Abstract
The endothelial cell protein C (PC) receptor (EPCR) facilitates PC activation by the thrombin-thrombomodulin complex. A soluble form of this receptor (sEPCR) found in plasma inhibits both activated PC (aPC) activity and PC activation by competing for PC with membrane-associated EPCR. Elevated sEPCR levels are found in approximately 20% of healthy subjects, but the mechanisms underlying this interindividual variability are unknown. We measured sEPCR levels in 100 healthy male volunteers, and observed 2 phenotypic groups of subjects. The temporal stability of sEPCR levels suggested genetic control. Extensive analysis of the EPCR gene in these subjects revealed 13 polymorphisms in complete linkage disequilibrium; these defined 3 haplotypes, 1 of which (A3) was strongly associated with high sEPCR levels. The high constitutive sEPCR levels observed in A3 haplotype carriers might reduce the efficiency of the PC system and predispose these subjects to venous thrombosis. By studying 338 patients with venous thrombosis and 338 age- and sex-matched healthy subjects, we found that the A3 haplotype was overrepresented in the patients. In multivariate analysis, subjects carrying the A3 haplotype had an increased risk of thrombosis (odds ratio [OR] = 1.8; P = .004). Thus, the A3 haplotype, which is associated with elevated plasma sEPCR levels, is a candidate risk factor for venous thrombosis.
Introduction
The protein C (PC) system is an important natural anticoagulant mechanism. PC, a vitamin K–dependent zymogen, is activated at the endothelial surface when thrombin binds to thrombomodulin, a protein that transforms the procoagulant enzyme into a potent activator of PC. In the presence of its cofactor, protein S, activated protein C (aPC) inactivates factors Va and VIIIa, thereby reducing thrombin generation.1
Another factor contributing to PC activation—endothelial cell activated protein C receptor (EPCR)—was discovered more recently at the surface of endothelial cells.2 This receptor, which can bind PC or aPC with the same affinity (dissociation constant [Kd] = 30 nM), is mainly expressed on endothelial cells of large vessels.3-5
Functional studies performed in vitro showed a 3- to 5-fold increase in the PC activation rate by the membrane thrombinthrombomodulin complex when PC is bound to its receptor.6 This increase results from a significant effect of EPCR on the MichaelisMenten constant (Km) for PC activation by the thrombinthrombomodulin complex. Indeed, without EPCR intervention, this Km is significantly higher (1 μM) than the circulating concentration of PC (60-70 nM). By presenting PC to the thrombin-thrombomodulin complex, and owing to its lateral mobility, EPCR reduces the Km and thereby allows the interaction to occur.
In view of these functions, EPCR was expected to intervene in the physiologic regulation of coagulation. Evidence for an important role of this type came from baboon studies,7,8 in which an 88% reduction in aPC generation induced by thrombin infusion was observed in animals that had been pretreated with anti-EPCR antibodies blocking the PC/EPCR interaction.
EPCR is a 46-kDa type 1 transmembrane glycoprotein homologous to major histocompatibility complex class I/CD1 family proteins.2,9,10 This 221-amino-acid (aa) protein comprises an extracellular domain, a 25-aa transmembrane domain, and a short (3 aa) intracytoplasmic sequence. The gene is located on chromosome 20,11 at position q11.2; it spans 8 kilobase (kb) and comprises 4 exons.11,12 The first exon encodes the 5′ untranslated region and the signal peptide; exons 2 and 3, most of the extracellular domain; and exon 4, the remaining parts of the protein and the 3′ untranslated region. The proximal part of the promoter was recently functionally characterized.13
Several authors have reported the presence in plasma of a soluble form of EPCR (sEPCR),14,15 which probably lacks the transmembrane domain and cytoplasmic tail. This sEPCR is detected as a single species of 43 kDa, resulting from shedding of membrane EPCR by the action of a metalloprotease,16 which is stimulated by thrombin and by some inflammatory mediators.17 Soluble EPCR binds PC and aPC with similar affinity,18,19 but its binding to aPC inhibits the anticoagulant activity of aPC by blocking its binding to phospholipids and by abrogating its ability to inactivate factor Va.20 By contrast with the membrane-associated form of EPCR, PC binding to sEPCR does not result in enhanced aPC generation by the thrombin-thrombomodulin complex.19 A recombinant sEPCR has recently been crystallized.21
Dysfunctional EPCR-dependent activation of PC would potentially be thrombogenic. A loss of function could result from mutations leading to decreased expression of membrane EPCR. A 23–base pair (bp) insertion has been reported to impair EPCR functions by leading to the synthesis of a truncated protein that is not expressed on endothelial surfaces.22 Although initially identified in thrombophilic subjects,23 the role of this mutation in thrombosis is difficult to assess because its allelic frequency is low.23-26 Point mutations were recently described within the promoter region27 of the gene in 4 thrombophilic subjects, but the involvement of these mutations in gene regulation could not be clearly demonstrated.
Another possible mechanism leading to dysfunction of the EPCR-mediated coagulation-regulating mechanism consists of mutations (or polymorphisms) leading to increased levels of sEPCR. Indeed, increased sEPCR levels may be prothrombotic, as sEPCR can inhibit aPC activity, as well as PC activation, by competing for PC with membrane-associated EPCR.
Recently, 2 studies have shown that sEPCR levels vary widely among healthy subjects.28,29 While sEPCR levels are between 75 and 178 ng/mL in 80% of subjects, the remaining 20% of subjects have values between 200 and 700 ng/mL. This bimodal distribution has repeatedly been reported in both French and Italian populations.29
The aim of this study was to seek a genetic explanation for the bimodal distribution of sEPCR levels. We first measured plasma sEPCR levels in 100 healthy male volunteers and confirmed the expected bimodal distribution. We then extensively analyzed the EPCR gene of the same subjects. We identified several polymorphisms that were in complete linkage disequilibrium, defining 3 haplotypes. One of these haplotypes was associated with increased sEPCR levels, offering the first evidence that interindividual variations in sEPCR levels are genetically regulated.
As sEPCR can inhibit both aPC generation and aPC activity, we examined whether the haplotype associated with high sEPCR levels carried an increased risk of venous thrombosis. On comparing 338 subjects with thrombosis and 338 age- and sex-matched healthy controls, we observed a significantly higher allelic frequency of this haplotype in the cases, suggesting that it may be a risk factor for venous thrombosis.
Patients, materials, and methods
Materials
Evacuated tubes were from Becton Dickinson (Le Pont de Claix, France). The Qiamp Maxi kit was from Qiagen (Courtaboeuf, France). The sEPCR Asserachrom kit was kindly supplied by Stago Laboratories (Asnières, France). The DNA sequencing kit (Big Dye Terminator V3.0 Cycle Sequencing Ready Reaction with AmpliTaq DNA Polymerase FS) and the ABI Prism 3700 sequencer were from Applied Biosystems (Applera, Courtaboeuf, France). The deoxynucleoside triphosphate (dNTP) mix was from Amersham Biosciences Europe (Orsay, France). Oligonucleotides were from Proligo (Paris, France). Plates for amplified-product purification were from Millipore (Saint-Quentin en Yvelines, France). PstI restriction endonuclease was from New England Biolabs (Ozyme, Saint Quentin en Yvelines, France). Agarose was from Life Technologies (Invitrogen, Cergy-Pontoise, France).
Healthy subjects and patients
Recruited and studied at the Clinical Investigations Center of Hôpital Européen Georges Pompidou were 100 unrelated healthy white male volunteers aged from 18 to 35 years. This population has been described in detail elsewhere.30,31 Briefly, the volunteers were nonsmokers and had not taken any medication for at least 10 days before blood sampling. Volunteers with a personal or family history of excessive bleeding or thrombosis were excluded. The subjects underwent a physical examination and routine laboratory tests,30,31 including C-reactive protein and F1+2 assay.
Blood was collected from all volunteers by venipuncture in tubes containing 0.11 M sodium citrate (1 vol/9 vol) on day 1 (visit 1) and day 7 (visit 2). Plasma was obtained by centrifugation at 2300g for 20 minutes, and was immediately subjected to routine laboratory tests or stored at –80°C until use. Genomic DNA was isolated from peripheral blood mononuclear cells using the Qiamp Maxi kit according to the manufacturer's instructions.
A group of 338 patients, matched for age and sex with 338 controls, was studied in a second phase. These subjects had participated in a case-control study, the PAris THRombosis Study (PATHROS), designed to seek genetic risk factors for venous thromboembolism (VTE). The inclusion and exclusion criteria applied to cases, and their clinical and biologic characteristics, have been extensively described elsewhere.32 Briefly, the patients had had at least one episode of objectively diagnosed deep venous thrombosis (documented by compression and ventilation lung ultrasonography or venography) and/or pulmonary embolism (documented by perfusion and ventilation lung scanning, convention pulmonary angiography, or computed tomographic angiography). The controls were healthy European subjects recruited from a health care center to which they had been referred for a routine checkup. Subjects with a history of VTE, arterial disease, or known malignancy were excluded on the basis of a medical questionnaire. To avoid a possible bias due to the fact that controls came from a health care center, we checked that the frequencies of factor V and prothrombin 20210G>A mutations were similar to those observed in other control populations.32,33
The study protocols were approved by our local ethics committee.
Blood was collected and plasma prepared as previously described.32 DNA was extracted from white blood cells using a standard method.34 Factor V Arg506Gln and prothrombin gene 20210G>A mutations were identified as previously described.35 All samples were obtained with the participants' informed consent.
Soluble EPCR assay
Soluble EPCR (sEPCR) levels were determined in plasma by using sEPCR Asserachrom enzyme-linked immunosorbent assay (ELISA) kits from the same batch, according to the manufacturer's instructions.
EPCR gene screening for polymorphisms
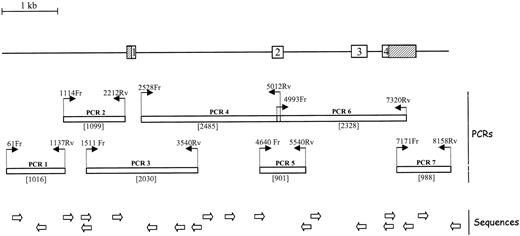
The nucleotides (nt) of the EPCR gene were numbered according to the sequence available under GenBank accession number AF106202. As shown in Figure 1, 7 amplification fragments spanned more than 99% of the EPCR gene. The location of the amplification primers relative to the EPCR gene sequence, as well as their nucleotide sequences, is indicated in Figure 1 and its legend. Each amplification product was purified by filtration on Millipore plates and sequenced with a DNA sequencing kit according to the manufacturer's instructions; the sequencing products were analyzed on ABI Prism 3700 sequencer.
Location of the amplification and sequencing primers on the EPCR gene. Exons are symbolized by boxes. The 5′ part of exon 1 and the 3′ part of exon 4, which are noncoding, are striped. The primer pairs used to amplify nearly the entire gene in 7 PCR runs are indicated, and the size of the amplification products (in base pairs) is indicated between brackets. The oligonucleotides are numbered according to the position of their 5′ nucleotide on sequence AF 106202 (Genbank accession number), followed by Fr for sense primers or Rv for antisense primers. The sequences of the amplification primers are indicated, from 5′ to 3′: PCR1: 61Fr GCTGAAGTGGGCGGATCACC and 1137Rv TCTAGCCTGGGTCATGCGGC; PCR2: 1114Fr TCTTGCCGCATGACCCAGGC and 2212Rv GGAAGGAGGCCAGGAGATGG; PCR3: 1511Fr CTCTTACTAAGGGTGACGCG and 3540Rv TCTGATGCCCCACGAGACAC; PCR4: 2528Fr TCTCTACAGGGCAGGCAGAG and 5012Rv TCGTGGTGTTGGTGTCTGGG; PCR5: 4640Fr AGGAGTGTCTCTTCCACTGC and 5540Rv CTTGTATGAGAAGTGGCTGG; PCR6: 4993Fr CCCAGACACCAACACCACGAT and 7320Rv GTCTGTCTTTGGAGGATGGG; and PCR7: 7171Fr AGAGGTGGACAAAGTACTTGG and 8158Rv GGAAGCCAGCATTTCCAGGG. The positions of the primers used to sequence the amplified regions are shown schematically; the full sequences are available on request.
Location of the amplification and sequencing primers on the EPCR gene. Exons are symbolized by boxes. The 5′ part of exon 1 and the 3′ part of exon 4, which are noncoding, are striped. The primer pairs used to amplify nearly the entire gene in 7 PCR runs are indicated, and the size of the amplification products (in base pairs) is indicated between brackets. The oligonucleotides are numbered according to the position of their 5′ nucleotide on sequence AF 106202 (Genbank accession number), followed by Fr for sense primers or Rv for antisense primers. The sequences of the amplification primers are indicated, from 5′ to 3′: PCR1: 61Fr GCTGAAGTGGGCGGATCACC and 1137Rv TCTAGCCTGGGTCATGCGGC; PCR2: 1114Fr TCTTGCCGCATGACCCAGGC and 2212Rv GGAAGGAGGCCAGGAGATGG; PCR3: 1511Fr CTCTTACTAAGGGTGACGCG and 3540Rv TCTGATGCCCCACGAGACAC; PCR4: 2528Fr TCTCTACAGGGCAGGCAGAG and 5012Rv TCGTGGTGTTGGTGTCTGGG; PCR5: 4640Fr AGGAGTGTCTCTTCCACTGC and 5540Rv CTTGTATGAGAAGTGGCTGG; PCR6: 4993Fr CCCAGACACCAACACCACGAT and 7320Rv GTCTGTCTTTGGAGGATGGG; and PCR7: 7171Fr AGAGGTGGACAAAGTACTTGG and 8158Rv GGAAGCCAGCATTTCCAGGG. The positions of the primers used to sequence the amplified regions are shown schematically; the full sequences are available on request.
Screening of 40 subjects from a given population is sufficient to identify polymorphisms having a frequency of 5% or more, with a confidence interval (CI) of 95%. Thus, the entire EPCR gene of the first 48 consecutive healthy volunteers was screened for polymorphisms as described above. Then, the polymorphic sites identified in these 48 subjects were screened for in 52 additional healthy subjects, with primers targeting these sites.
Haplotype A3 identification in the PATHROS population
A rapid method of A3 haplotype identification was developed using the G at nt 6936 of the EPCR gene as marker. The region surrounding nucleotide 6936 was amplified using a mutagenic 35-mer 6936 mutagen (5′-CCTACACTTCGCTGGTCCTGGGCGTCCTGGTctGC-3′) as upstream primer, and a 22-mer 7190Rv (5′-CAAGTACTTTGTCCACCTCTCC-3′) as downstream primer. The upstream primer bore 2 foreign nucleotides (underlined lowercase characters in the preceding sequence), thereby allowing amplified fragments bearing an A at position 6936 of the EPCR gene (thus corresponding to haplotype A1 or A2) to be cleaved by the restriction endonuclease PstI, whereas amplified fragments bearing a G (and corresponding to haplotype A3) remained undigested. With 20 units of PstI, 20 μL of the 290-bp amplification product was incubated overnight at 37°C, and digestion was checked by electrophoresis on 2% agarose gel.
Statistical analysis
Continuous variables are reported as means and standard deviation or as medians and range (according to their distribution), and categoric variables are reported as counts and percentages. Skewed variables were log-transformed before analysis. Individual subjects' sEPCR plasma concentrations at visits 1 and 2 were compared using a concordance test.36 The chi-square test was used to compare the observed genotype frequencies with the Hardy-Weinberg equilibrium prediction. The association between the genotype and the biologic phenotype (sEPCR level) was tested using analysis of variance. Comparisons between case and control subjects were based on student unpaired t test for continuous variables, and the chi-square test or Fisher exact test for categoric variables. Multivariate analysis was used to determine the odds ratio (OR), based on multiple logistic regression. Statistical tests were run on Statview5 statistical software (SAS, Cary, NC), and differences with P values less than .05 were considered statistically significant.
Results
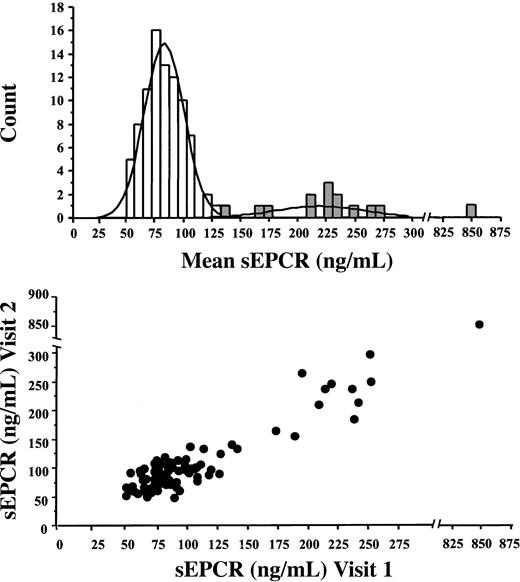
To assess the intraindividual variability of plasma sEPCR levels, we tested 2 blood samples, obtained one week apart, from each of 100 healthy male volunteers, except for 2 subjects who did not attend visit 2. At least 2 phenotypic groups were identified. Values in both groups had a gaussian distribution (Figure 2, upper panel); sEPCR levels were less than 137.5 ng/mL (3 nM) at both visits in 84 subjects, and more than 138.5 ng/mL at both visits in 14 subjects. Plasma sEPCR levels concorded between the 2 visits (R2 = 0.95, P < .0001) (Figure 2, lower panel). One subject had a very high sEPCR level (854 ng/mL) at both visits.
Distribution and concordance of sEPCR in 100 healthy male volunteers. Upper panel: distribution of sEPCR levels. Lower panel: concordance of sEPCR levels at 2 visits.
Distribution and concordance of sEPCR in 100 healthy male volunteers. Upper panel: distribution of sEPCR levels. Lower panel: concordance of sEPCR levels at 2 visits.
As plasma sEPCR levels may be influenced by inflammation, C-reactive protein (CRP) levels were also determined in all the subjects. The results (CRP values always less than 5 mg/mL) ruled out a role of inflammation in the bimodal distribution of sEPCR levels. F1+2 levels in the 84 subjects with lower sEPCR levels (median, 1.03 nM; range, 0.56-3.45 nM) were similar to those in the 14 subjects with higher sEPCR levels (median, 1.28 nM; range, 0.68-3.12 nM) (P = .57), ruling out elevated thrombin generation in the latter group.
The existence of different phenotypic groups of sEPCR expression, together with the stability of individual levels over time, pointed to genetic control of the sEPCR level. We therefore analyzed the EPCR gene in 48 consecutive healthy volunteers, from nucleotides 80 to 8100 (ie, 99% of the available AF106202 sequence, corresponding to ∼ 2300 nucleotides upstream of the ATG codon, the exons, the introns, and ∼ 1500 nt downstream of the stop codon). We found 16 single nucleotide polymorphisms (SNPs) located throughout the gene. The first was a C to G transversion of nucleotide (nt) 1651, located within the promoter region. There were 6 other SNPs located in intron 1 that affected nt 3610 (T to C transition), nt 3787 (T to C transition), nt 3877 (A to G transition), nt 4216 (C, G, or A), nt 4414 (T to C transition), and nt 4868 (C to T transition). There were 4 SNPs located in intron 2 that affected nt 5233 (A to G transition), nt 5760 (C to T transition), nt 6146 (G to A transition), and nt 6333 (C to T transition). Exon 4 contained 2 SNPs, with an A to G transition at nt 6936, changing Ser 219 to Gly,12 and a C to G transversion at nt 7014, in the noncoding part of exon 4. Finally, 3 SNPs were located in the 3′ untranslated (3′-UTR) part of the gene; they consisted of a C to G transversion at nt 7966,aGtoA transition at nt 7968, and an A to G transition at nt 7999. These polymorphisms had allelic frequencies higher than 0.05, except for 4414C and 6146A (0.041 and 0.036, respectively).
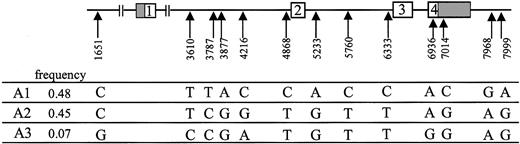
Using primers targeting the 14 frequent polymorphic positions, we amplified and sequenced the corresponding regions of the EPCR gene in the other 52 healthy volunteers in order to determine the allelic frequencies of the polymorphisms. All but one (nt 7966) of the 14 frequent SNPs were in complete linkage disequilibrium. These 13 SNPs defined 3 haplotypes, which we designated A1, A2, and A3. As shown in Figure 3, A1 and A2 were major haplotypes, with allelic frequencies of 0.48 and 0.45, respectively.
The 3 EPCR gene haplotypes. The 13 polymorphisms found to be in complete linkage disequilibrium defined 3 haplotypes designated A1, A2, and A3. The nucleotides are numbered according to the Genbank sequence (accession number AF106202).
The 3 EPCR gene haplotypes. The 13 polymorphisms found to be in complete linkage disequilibrium defined 3 haplotypes designated A1, A2, and A3. The nucleotides are numbered according to the Genbank sequence (accession number AF106202).
The A1 haplotype consisted of a combination of T at nt 3787, A at nt 3877, C at nt 4216, C at nt 4868, A at nt 5233, C at nt 5760, C at nt 6333, C at nt 7014, G at nt 7968, and A at nt 7999. The A2 haplotype was a combination of C at nt 3787, G at nt 3877, G at nt 4216, T at nt 4868, G at nt 5233, T at nt 5760, T at nt 6333, G at nt 7014, A at nt 7968, and G at nt 7999. A3, the least common haplotype (allelic frequency 0.07), differs from the A2 haplotype at 4 nucleotide positions (G at nt 1651, C at nt 3610, A at nt 4216, and G at nt 6936). The allelic frequencies of the 3 haplotypes were in Hardy-Weinberg equilibrium (χ2 test, P > .05).
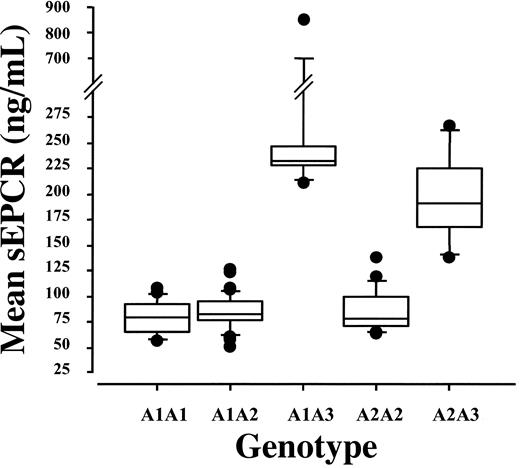
To establish whether the plasma sEPCR level is genetically regulated, we compared the plasma sEPCR level with the sEPCR genotype (Figure 4). As plasma sEPCR levels were stable between the 2 visits, we used the mean value for each subject. As shown in Figure 4, sEPCR levels were significantly higher in subjects carrying one A3 allele (A1 A3 or A2 A3) than in subjects carrying no A3 allele. No significant difference in sEPCR levels was observed between A1 A1 or A2 A2 homozygotes and A1 A2 heterozygotes. The mean sEPCR level in subjects having at least one A3 allele was 264 ± 174 ng/mL (range, 138.5 to 854 ng/mL; 218.9 ± 39.36 ng/mL [range, 138.5 to 274.9 ng/mL] after excluding the subject with a value of 854 ng/mL), compared with 83.6 ± 17.2 ng/mL (range, 50.5 to 137.5 ng/mL) in the other subjects (P < .0001, 95% CI). It is important to underline that all the subjects carrying the A3 haplotype had elevated sEPCR levels at both visits, one week apart. Interestingly, none of the 100 volunteers was an A3 A3 homozygote.
Plasma sEPCR levels according to the genotype in 100 healthy male volunteers. The mean ± SD (n, range; median) was 79.4 ± 16.6 ng/mL (20, 56.4-108.2; 79.5), 84.6 ± 16.3 (48, 50.5-126; 82.9), 314 ± 218.7 (8, 211.3-854.4; 235.1), 85.5 ± 20.3 (18, 63.3-137.5; 77.8), and 196.7 ± 46.2 ng/mL (6, 138.4-266.5; 190.5) for A1 A1, A1 A2, A1 A3, A2 A2, and A2 A3 subjects, respectively. When excluding the subject with atypical high sEPCR levels (854 ng/mL), mean ± SD (n, range; median) of A1 A3 subjects was 237.9 ± 20.2 ng/mL (7, 211.3-274.9; 233.1).
Plasma sEPCR levels according to the genotype in 100 healthy male volunteers. The mean ± SD (n, range; median) was 79.4 ± 16.6 ng/mL (20, 56.4-108.2; 79.5), 84.6 ± 16.3 (48, 50.5-126; 82.9), 314 ± 218.7 (8, 211.3-854.4; 235.1), 85.5 ± 20.3 (18, 63.3-137.5; 77.8), and 196.7 ± 46.2 ng/mL (6, 138.4-266.5; 190.5) for A1 A1, A1 A2, A1 A3, A2 A2, and A2 A3 subjects, respectively. When excluding the subject with atypical high sEPCR levels (854 ng/mL), mean ± SD (n, range; median) of A1 A3 subjects was 237.9 ± 20.2 ng/mL (7, 211.3-274.9; 233.1).
To evaluate the possible influence of sEPCR levels on the risk of venous thromboembolism, we investigated a cohort of 338 patients matched for age and sex with 338 healthy controls from the PATHROS study. The patients were 162 men (47.9%) and 176 women (52.1%). Mean age was 46 ± 13 years in the control group and 48 ± 15 years in the patient group (P = .06). The main characteristics and clinical events of the patients and controls are shown in Table 1.
Characteristics of the PATHROS case-control study population
. | Cases, n = 338 . | Controls, n = 338 . | P . |
|---|---|---|---|
| Ratio of men/women, n | 162/176 | 162/176 | — |
| Age, y | 48 ± 15 | 46 ± 13 | .06 |
| OC or HRT in females, % | 28.9 | 40.2 | — |
| Pulmonary embolism, % | 43.5 | — | — |
| Recurrent thrombosis, % | 33.2 | — | — |
| Primary thrombosis,* % | 25.0 | — | — |
| Factor V Arg506Gln, % | 18.6 | 4.2 | < .0001 |
| Factor II 20210G>A mutation, % | 11.9 | 4.5 | .0003 |
. | Cases, n = 338 . | Controls, n = 338 . | P . |
|---|---|---|---|
| Ratio of men/women, n | 162/176 | 162/176 | — |
| Age, y | 48 ± 15 | 46 ± 13 | .06 |
| OC or HRT in females, % | 28.9 | 40.2 | — |
| Pulmonary embolism, % | 43.5 | — | — |
| Recurrent thrombosis, % | 33.2 | — | — |
| Primary thrombosis,* % | 25.0 | — | — |
| Factor V Arg506Gln, % | 18.6 | 4.2 | < .0001 |
| Factor II 20210G>A mutation, % | 11.9 | 4.5 | .0003 |
OC indicates oral contraceptive; HRT, hormonal replacement treatment; and —, no event.
Excluding the following acquired risk factors: pregnancy, cancer, surgery, and immobilization.
The factor V Arg506Gln mutation was detected in 18.6% of cases and 4.2% of controls (P < .0001), and the prothrombin 20210G>A mutation was detected in 11.9% of cases and 4.3% of controls (P = .0003). The observed frequencies of these polymorphisms in our control population and the associated risks for venous thromboembolism disease are similar to those reported in other independent studies.33 Therefore, the recruitment of control subjects in a health care center did not appear to be a major selection bias for the study of genetic risk factors.
To identify subjects bearing an A3 allele, we developed a rapid screening method for this haplotype (for details see Figure 5).
Rapid A3 haplotype identification method. Upper panel: schematic representation of the part of the human EPCR gene exon 4 containing G 6936, which identifies the A3 haplotype. The 6936 mutagen primer contains 2 foreign nucleotides at positions n–4 and n–3 from the 3′ end (indicated by asterisks) in order to create a restriction site for the endonuclease PstI when the amplified fragment contains an A at position 6936, which corresponds to haplotype A1 or A2; the amplified fragment containing a G, which corresponds to haplotype A3, remains undigested. After genomic amplification using this primer and the 7190Rv primer, the PCR-amplified fragment contains a PstI site (CTGCA/G; underlined) when nucleotide 6936 is an A. In the amplified fragment, the part corresponding to the primer is shown in lower letters. Lower panel: 2% agarose gel electrophoresis of digested PCR products obtained using 6936 mutagen and 7190Rv primers. Lanes i, ii, iii: subjects homozygous for an A at position 6936 (A1/A1, A2/A2, and A1/A2, respectively); a restriction site for PstI was created, allowing the amplified fragment to be completely digested into 2 fragments of 254 and 36 bp (the latter is not visible on the gel). Lane iv: subject homozygous foraGat position 6936 (A3/A3): no PstI restriction site is available, and the fragment remains undigested at 290 bp. Lanes v-vi: subjects heterozygous A/G at position 6936 (A1/A3 or A2/A3; ie, A3 “heterozygotes”): both patterns are visible, corresponding to the undigested (290 bp) and digested (254 bp) amplified fragments. Lane vii: undigested PCR-amplified fragment.
Rapid A3 haplotype identification method. Upper panel: schematic representation of the part of the human EPCR gene exon 4 containing G 6936, which identifies the A3 haplotype. The 6936 mutagen primer contains 2 foreign nucleotides at positions n–4 and n–3 from the 3′ end (indicated by asterisks) in order to create a restriction site for the endonuclease PstI when the amplified fragment contains an A at position 6936, which corresponds to haplotype A1 or A2; the amplified fragment containing a G, which corresponds to haplotype A3, remains undigested. After genomic amplification using this primer and the 7190Rv primer, the PCR-amplified fragment contains a PstI site (CTGCA/G; underlined) when nucleotide 6936 is an A. In the amplified fragment, the part corresponding to the primer is shown in lower letters. Lower panel: 2% agarose gel electrophoresis of digested PCR products obtained using 6936 mutagen and 7190Rv primers. Lanes i, ii, iii: subjects homozygous for an A at position 6936 (A1/A1, A2/A2, and A1/A2, respectively); a restriction site for PstI was created, allowing the amplified fragment to be completely digested into 2 fragments of 254 and 36 bp (the latter is not visible on the gel). Lane iv: subject homozygous foraGat position 6936 (A3/A3): no PstI restriction site is available, and the fragment remains undigested at 290 bp. Lanes v-vi: subjects heterozygous A/G at position 6936 (A1/A3 or A2/A3; ie, A3 “heterozygotes”): both patterns are visible, corresponding to the undigested (290 bp) and digested (254 bp) amplified fragments. Lane vii: undigested PCR-amplified fragment.
Using this method, we identified 89 patients (26.3%) carrying at least one A3 allele (4 were homozygous and 85 “heterozygous”); 60 controls (17.7%) carried an A3 allele (2 were homozygous and 58 “heterozygous”) (P = .009) (Table 2). The allelic frequency of the A3 allele was 0.092 in the control subjects and 0.138 in the patients.
Repartition of A3 haplotype in cases and controls in the PATHROS study, in the whole population or according to sex
. | Total . | . | Men . | . | Women . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | Cases . | Controls . | Cases . | Controls . | Cases . | Controls . | |||
| n | 338 | 338 | 162 | 162 | 176 | 176 | |||
| Age, mean ± SD, y | 48 ± 15 | 46 ± 13 | 52 ± 14 | 49 ± 12 | 45 ± 14 | 44 ± 13 | |||
| No. of subjects with | |||||||||
| No A3 allele | 249 | 278 | 116 | 136 | 133 | 142 | |||
| 1 A3 allele | 85 | 58 | 45 | 26 | 40 | 32 | |||
| 2 A3 alleles | 4 | 2 | 1 | 0 | 3 | 2 | |||
| A3 carriers, % | 26.3* | 17.7 | 28.4† | 16 | 24.4‡ | 19.3 | |||
| Allele frequency | |||||||||
| A3 | 0.138 | 0.092 | 0.145 | 0.08 | 0.13 | 0.1 | |||
| A1 + A2 | 0.862 | 0.908 | 0.855 | 0.092 | 0.87 | 0.9 | |||
. | Total . | . | Men . | . | Women . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | Cases . | Controls . | Cases . | Controls . | Cases . | Controls . | |||
| n | 338 | 338 | 162 | 162 | 176 | 176 | |||
| Age, mean ± SD, y | 48 ± 15 | 46 ± 13 | 52 ± 14 | 49 ± 12 | 45 ± 14 | 44 ± 13 | |||
| No. of subjects with | |||||||||
| No A3 allele | 249 | 278 | 116 | 136 | 133 | 142 | |||
| 1 A3 allele | 85 | 58 | 45 | 26 | 40 | 32 | |||
| 2 A3 alleles | 4 | 2 | 1 | 0 | 3 | 2 | |||
| A3 carriers, % | 26.3* | 17.7 | 28.4† | 16 | 24.4‡ | 19.3 | |||
| Allele frequency | |||||||||
| A3 | 0.138 | 0.092 | 0.145 | 0.08 | 0.13 | 0.1 | |||
| A1 + A2 | 0.862 | 0.908 | 0.855 | 0.092 | 0.87 | 0.9 | |||
indicates P = .009; †, P = .011; and ‡, P = .3.
Surprisingly, the A3 haplotype distribution was sex-related, prompting us to analyze men and women separately (Table 2). The A3 haplotype distribution remained significantly different (P = .011) between male cases (28.4% were A3 carriers; allelic frequency, 0.145) and male controls (16% were A3 carriers; allelic frequency, 0.08). In contrast, there was no significant difference (P = .3) between female cases (24.4% of A3 carriers; allelic frequency, 0.13) and female controls (19.3% of A3 carriers; allelic frequency, 0.1).
This latter finding led us to examine whether the A3 haplotype was also associated with higher plasma sEPCR levels in women. We therefore assayed sEPCR in plasma from the 176 female controls (Figure 6).
Plasma sEPCR levels according to the presence or absence of A3 alleles, in a series of 176 healthy female controls from the PATHROS case-control study. Upper panel: distribution of sEPCR levels. Lower panel: correlation between the genotype and the sEPCR level.
Plasma sEPCR levels according to the presence or absence of A3 alleles, in a series of 176 healthy female controls from the PATHROS case-control study. Upper panel: distribution of sEPCR levels. Lower panel: correlation between the genotype and the sEPCR level.
As in the 100 healthy male volunteer population, the healthy female controls from the PATHROS study who carried the A3 haplotype had elevated sEPCR levels. In addition, sEPCR levels correlated with the number of A3 alleles: values were 77.5 ± 20.6 ng/mL (mean ± 1 SD) in women with no A3 alleles (n = 142), 211.1 ± 48.9 ng/mL in women with one A3 allele (n = 32), and 483.2 ± 47.4 ng/mL in the 2 women who were homozygous for the A3 haplotype (P < .0001). Thus, as in healthy men, the A3 haplotype is associated with elevated sEPCR levels in healthy women.
Discussion
On studying 100 healthy male volunteers, we observed strong interindividual variability in the plasma sEPCR values, which remained stable at a 1-week interval in a given subject. The distribution of plasma sEPCR values was bimodal, as previously reported.28,29 As F1+2 levels were not significantly different between the volunteers with the higher sEPCR levels and those with lower sEPCR levels, and as CRP levels were normal in all the volunteers, the observed bimodal distribution was not due to thrombin production or inflammation, both of which have been reported to augment sEPCR levels.17 Thus, plasma sEPCR levels were higher than 138 ng/mL in 14% of the healthy men studied here, a frequency slightly lower than that reported by Stearns-Kurosawa et al.28,29
The temporal stability of individual sEPCR levels strongly suggested that a genetic mechanism accounted for the observed bimodal distribution. We therefore extensively sequenced the EPCR gene of the 100 volunteers. We identified 16 polymorphisms, of which 13 were in complete linkage disequilibrium, defining 3 different haplotypes (A1, A2, and A3). Soluble EPCR levels correlated with the low allelic frequency A3 haplotype: sEPCR values were less than 137.5 ng/mL in men with no A3 alleles, and between 138.5 and 854 ng/mL in men carrying one A3 allele. One subject had an unusually high sEPCR level (854 ng/mL). This subject was an A1 A3 heterozygote and did not carry any other variations in the promoter region or any other region of the gene. The explanation for this very high plasma level is unclear. Incidentally, we never found the 23-bp insertion initially described by Biguzzi et al22 when screening healthy and thrombophilic subjects. This further confirms the rarity of this mutation.23-26
To determine whether the A3 haplotype is associated with an increased risk of thrombosis, we studied 338 patients with a history of deep venous thrombosis and 338 age- and sex-matched healthy subjects.
The A3 haplotype was overrepresented in the patients, with an allelic frequency of 0.138 compared with 0.092 in the controls (P = .009), suggesting that the A3 haplotype may increase the risk of thrombosis. Indeed, the OR was 1.7 in univariate analysis (95% CI, 1.2 to 2.4; P = .007) and 1.8 in multivariate analysis, taking into account sex, age, and the factor V Leiden and prothrombin 20210A mutations (95% CI, 1.2 to 2.6; P = .004) (Table 3). When restricted to subjects with a first thrombotic event, similar results were obtained: the ORs were 1.6 (95% CI, 1.1 to 2.5; P = .017) and 1.7 (95% CI, 1.1 to 2.6; P = .014) in univariate and multivariate analysis, respectively. Conversely, no statistically significant effect was observed on thrombosis recurrence.
OR in total population, or according to sex
. | Total . | Men . | Women . |
|---|---|---|---|
| Univariate analysis (P) | 1.7 (.007) | 2.1 (.008) | 1.4 (.25) |
| [95% CI] | [1.2-2.4] | [1.2-3.6] | [0.8-2.3] |
| Multivariate analysis* (P) | 1.8 (.004) | 2.5 (.0017) | 1.3 (.37) |
| [95% CI] | [1.2-2.6] | [1.4-4.5] | [0.8-2.2] |
. | Total . | Men . | Women . |
|---|---|---|---|
| Univariate analysis (P) | 1.7 (.007) | 2.1 (.008) | 1.4 (.25) |
| [95% CI] | [1.2-2.4] | [1.2-3.6] | [0.8-2.3] |
| Multivariate analysis* (P) | 1.8 (.004) | 2.5 (.0017) | 1.3 (.37) |
| [95% CI] | [1.2-2.6] | [1.4-4.5] | [0.8-2.2] |
Multivariate analysis for sex, age, factor V Leiden mutation, and prothrombin 20210A mutation. CI indicates confidence interval.
Surprisingly, the distribution of the A3 haplotype was sex-related, being significantly more frequent in male cases than in male controls (28.4% versus 16%, P = .01), whereas no such difference was found between female cases and female controls (24.4% versus 19.3%, P = .3). Men carrying 1 or 2 A3 alleles were at an increased risk of thrombosis, with an OR of 2.1 (95% CI, 1.2 to 3.6; P = .008) in univariate analysis and 2.5 (95% CI, 1.4 to 4.5; P = .0017) in multivariate analysis; the corresponding OR values in females were 1.4 (95% CI, 0.8 to 2.3; P = .25) in univariate analysis and 1.3 (95% CI, 0.8 to 2.2; P = .37) in multivariate analysis. Thus, the A3 haplotype appeared to increase the risk of thrombosis in men but not in women, although the sEPCR level is also increased in women carrying the A3 haplotype.
It is noteworthy that the frequency of hormonal treatment (oral contraception or replacement therapy) was higher in the female controls than in the female cases. This hinders the interpretation of our results in the absence of data on the possible interaction of the A3 allele with hormonal treatment. In addition, because of subgroup analysis with sample size reduction and decreased statistical power, a true effect in women may have been missed. However, we cannot exclude a hazard effect, the women cases having by chance a few percent less carriers than the male cases, and the women controls a few percent more carriers than male controls, which reduced the power to find a significant effect in women.
The mechanism linking elevated sEPCR levels to venous thrombosis remains to be determined. In healthy subjects, the mean physiologic sEPCR concentration being around 3 nM, and thus well below the concentration (∼ 70 nM) of circulating PC and the Kd (30 nM) of the EPCR/PC and EPCR/aPC interactions, elevated sEPCR concentrations are unlikely to markedly affect the interaction with PC. However, in subjects with increased circulating sEPCR levels due to the A3 haplotype (250-854 ng/mL represents ∼ 6-20 nM sEPCR), the local sEPCR concentration at the endothelial surface may approach or exceed the Kd of the PC interaction, especially in the presence of other factors such as inflammation and thrombin generation, which can trigger sEPCR production. Increased sEPCR concentrations result in decreased aPC generation and inhibit generated aPC, with possible implications for the regulation of coagulation, since low circulating aPC level has been shown to be a risk factor for venous thromboembolism.37
Several polymorphisms belonging to the haplotypes described here have already been identified separately in other studies. The A>G polymorphism affecting nt 6936 has been described in 2 different studies, both of which showed a very similar frequency of the G allele (corresponding to the A3 haplotype) in thrombophilic and control white subjects.38,39 This apparent discrepancy may be due to the heterogeneity of the populations studied, or to the fact that the subjects studied were not matched for sex and age.
In a recent study, Espana et al38 reported a second polymorphism (7763C>G) corresponding to nt 7014 polymorphism described here. Interestingly, they found that patients carrying the 7014G allele had lower aPC levels (0.98 ± 0.4 ng/mL and 0.99 ± 0.31 in patients with genotypes GC and GG, respectively) than noncarriers (corresponding to A1 A1 homozygotes according to the haplotypes we described here), who had aPC levels of 1.43 ± 0.61 ng/mL. Among these subjects with lower aPC levels, 33 (18%) carried the A3 haplotype, as they were 6936A>G heterozygotes (7685A>G in the numbering system used by Espana et al38 ). This is consistent with our hypothesis that reduced aPC generation is associated with the A3 haplotype. It would have been interesting to measure aPC levels in our healthy volunteers, but appropriate blood samples were not collected.
The molecular mechanism by which the A3 haplotype increases the plasma sEPCR level remains to be identified. Several polymorphisms defining the A3 haplotype are located within intronic regions, but none affects a splice site, activates a cryptic splice site, or creates an alternative splice site leading to the secretion of a truncated protein lacking the transmembrane region. Moreover, mRNA analysis of the A3 haplotype showed normal sequences at the exon 3–exon 4 junction (data not shown), ruling out a splice variant deleting the C-terminal part of the protein and, thus, the transmembrane domain. Polymorphisms in promoter regions, in intronic enhancer regions, or 3′ UTR region that augment transcription efficiency and protein synthesis, have been described in other genes.40 The SNP affecting nt 1651 is located 886 nt upstream of the ATG codon. Nothing is known of the role of this promoter region in transcription efficiency, but this nt could conceivably belong to a transcription factor binding site; alternatively, the C to G substitution might stimulate an enhancer region (or abolish a repressor region). It is also possible that nt 3610C (located in a T track at the beginning of an Alu sequence) or the nt 4216A polymorphism, both of which are located in intron 1, affects an enhancer region. Another possible explanation for the increased sEPCR levels associated with the A3 haplotype is a putative conformational change in the protein due to the Ser 219 to Gly substitution (resulting from the 6936 A to G mutation). This residue is located in the transmembrane domain,9 near another glycine residue, and these 2 adjacent Gly residues may destabilize the helical transmembrane domain and thus might change the exposure of the cleavage site, resulting in a protein that is more sensitive to metalloproteinase cleavage.
One final hypothesis is that 1 (or several) of the 4 nt changes characterizing the A3 haplotype stabilizes the mRNA generated by the A3 allele, leading to increased protein synthesis.
Several acquired factors, such as sepsis and systemic lupus erythematosus,15 as well as thrombin generation,17 are reported to increase plasma sEPCR levels. The present study offers the first evidence that genetic control, mediated by a specific haplotype of the EPCR gene, contributes to sEPCR levels. The A3 haplotype is the least frequent of the 3 possible EPCR haplotypes. From an evolutionary standpoint, the fact that the A3 haplotype differs from the A2 haplotype by only 4 nucleotides indicates that this allele is the youngest of the 3. This could explain observed differences in allele distribution according to ethnic origin. The A3 allele is probably mainly observed in white subjects; indeed, Hayashi et al never observed a Gly-encoding sequence at position 219 of the EPCR gene in a study of 30 Japanese subjects.11
Our results thus confirm an in vivo function of EPCR in the regulation of coagulation in humans. In particular, we found that the A3 haplotype was associated both with increased plasma sEPCR levels and with an increased risk of venous thrombosis in men. This candidate inherited risk factor for thrombosis warrants studies in other populations.
Prepublished online as Blood First Edition Paper, October 23, 2003; DOI 10.1182/blood-2003-07-2520.
Supported in part by a grant from Programme Hospitalier de Recherche Clinique (Ministère chargé de la Santé, PHRC AOR01023, sponsor: INSERM)
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Diagnostica Stago Laboratories for kindly providing the sEPCR Asserachrom kits. We are grateful to Philippe Coudol (Genetics Department, Hôpital Européen Georges Pompidou) for his skillful help with sequencing. We also thank the nursing staff of the Clinical Investigation Center 9201-Inserm AP-HP of Hôpital Européen Georges Pompidou, and Alvine Bissery for statistical help.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal