. CFSE-labeled human CD4+ T cells were pretreated with media or with varying concentrations of SDF-1α at 37°C for one hour. After washing, human CD4+ T cells were mixed with HIV-1 NL4-3–infected or mock-infected Jurkat cells and cocultured at 37°C for 16 to 20 hours. The expression of L-selectin, CXCR4, and CD4 was analyzed by flow cytometry (gated on CFSE+ cells). Thick lines represent the expression of L-selectin, CXCR4, and CD4 on human CD4+ T cells cocultured with HIV-1 NL4-3–infected Jurkat cells; thin lines represent the expression of L-selectin, CXCR4, and CD4 on CD4+ T cells cocultured with mock-infected Jurkat cells; dotted lines represent the isotype control. The apparent decrease in expression of CXCR4 in the presence of AMD3100 is due to binding of AMD3100 to the same epitope as 12G5, the anti-CXCR4 Ab.21 (D) SDF-1α does not induce L-selectin shedding. Human CD4+ T cells were treated with media (thin line) or 2.0 μg/mL SDF-1α (thick line) at 37°C. The expression of L-selectin, CD69, CXCR4, and CD4 was analyzed after 4 hours or 24 hours of culture by flow cytometry. Dotted lines represent the isotype control. A representative experiment of 3 is shown.
AMD3100, but not SDF-1α, completely inhibits HIV-induced L-selectin shedding on resting CD4+ T cells. CFSE-labeled human CD4+ T cells (2 × 106) were treated with medium, 1 μg/mL AMD3100, 10 μg/mL SDF-1α,or10 μg/mL BSA at 37°C for one hour, and cocultured with 2 × 106 HIV-1 NL4-3–infected or mock-infected Jurkat cells at 37°C for 16 to 20 hours. (A) The expression of L-selectin and CD69 on CFSE+ cells was determined by flow cytometry. Results shown are representative of 3 experiments. (B) As in Figure 1A, showing the average ± SEM of 3 independent experiments. *P < .05; **P > .05. (C) Dose response of SDF-1α in induction of L-selectin shedding. CFSE-labeled human CD4+ T cells were pretreated with media or with varying concentrations of SDF-1α at 37°C for one hour. After washing, human CD4+ T cells were mixed with HIV-1 NL4-3–infected or mock-infected Jurkat cells and cocultured at 37°C for 16 to 20 hours. The expression of L-selectin, CXCR4, and CD4 was analyzed by flow cytometry (gated on CFSE+ cells). Thick lines represent the expression of L-selectin, CXCR4, and CD4 on human CD4+ T cells cocultured with HIV-1 NL4-3–infected Jurkat cells; thin lines represent the expression of L-selectin, CXCR4, and CD4 on CD4+ T cells cocultured with mock-infected Jurkat cells; dotted lines represent the isotype control. The apparent decrease in expression of CXCR4 in the presence of AMD3100 is due to binding of AMD3100 to the same epitope as 12G5, the anti-CXCR4 Ab.21 (D) SDF-1α does not induce L-selectin shedding. Human CD4+ T cells were treated with media (thin line) or 2.0 μg/mL SDF-1α (thick line) at 37°C. The expression of L-selectin, CD69, CXCR4, and CD4 was analyzed after 4 hours or 24 hours of culture by flow cytometry. Dotted lines represent the isotype control. A representative experiment of 3 is shown.
Is binding of CXCR4 by its ligand sufficient to induce L-selectin shedding? As discussed above, AMD3100 blocks both membrane fusion and CXCR4 signaling. In contrast, although SDF-1α inhibits fusion, binding of SDF-1α transduces signals through CXCR4. We reasoned that SDF-1α itself might induce L-selectin shedding. In contrast, as shown in Figure 2D, SDF-1α alone did not lead to decreased expression of L-selectin on resting CD4+ T cells, although it did cause CXCR4 internalization. Thus, both CD4 and CXCR4 are required for HIV-induced L-selectin shedding.
CD4 and CXCR4 are also required for HIV-1 infection. Although primary resting CD4+ T cells do not support productive infection, these cells do support early postentry events of HIV-1 infection26,27 , as shown in Supplemental Figure S1 (available at the Blood website; see the Supplemental Figures link at the top of the online article). In order to confirm that L-selectin shedding is due to “early” events following envelope binding, AZT (3′-azido-3′-deoxythymidine) was used to block reverse transcription. As shown in Supplemental Figure S2, AZT does not block HIV-induced L-selectin shedding on resting CD4+ T cells. Thus, our data suggest that early CD4/CXCR4-mediated events prior to reverse transcription are sufficient for HIV-induced L-selectin shedding.
In summary, these data together with our previously published findings suggest that the chemokine receptor, CXCR4, as well as CD4, regulates HIV-1–induced L-selectin shedding on resting CD4+ T cells. L-selectin shedding may be mediated by calciumcalmodulin–induced conformational changes in L-selectin.28 Our preliminary data show that T cells from mice transgenic for a mutated CD4 lacking the cytoplasmic tail, and thus lacking the signaling domain of CD4, do not shed L-selectin in response to CD4 ligation. Intriguingly, CXCR4 blockade by the inert AMD3100, but not by SDF-1α, inhibits HIV-1–induced L-selectin shedding. While the failure of SDF-1α to block shedding might be due to its degradation or to induced CXCR4 internalization, it is possible that signals transduced via SDF-1α binding contribute to L-selectin shedding. We suggest that the L-selectin shedding and aberrant homing induced by HIV-1 are dependent upon signals mediated by both CD4 and CXCR4.
Prepublished online as Blood First Edition Paper, October 23, 2003; DOI 10.1182/blood-2003-02-0576.
Supported by National Institutes of Health (NIH) RO1 AI35513, NIH RO1 AI40003, the University of Pennsylvania Center for AIDS Research, the Bender Foundation, and the NIH AIDS Research and Reference Reagent Program.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Nithianandan Selliah for his critical review and thoughtful comments and Mrs Lisa M. Sudell for her kind help in the preparation of the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal