Abstract
Radioimmunotherapy (RIT) has emerged as an effective treatment for lymphoma, however the underlying mechanisms are poorly understood. We therefore investigated the relative contributions of antibody and targeted radiation to the clearance of tumor in vivo, using 2 different syngeneic murine B-cell lymphoma models. Although RIT with 131I–anti–major histocompatibility complex class II (MHCII) was effective in targeting radiation to tumor, no improvement in survival was seen by escalating the radiation dose alone and there were no long-term survivors. In contrast, using the combination of 131I anti-MHCII in the presence of unlabeled anti-idiotype (anti-Id), 100% prolonged disease-free survival was seen in both B-cell lymphoma models at the higher radiation dose. Using in vivo tracking we show that treatment with radiation plus anti-Id monoclonal antibody (mAb) results in a substantially greater reduction of splenic tumor cells than with either treatment alone. Prolonged survival could also be achieved using 131I anti-MHCII plus the signaling anti-CD19 mAb. Furthermore, the ability of these anti–B-cell mAbs to improve survival with targeted radiotherapy appeared to correlate with their ability to initiate intracellular signal transduction. Together these data illustrate that using 1 mAb to target radiation to tumor and a second to induce cell signaling is an effective new strategy in RIT.
Introduction
Monoclonal antibodies (mAbs) offer new therapeutic choices for patients with hematologic malignancies.1,2 Although the single-agent activity of most mAbs has generally been modest and short lived, the different mechanisms of action and nonoverlapping toxicity of mAbs has enabled them to be combined with chemotherapy and radiotherapy to greatly improve response rates.3 Radioimmunotherapy (RIT) involves the conjugation of radioisotopes to mAbs and has resulted in significantly superior response rates to unlabeled mAbs.4 A variety of different mAbs, delivery schedules, radioisotopes, and doses of radioactivity have been used in RIT, resulting in impressive partial and complete responses in the treatment of non-Hodgkin lymphoma (NHL).2,5-12 It now seems highly likely that RIT will play a significant role in the treatment of some NHL patients following the United States Food and Drug Administration (US FDA) approval of 90Y-ibritumomab tiuxetan and 131I-tositumomab.
Despite these clinical successes there remains considerable uncertainty regarding the optimal treatment approach in using RIT in NHL patients. One such issue is whether a radiation dose response exists and whether higher or lower doses of radioactivity should be used. Press and colleagues10,13 have adopted a high-dose myeloablative approach with peripheral blood stem cell transplantation support. In contrast, other groups including Kaminski and coworkers12 have elected to use a lower nonmyeloablative dose. Both approaches have resulted in impressive durable clinical responses and there is currently no randomized evidence to support the superiority of either approach.14 Using higher doses of radiation and stem cell transplantation does, however, create a number of radiation protection and logistical issues that make widespread use of this approach more difficult. A clear benefit of using higher doses of radiation would therefore have to be demonstrated before higher doses of radiation could enter routine clinical practice.
Furthermore, the mechanisms involved in clearance of tumor by RIT remains poorly understood and some mAbs, such as Lym-1 (binding to human leukocyte antigen [HLA]–DR) have often been considered simply as vectors for delivering radiation to tumor or “systemic radiotherapy.”15 Recent preclinical and clinical data have, however, clarified that some mAbs are intrinsically therapeutically active and may operate by interacting with the host immune system or through a direct cytotoxic effect via cell surface signaling.1,16,17 An understanding of the mechanisms underlying the clinical responses is likely to be important in the further development and optimization of this treatment approach. Dissecting the mechanism of action of RIT and attributing the relative importance of mAbs, targeted radiation, and nonspecific whole-body irradiation to the therapeutic efficacy is difficult or impossible in a clinical setting. Such fundamental issues are therefore ideally approached in syngeneic tumor models. In this situation tumor develops in the presence of an intact immune system and the targeting mAb is able to cross-react with appropriate normal tissues in a manner analogous to the clinical situation and contrasting with the limitations of human severe combined immunodeficiency (SCID) xenograft models where there is no such host immunity or specific cross-reaction of mAbs with normal tissues.
We have previously reported the importance of antibody specificity in the successful eradication of tumor with RIT.16 In this study, we have demonstrated that a radiation dose response exists for RIT in the presence of cell surface signaling in B-cell lymphomas in vivo. We have also characterized the relative contributions made by targeted irradiation and mAb effector mechanisms using two different mAbs to fulfill different functions and work cooperatively to successfully eradicate lymphoma in vivo. In this situation, the characteristics of one mAb are exploited to target radiation effectively to the tumor while the second mAb provides intracellular signaling by ligating key cell surface receptor molecules. We have shown that both anti-idiotype (anti-Id) and anti-CD19 mAbs initiate cell surface signaling and this appears to dramatically enhance the clearance of tumor when combined with targeted radiation delivered by 131I–anti–major histocompatibility complex class II (MHCII). We believe that these results provide new insights into the mechanisms of action of RIT and should influence the design of further clinical studies integrating combinations of mAbs to successfully treat lymphoma.
Materials and methods
Animals and cell lines
Mice were supplied by Harlan UK Limited (Blackthorn, United Kingdom) and maintained in local animal facilities. BCL118 and A3119 are B-cell lymphomas that arose spontaneously in mice and are maintained by in vivo passage in BALB/c and CBA/H mice, respectively. πBCL1 is a variant of the BCL1 lymphoma that can be maintained in culture as well as in vivo.20 Cell culture was performed in RPMI (Life Technologies, Paisley, United Kingdom) supplemented with glutamine (2 mM), pyruvate (1 mM), penicillin and streptomycin (100 IU/mL), fungizone (2 μg/mL), 2-mercaptoethanol (50 μM) (VWR International, Poole, United Kingdom), and 10% fetal calf serum (FCS; Myoclone; Life Technologies).
Antibodies and iodination
A list of the mAbs and their sources is given in Table 1.21-23 Details of their production and purification is given elsewhere.17 The 125I and 131I radioisotopes were supplied from Amersham International (Little Chalfont, United Kingdom). The mAbs were iodinated using Iodobeads (Pierce Chemicals, Rockford, IL) according to the manufacturer's instructions and dialyzed to remove unbound iodine. Labeling efficiency varied between 82.5% and 98.5% as measured by thin layer chromatography and high performance liquid chromatography. The specific activity was 37 to 185 MBq/mg (1.0-5.0 mCi/mg). The immunoreactivity of iodinated antibodies was assessed according to the method described by Elliott et al.24
Antimouse monoclonal antibodies used in this work
Antibody clone . | Specificity . | Isotype . | Affinity, Ka; M-1* . | Saturation level† . | Source . |
|---|---|---|---|---|---|
| Mc10-6A5 | BCL1 Id | Ratγ2a | 1 × 109 | 0.9 × 105 | George et al21 |
| Mc39-16 | A31 Id | Ratγ2a | 5 × 108 | 0.4 × 105 | Tenovus |
| ID3 | CD19 | Ratγ2a | 3 × 108 | 0.4 × 105 | Krop et al22 |
| NIMR6 | CD22 | Ratγ1 | 1.2 × 108 | 0.6 × 105 | Torres et al23 |
| TI2-3 | MHCII | Ratγ1 | 5.3 × 108 | 1.8 × 105 | Illidge et al16 |
| 1A5E8 | CD38 | Ratγ1 | 1.5 × 108 | 0.4 × 105 | Parkhouse‡ |
Antibody clone . | Specificity . | Isotype . | Affinity, Ka; M-1* . | Saturation level† . | Source . |
|---|---|---|---|---|---|
| Mc10-6A5 | BCL1 Id | Ratγ2a | 1 × 109 | 0.9 × 105 | George et al21 |
| Mc39-16 | A31 Id | Ratγ2a | 5 × 108 | 0.4 × 105 | Tenovus |
| ID3 | CD19 | Ratγ2a | 3 × 108 | 0.4 × 105 | Krop et al22 |
| NIMR6 | CD22 | Ratγ1 | 1.2 × 108 | 0.6 × 105 | Torres et al23 |
| TI2-3 | MHCII | Ratγ1 | 5.3 × 108 | 1.8 × 105 | Illidge et al16 |
| 1A5E8 | CD38 | Ratγ1 | 1.5 × 108 | 0.4 × 105 | Parkhouse‡ |
Estimates of the Ka were calculated from binding curves by determining the concentration of antibody required to achieve half-maximum binding capacity.
Estimates of the average number.
Generous gift from Michael E. Parkhouse (Pirbright Laboratory, Woking, United Kingdom).
Biodistribution studies
Groups of BALB/c or CBA/H mice were injected via the tail vein with 105 BCL1 or 106 A31 cells, respectively. Mice were given Lugol solution (0.125 g KI, 0.0625 g elemental iodine in 100 mL water) in their drinking water 3 days before the biodistribution. Ten days after tumor inoculation animals received 500 μgof 125I-labeled mAbs by intravenous injection. Biodistribution of each radioactive mAb (anti-Id, anti-CD19, anti-MHCII) was compared with an isotype-matched control antibody. Animals were killed 1, 8, 24, 48, and 96 hours after receiving the radioactive mAbs and the blood, spleen, liver, kidneys, and lungs were assessed.
The weight and radioactive counts of the dissected organs were measured, and the percentage of the injected dose per gram of tissue (% ID/g) was calculated as described by Badger et al.25 The effective half-life and biodistribution of the labeled antibodies were determined. Assuming total absorption of the nonpenetrating radiation component with a homogeneous distribution, both the whole body and the organ doses were calculated by integrating the area under the retention curve.26
Immunotherapy
Age- and sex-matched mice were inoculated by intravenous injection with 106 A31 or 105 BCL1 lymphoma cells on day 0 and treated with external beam radiation therapy (EBRT) plus mAb, or mAb alone, 10 days later. EBRT was delivered using a modified 225 kV X-ray unit (Gulmay Medical, Chertsey, United Kingdom) at a dose rate of 0.11 Gy/minute. Intravenous mAb was given 4 hours after EBRT. Animals were fed acidified water (pH 2.5; 1 N HCl) supplemented with neomycin sulfate (2 g/L; Sigma-Aldrich, Poole, United Kingdom) starting 3 days prior to EBRT and continuing for 2 weeks afterward to prevent the risk of opportunistic infection. In addition, animals were housed in a sterile maximizer unit (Techniplast, Northants, United Kingdom).
For RIT experiments, 500 μg (anti-CD19, anti-CD38, anti-MHCII, or anti-BCL1 Id) or 100 μg (anti-A31 Id) 131I-labeled mAbs carrying 1.85 MBq to 18.5 MBq was injected intravenously per mouse. For therapies combining RIT with unlabeled mAbs, mice were injected intravenously with unlabeled anti-BCL1 and anti-A31 Id antibodies (doses as mentioned above) 2 to 3 hours prior to the injection of the 131I-labeled anti-MHCII. Parallel groups of mice were treated with unlabeled antibodies alone or 131I anti-MHCII alone for comparison. For all RIT therapies mice were given Lugol solution (as described in “Biodistribution studies”).
Animal survival was monitored daily, and the results were analyzed using the χ2 test of Peto.27 Animal immunotherapy was approved by the local ethical committee and was performed under a United Kingdom Home Office project license.
Hematologic toxicity of RIT
RIT toxicity experiments were carried out to evaluate the hematologic impact of the 131I-labeled anti-MHCII antibody. Groups of BALB/c mice (12 mice/group) were injected via the tail vein with 105 BCL1 cells and given Lugol solution. Ten days after tumor inoculation animals received 500 μg of either 9.25 MBq or 18.5 MBq 131I-labeled anti-MHCII mAbs by intravenous injection 2 to 3 hours after unlabeled anti-BCL1 Id. Parallel groups of untreated or non–tumor-bearing mice were given the same therapy as controls. Animals were killed on days 1, 7, 14, 21, 28, and 35 after receiving the radioactive mAbs. Blood samples were obtained from cardiac puncture and analyzed using a Sysmex XE-2100 Blood Cell Analyzer (Sysmex, Milton Keynes, United Kingdom).
In vivo tracking
To measure tumor growth following treatment and chart the progress of any immunologic response, groups of CBA/H mice were inoculated with 106 A31 tumor cells intravenously on day 0 and treated on day 10 with EBRT, mAb (anti-Id, 100 μg intravenously), or a combination of both. Mice were culled 5 days after treatment, and the spleens were removed, homogenized, and then assessed by flow cytometry. Tumor cells were detected by staining using fluorescein isothiocyanate (FITC)–labeled Mc39-16 (rat anti-A31 Id)17 and phycoerythrin (PE)–labeled-ID3 (rat antimouse CD19). Cells were stained with mAbs (50 μg/mL) on ice for 20 minutes, washed, and analyzed on a FACS Calibur (Becton Dickinson, Mountain View, CA). To characterize the immunologic response to treatment, homogenates were labeled with either PE-YTS169 (rat antimouse CD8) or FITC-YTA1.3.2 (rat antimouse CD4)28 to determine the number of splenic CD8+ or CD4+ T cells, respectively.
Phosphotyrosine detection by Western blot
Up-regulation of intracellular protein tyrosine phosphorylation was measured by Western blot as described by Vuist et al.29 Briefly, BCL1 tumor cells were warmed for 15 minutes at 37°C and equal volumes of rat antimouse mAb solutions were added to a final concentration of 20 μg/mL and incubated for 2 minutes. Hyper–cross-linking of the primary mAbs was performed by adding sheep antirat immunoglobulin G (IgG) or equal amount of control Abs to a final concentration of 50 μL/mL. The samples were incubated for a further 2 minutes and washed once using 900 μL/sample of ice-cold phosphate-buffered saline (PBS) containing 1 mM Na3VO4. In experiments where mAbs were combined with EBRT, samples were irradiated with 5 Gy using a 137Cs source (Gamma cell 1000; Kanata, ON, Canada) directly after hyper–cross-linking. Cell pellets were lysed on ice in 1% Triton-X 100 buffer. The cell lysates were then centrifuged for 15 minutes at 16 100g, and 20 μL of the supernatant was mixed with 10 μL loading buffer, and boiled for 5 minutes before being subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Proteins in the gel were transferred to nitrocellulose and the blot was incubated overnight at 4°C in 5% bovine serum albumin (BSA). Phosphotyrosine was detected by incubating the blot with mAb 4G10 and horseradish peroxidase (HRP)–labeled goat antimouse antibody. Enhanced chemiluminescence (ECL) reagents were added as directed by the manufacturer (Pierce Chemicals) and the blot was exposed to X-ray films for developing.
Immunoprecipitation analysis for tyrosine phosphorylation of Syk
To detect Syk tyrosine phosphorylation, Triton X-100 lysates prepared as detailed in the previous paragraph were first incubated with 4G10 (2 μg/sample) at 4°C overnight. Protein G–coated Sepharose beads (Amersham Biosciences, Buckinghamshire, United Kingdom) preblocked with 5% (wt/vol) BSA were then added to the samples (15 μL/sample) and incubated for a further 60 minutes at 4°C. The resulting beads were then washed 4 times with cold lysis buffer and boiled in sample buffer. The precipitated proteins were separated by SDS-PAGE and immunoblotted as described in the previous paragraph with the anti-Syk antibody C-20 (Santa Cruz Biotechnology, Santa Cruz, CA).
Measurement of intracellular-free calcium
Intracellular-free calcium was measured using the cell-permeable fluorescent probe Indo-1-am (Sigma-Aldrich) using well-established methodology.30 Cultured πBCL1 lymphoma cells (107 /mL) were washed twice in serum-free media and loaded with Indo-1-AM at 5 μM for 30 minutes at 37°C. Cells were then washed twice in serum-free RPMI and stored in the dark at room temperature until required. For the assay, 250 μL of cells was warmed to 37°C, 2 μg/mL of mAbs was added, and the assay was immediately analyzed using flow cytometry on a FACS Vantage (Becton Dickinson). Where appropriate, hyper–cross-linking sheep antirat or rabbit antihamster IgG was added at a final concentration of 20 μg/mL immediately before analysis. Intracellular calcium mobilization was estimated by determining the fluorescence ratio of Indo-1–bound calcium (398 nm, FL5) to free calcium (482 nm, FL4) against time. The response was measured over 300 seconds and recorded using the Cell Quest software (Becton Dickinson).
Results
External beam radiation therapy (EBRT) and anti-Id mAbs have an additive effect in vivo
In our previous work we showed that 131I anti-Id was effective at clearing tumor in the BCL1 model and providing long-term survival.16 In the current study we attempted to separate the actions of mAbs from that of the radiation and initially investigated this using EBRT and unlabeled anti-Id mAbs in two different syngeneic B-cell lymphoma models (A31 and BCL1). Mice were inoculated with either 105 (BCL1) or 106 (A31) tumor cells and treated 10 days later with a single fraction of 5 Gy whole-body EBRT. In both tumor models, EBRT alone resulted in only a modest improvement in animal survival of around 10 to 20 days (Figure 1A-B). Larger doses of EBRT coupled with bone marrow transplantation did not significantly increase survival over and above the level seen with 5 Gy (data not shown), confirming the similar resistance of both lymphoma models to EBRT alone. In the BCL1 model, anti-Id mAb alone produced an increase in survival of around 8 days. The addition of anti-Id mAb to EBRT produced a small increase in survival of around 3 to 5 days longer than that seen with EBRT alone and around 10 days longer than with anti-Id alone (P < .01). In contrast, the A31 tumor was much more sensitive to the effects of anti-Id mAb and improvements in survival of around 40 days were seen over control animals with mAb alone, in keeping with results previously published by our group.17 However, when anti-Id mAb was given in combination with 5 Gy EBRT, long-term survival (> 100 days) was observed (Figure 1B).
EBRT and anti-Id mAb therapy in BCL1 and A31 lymphoma. Groups of 5 age- and sex-matched BALB/c (A) and CBA (B) mice were inoculated with BCL1 (105) or A31 (106) lymphoma cells, respectively, by intravenous injection on day 0. Mice were then treated on day 10 with 5 Gy EBRT, with or without anti-Id mAb (500 μg BCL1; 100 μg A31) given by intravenous injection 3 to 4 hours after EBRT. Survival was monitored daily. Both the BCL1 and A31 tumors show similar results to the effects of EBRT with modest increases in survival of around 15 to 20 days after 5 Gy. The differences in sensitivity to anti-Id therapy between the BCL1 and A31 models can be clearly seen, with the anti-Id alone providing 40 days additional survival over control animals, but no long-term survivors in the A31 model (B). In contrast, in the BCL1 model, anti-Id provided very modest improvements in survival of around 8 days with EBRT providing approximately 15 days over the control animals. The addition of anti-Id mAb to 5 Gy EBRT in the A31 model however provided long-term protection with a highly significant increase in survival over controls and anti-Id alone (P < .01); however, the addition of anti-Id to 5 Gy EBRT in the BCL1 model provided just 2 to 3 days over EBRT alone. This experiment is representative of 1 of 3 identical experiments performed.
EBRT and anti-Id mAb therapy in BCL1 and A31 lymphoma. Groups of 5 age- and sex-matched BALB/c (A) and CBA (B) mice were inoculated with BCL1 (105) or A31 (106) lymphoma cells, respectively, by intravenous injection on day 0. Mice were then treated on day 10 with 5 Gy EBRT, with or without anti-Id mAb (500 μg BCL1; 100 μg A31) given by intravenous injection 3 to 4 hours after EBRT. Survival was monitored daily. Both the BCL1 and A31 tumors show similar results to the effects of EBRT with modest increases in survival of around 15 to 20 days after 5 Gy. The differences in sensitivity to anti-Id therapy between the BCL1 and A31 models can be clearly seen, with the anti-Id alone providing 40 days additional survival over control animals, but no long-term survivors in the A31 model (B). In contrast, in the BCL1 model, anti-Id provided very modest improvements in survival of around 8 days with EBRT providing approximately 15 days over the control animals. The addition of anti-Id mAb to 5 Gy EBRT in the A31 model however provided long-term protection with a highly significant increase in survival over controls and anti-Id alone (P < .01); however, the addition of anti-Id to 5 Gy EBRT in the BCL1 model provided just 2 to 3 days over EBRT alone. This experiment is representative of 1 of 3 identical experiments performed.
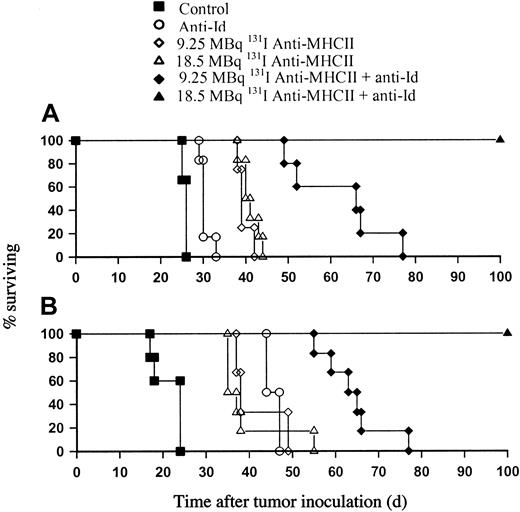
A radiation dose response exists for RIT of lymphoma in the presence of anti-Id mAb
Given the modest improvement in survival achieved with EBRT and mAb in the BCL1 tumor model, we next investigated whether using RIT to deliver targeted radiation could improve upon the results achieved with EBRT. We have previously shown that 131I-conjugated anti-MHCII mAb was an extremely effective mAb for delivering radiation to tumor in the BCL1 model.16 We therefore investigated the therapeutic effect of using the combination of 131I anti-MHCII mAb and unlabeled anti-Id mAb (Figure 2A-B). These experiments led us to make two important observations. First, there does not appear to be a radiation dose response with targeted radiation alone, as similar levels of animal survival were seen with 9.25 and 18.5 MBq of 131I anti-MHCII mAb in both the A31 and BCL1 models. It is important to note that the unlabeled anti-MHCII mAb had no therapeutic effect and that 2.31 MBq of anti-MHCII mAb had a similar poor performance to that achieved with 18.5 MBq of 131I-labeled control (irrelevant) mAb of around 5 to 7 days further protection than that seen with untreated animals (data not shown). Therefore although a dose response was seen, as the dose of 131I anti-MHCII was increased from 2.31 MBq to 9.25 MBq and survival improved to around 15 to 20 days over controls, no improvement in survival was seen in either the A31 or BCL1 tumor models above the 9.25-MBq dose level. This lack of efficacy confirms the effective tumor targeting of the 131I anti-MHCII and argues against a nonspecific whole-body irradiation effect. The second most striking observation was the impressive therapeutic efficacy of combining 131I anti-MHCII with unlabeled anti-Id mAb (Figure 2A-B). In contrast to the results seen with the combination of EBRT and anti-Id in the BCL1 model, when 9.25 MBq 131I anti-MHCII was given instead of EBRT with unlabeled anti-Id, an increase of approximately 30 days survival was seen over and above that seen with either the 131I anti-MHCII or anti-Id alone. Furthermore, in the presence of anti-Id mAb there was a clear radiation dose response with animal survival improving as the dose increased from 9.25 MBq to 18.5 MBq 131I anti-MHCII. While there were no long-term survivors seen with 9.25 MBq 131I anti-MHCII plus anti-Id, when the dose of 131I was increased to 18.5 MBq, 100% of animals survived longer than 100 days. This important observation was also reproduced using the A31 model and a similar dose response was again seen, with 18.5 MBq 131I anti-MHCII plus anti-Id treatment consistently producing 100% long-term disease-free survivors (Figure 2B).
Dose response of RIT in B-cell lymphoma models. Unlabeled anti-Id mAb was added to 131I-labeled anti-MHCII mAb (RIT) in the treatment of murine lymphomas. (A) Animals were inoculated with 105 BCL1 cells by intravenous injection and treated with 500 μg anti-Id mAb per animal given 2 hours before RIT on day 10. (B) For the A31 model, animals were inoculated with 106 tumor cells and treated with 100 μg anti-Id mAb by intravenous injection 8 days later. This experiment is representative of 1 of 3 similar experiments. In both models, 9.25 MBq 131I anti-MHCII and 18.5 MBq 131I anti-MHCII provided very similar levels of protection. In contrast, with the addition of anti-Id mAb, a clear dose response was seen in both BCL1 and A31 models with 18.5 MBq 131I anti-MHCII in conjunction with anti-Id providing highly significant improvements in survival over the 9.25 MBq RIT dose and untreated controls (P < .01).
Dose response of RIT in B-cell lymphoma models. Unlabeled anti-Id mAb was added to 131I-labeled anti-MHCII mAb (RIT) in the treatment of murine lymphomas. (A) Animals were inoculated with 105 BCL1 cells by intravenous injection and treated with 500 μg anti-Id mAb per animal given 2 hours before RIT on day 10. (B) For the A31 model, animals were inoculated with 106 tumor cells and treated with 100 μg anti-Id mAb by intravenous injection 8 days later. This experiment is representative of 1 of 3 similar experiments. In both models, 9.25 MBq 131I anti-MHCII and 18.5 MBq 131I anti-MHCII provided very similar levels of protection. In contrast, with the addition of anti-Id mAb, a clear dose response was seen in both BCL1 and A31 models with 18.5 MBq 131I anti-MHCII in conjunction with anti-Id providing highly significant improvements in survival over the 9.25 MBq RIT dose and untreated controls (P < .01).
Biodistribution and myelotoxicity of 131I anti-MHCII RIT
We next wished to investigate whether the impressive therapeutic results seen using 131I anti-MHCII plus anti-Id compared with EBRT plus anti-Id in the BCL1 model were due to an increased delivery of targeted radiation by the 131I anti-MHCII mAb to the tumor-bearing organs. We had previously shown that MHCII had a higher level of surface expression than CD19 or Id. In addition we demonstrated in vivo that the MHC antigen complex unlike CD19 or Id did not modulate on binding mAbs.16 We therefore hypothesized that anti-MHCII would deliver more radiation to the tumor-bearing organs and thus performed biodistribution experiments to calculate the radiation dose delivered to both normal and tumor-bearing organs. Figure 3 demonstrates the biodistribution of a panel of B-cell–specific mAbs given 10 days after tumor. At specific times, over the next 4 days (Figure 3), the animals were culled and the major organs removed to determine the percent injected radioactive dose per gram (% ID/g) of organ tissue. The ability of a single trace-labeled (0.74 MBq) dose of 500 μg of 125I-labeled anti-MHCII to selectively target the major tumor-bearing organ (spleen) is clearly shown. In contrast, the B-cell–specific mAbs (anti-CD19 and anti-Id) were considerably poorer at delivering radiation to the spleen and provided similar targeting of radiation (% ID/g) to that seen with an irrelevant isotype-matched (control) mAb. The approximate radiation doses delivered by these mAbs were calculated using the medical internal radiation dosimetry (MIRD) method and the doses are presented in Table 2. The results confirm the ability of anti-MHCII mAbs to deliver larger doses of radiation per MBq of radiolabeled mAbs infused to the spleen and less nonspecific irradiation to the whole body. For an infusion of 18.5 MBq 131I anti-MHCII, the dose delivered to the spleen is estimated to be around 18 Gy, which is 3 to 6 times greater than that delivered by anti-CD19 (5.14 Gy), the irrelevant (4.5 Gy), and the anti-Id (3.4 Gy) mAbs (Figure 3A-D). This contrasts to the whole-body dose where anti-MHCII delivered around 2.9 Gy, whereas the other mAbs delivered larger nonspecific doses: anti-Id (4.4 Gy), anti-CD19 (7.25 Gy), and the irrelevant mAb (5.8 Gy). Biodistribution studies performed in advanced disease (day 18) showed generally similar results with the exception that despite the presence of a greatly increased tumor burden, the dose of radiation delivered by anti-Id was actually decreased (data not shown). We repeated the same biodistribution studies in the A31 tumor model and found that the % ID/g of tissue studied for the anti-MHCII mAb was almost exactly the same as for the BCL1 model (data not shown).
Biodistribution of 125I mAb in BCL1 inoculated mice. Age- and sex-matched groups of mice (18 for each mAb group) were inoculated with 105 BCL1 cells intravenously and then treated with 125I mAb (500 μg) 10 days later. At the time intervals indicated, animals were killed and blood samples and various organs were removed for weighing and estimation of radioactive content. Results are expressed as the percentage of the injected dose of 125I mAb per gram of tissue recovered (% ID/g; see “Materials and methods”). Each bar shows the mean and range for 3 animals investigated and the results shown are representative of 1 of 2 identical experiments.
Biodistribution of 125I mAb in BCL1 inoculated mice. Age- and sex-matched groups of mice (18 for each mAb group) were inoculated with 105 BCL1 cells intravenously and then treated with 125I mAb (500 μg) 10 days later. At the time intervals indicated, animals were killed and blood samples and various organs were removed for weighing and estimation of radioactive content. Results are expressed as the percentage of the injected dose of 125I mAb per gram of tissue recovered (% ID/g; see “Materials and methods”). Each bar shows the mean and range for 3 animals investigated and the results shown are representative of 1 of 2 identical experiments.
Internal radiation dosimetry of RIT-treated mice (131I mAb, 0.5 mCi): Gy (mean ± range)
. | Control . | Anti-MHCII . | Anti-Id . | Anti-CD19 . |
|---|---|---|---|---|
| Whole body | 5.80 ± 0.17 | 2.90 ± 0.11 | 4.44 ± 0.23 | 7.25 ± 0.09 |
| Spleen | 4.50 ± 0.20 | 18.00 ± 1.02 | 3.40 ± 0.21 | 5.14 ± 0.13 |
| Liver | 3.04 ± 0.07 | 1.82 ± 0.17 | 5.14 ± 0.34 | 6.05 ± 0.44 |
| Lung | 7.63 ± 0.36 | 4.54 ± 0.22 | 8.57 ± 0.25 | 10.15 ± 0.24 |
| Kidney | 3.04 ± 0.15 | 3.80 ± 0.14 | 2.75 ± 0.12 | 7.60 ± 0.42 |
. | Control . | Anti-MHCII . | Anti-Id . | Anti-CD19 . |
|---|---|---|---|---|
| Whole body | 5.80 ± 0.17 | 2.90 ± 0.11 | 4.44 ± 0.23 | 7.25 ± 0.09 |
| Spleen | 4.50 ± 0.20 | 18.00 ± 1.02 | 3.40 ± 0.21 | 5.14 ± 0.13 |
| Liver | 3.04 ± 0.07 | 1.82 ± 0.17 | 5.14 ± 0.34 | 6.05 ± 0.44 |
| Lung | 7.63 ± 0.36 | 4.54 ± 0.22 | 8.57 ± 0.25 | 10.15 ± 0.24 |
| Kidney | 3.04 ± 0.15 | 3.80 ± 0.14 | 2.75 ± 0.12 | 7.60 ± 0.42 |
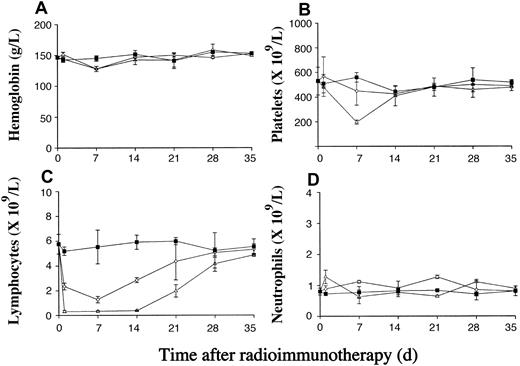
We next investigated the hematologic toxicity of 131I-labeled anti-MHCII plus anti-Id, which we had shown was highly effective at clearing lymphoma in vivo (Figure 2). The results are shown in Figure 4 and demonstrate that the toxicity of such treatment is mild, dose dependent, and reversible. The hemoglobin level is not significantly affected (Figure 4A), while there is a dose-dependent decrease in the platelet and white cell counts as the dose of 131I is increased from 9.25 to 18.5 MBq (Figure 4B-C). For 18.5 MBq 131I anti-MHCII plus anti-Id mAb, there is substantial fall in the platelet count from around 500 × 109/L to 200 × 109/L, which recovers to pretreatment level by 21 days after the therapy. A dose-dependent decrease in the lymphocyte count can be seen as early as 1 day after RIT, remaining suppressed for 14 days, with initial recovery being seen by day 21. In contrast, neutrophils appear more resistant to the effects of 131I-labeled anti-MHCII with the total numbers unaffected (Figure 4D).
Hematologic toxicity of RIT using 131I-labeled anti-MHCII. The hematologic toxicity of higher doses of 131I-labeled anti-MHCII mAb reveals a dose-dependent effect on the platelet (B) and white cell (C) counts, whereas the hemoglobin level (A) and neutrophil count (D) are not significantly affected. Groups of BALB/c mice (12 mice/group) were injected via the tail vein with 105 BCL1 cells. Ten days after tumor inoculation animals received 500 μg of 9.25MBq or 18.5 MBq 131I-labeled anti-MHCII mAb by intravenous injection 2 to 3 hours after the injection of unlabeled anti-BCL1 Id. In comparison, parallel groups of non–tumor-bearing BALB/c mice were given the same mAb therapy, and a group of untreated mice were set up as controls. Animals from respective groups were killed on days 1, 7, 14, 21, 28, and 35 after treatment and blood counts were measured as described in “Materials and methods.” Each point represents the mean value of 2 mice and the errors bars represent the range seen. ▪ indicates control; ⋄, 9.25 MBq 131I anti-MHCII + anti-Id; and ▵, 18.5 MBq 131I anti-MHCII + anti-Id.
Hematologic toxicity of RIT using 131I-labeled anti-MHCII. The hematologic toxicity of higher doses of 131I-labeled anti-MHCII mAb reveals a dose-dependent effect on the platelet (B) and white cell (C) counts, whereas the hemoglobin level (A) and neutrophil count (D) are not significantly affected. Groups of BALB/c mice (12 mice/group) were injected via the tail vein with 105 BCL1 cells. Ten days after tumor inoculation animals received 500 μg of 9.25MBq or 18.5 MBq 131I-labeled anti-MHCII mAb by intravenous injection 2 to 3 hours after the injection of unlabeled anti-BCL1 Id. In comparison, parallel groups of non–tumor-bearing BALB/c mice were given the same mAb therapy, and a group of untreated mice were set up as controls. Animals from respective groups were killed on days 1, 7, 14, 21, 28, and 35 after treatment and blood counts were measured as described in “Materials and methods.” Each point represents the mean value of 2 mice and the errors bars represent the range seen. ▪ indicates control; ⋄, 9.25 MBq 131I anti-MHCII + anti-Id; and ▵, 18.5 MBq 131I anti-MHCII + anti-Id.
Radiation dose delivered by RIT does not correlate with survival
Having demonstrated that the panel of anti–B-cell mAbs delivered very different doses of radiation to the tumor-bearing organs, we next sought to determine whether a relationship existed between the radiation dose delivered to tumor-bearing organs and the therapeutic results observed. Under conditions similar to those seen during the biodistribution studies, mice received 105 BCL1 cells intravenously and were treated 10 days later with unlabeled or 9.25 MBq 131I-labeled mAbs. Figure 5 confirms that while unlabeled anti-MHCII mAb gives no protection over controls, both anti-Id and anti-CD19 produce a small therapeutic effect, increasing survival with anti-Id by around 8 days. This activity was, however, further enhanced by the conjugation of 9.25 MBq 131I to the anti-CD19 mAb. Interestingly, all 3 radioimmunoconjugates produced a similar level of therapeutic performance (Figure 5). Therefore, despite 131I–anti-CD19 and 131I–anti-Id delivering significantly less radiation dose to tumor, these radioimmunoconjugates provided a similar level of therapy to 131I anti-MHCII. These data indicate that there did not appear to be a relationship between radiation dose delivered with single mAb and therapeutic response seen. The radiolabeled irrelevant mAb provided approximately 5 days improvement in survival over control animals as before (data not shown).
RIT of BCL1 lymphoma using a panel of anti–B-cell mAbs. BCL1 cells (105 intravenously) were given on day 0, and RIT treatments were administered by intravenous injection 10 days later (500 μg mAbs with or without 9.25 MBq conjugated 131I). Unlabeled anti-MHCII had no discernible therapeutic effect and anti-Id and anti-CD19 had very modest improvements in survival of just a few days. A similar therapeutic effect was seen for all 3 radioimmunconjugates (9.25MBq 131I-labeled anti-MHCII, anti-CD19, and anti-Id).
RIT of BCL1 lymphoma using a panel of anti–B-cell mAbs. BCL1 cells (105 intravenously) were given on day 0, and RIT treatments were administered by intravenous injection 10 days later (500 μg mAbs with or without 9.25 MBq conjugated 131I). Unlabeled anti-MHCII had no discernible therapeutic effect and anti-Id and anti-CD19 had very modest improvements in survival of just a few days. A similar therapeutic effect was seen for all 3 radioimmunconjugates (9.25MBq 131I-labeled anti-MHCII, anti-CD19, and anti-Id).
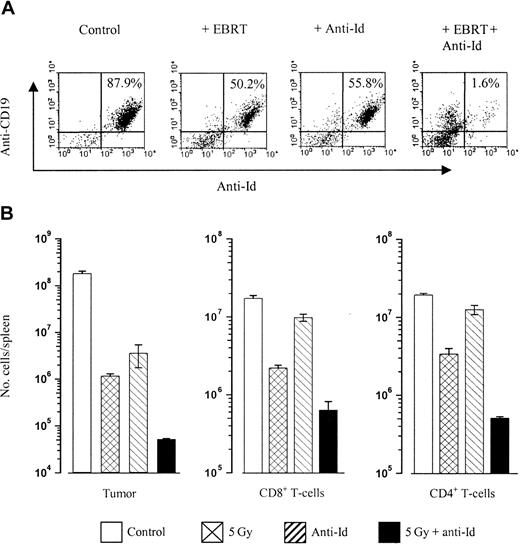
Anti-Id and radiation have a synergistic effect in clearing tumor in vivo
We next wished to further explore the mechanisms that might underlie the efficacy of combining radiotherapy and unlabeled anti-Id mAbs in clearing tumor in vivo that we had previously established (Figures 1B and 2). Tracking experiments were therefore performed to monitor the differences in tumor clearance in mice treated with radiation and anti-Id compared with mice treated with either treatment alone. In addition the possibility that these effects might be due to an up-regulated immune response were investigated. These experiments were performed in the A31 model where we had observed such impressive clearances of tumor with EBRT and anti-Id mAbs (Figure 1B) due to the problems of handling RIT mAb ex vivo. Mice were inoculated with 106 tumor cells intravenously on day 0 and treated as before on day 10 with anti-Id mAb alone or in combination with EBRT. Animals were subsequently killed on day 5 after treatment and the number of splenic tumor cells was determined using flow cytometry. Figure 6 shows that the tumor cell kill is similar in the 5 Gy– and anti-Id–treated groups, but there is a highly significant (P < .01) increase in tumor cell kill when these two treatments are given in combination. To assess whether this clearance of tumor may be due to activation of an immune response, we assessed the number of splenic CD4+ and CD8+ T cells in mice from each treatment group (Figure 6B). These data reveal that the number of CD4+ and CD8+ T cells actually decreases in mice treated with a combination of anti-Id and EBRT and suggests that the profound clearance of tumor does not appear to be due to a host cellular immune response but rather is the result of a synergistic or additive action between radiation and mAb.
In vivo clearance of tumor. Mice were inoculated with 106 A31 lymphoma cells intravenously on day 0 and treated on day 10 with EBRT (5Gy), anti-Id mAb (100 μg intravenously), or both together. Animals were culled 5 days later, spleens were removed and homogenized, and the relative number of tumor, CD8+, or CD4+ T cells were calculated. (A) Shown is the percentage of tumor cells present in the spleen of a representative mouse following each treatment, as measured by flow cytometry using PE-labeled anti-CD19 versus FITC-labeled anti-Id on a FACS Calibur. (B) Histogram illustrating the total number of tumor (Id+) cells, CD8+ T cells, and CD4+ T cells present in the spleens of mice following each treatment. The average number of cells found in each group are shown, with error bars indicating the SEM (n = 3). Data represent 1 of 3 independent experiments. Combination therapy results in a significantly increased depletion of tumor cells compared with controls (P < .01) or single-modality treated (P < .01) cohorts. However, CD8+ and CD4+ cell numbers are also reduced following combination therapy treatment compared with single treatment groups, indicating that clearance of tumor is not dependent on an enhanced T-cell response.
In vivo clearance of tumor. Mice were inoculated with 106 A31 lymphoma cells intravenously on day 0 and treated on day 10 with EBRT (5Gy), anti-Id mAb (100 μg intravenously), or both together. Animals were culled 5 days later, spleens were removed and homogenized, and the relative number of tumor, CD8+, or CD4+ T cells were calculated. (A) Shown is the percentage of tumor cells present in the spleen of a representative mouse following each treatment, as measured by flow cytometry using PE-labeled anti-CD19 versus FITC-labeled anti-Id on a FACS Calibur. (B) Histogram illustrating the total number of tumor (Id+) cells, CD8+ T cells, and CD4+ T cells present in the spleens of mice following each treatment. The average number of cells found in each group are shown, with error bars indicating the SEM (n = 3). Data represent 1 of 3 independent experiments. Combination therapy results in a significantly increased depletion of tumor cells compared with controls (P < .01) or single-modality treated (P < .01) cohorts. However, CD8+ and CD4+ cell numbers are also reduced following combination therapy treatment compared with single treatment groups, indicating that clearance of tumor is not dependent on an enhanced T-cell response.
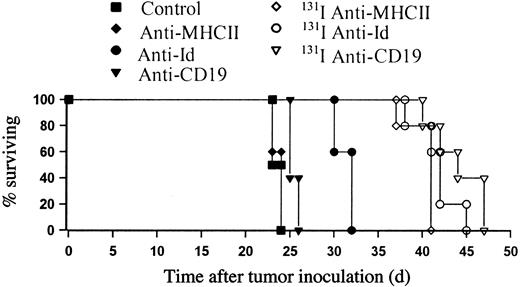
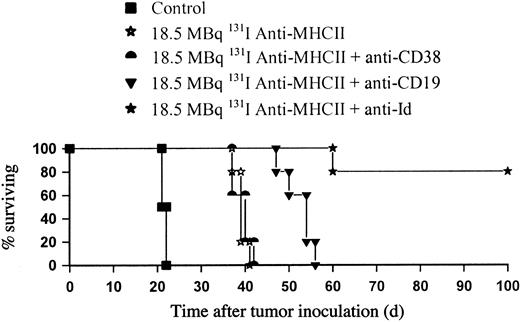
Anti-CD19 improves survival in combination with 131I anti-MHCII RIT
We then went on to investigate whether the long-term clearance of tumor achieved with anti-Id in combination with 131I anti-MHCII could be reproduced with other B-cell–specific mAbs, regarding the potential problems of translating these results with anti-Id mAb to the clinic. Figure 7 shows that when anti-CD19 mAb was substituted for anti-Id and used in combination with 131I anti-MHCII in the BCL1 model, impressive tumor protection was also seen of around 35 days longer than with control animals and 15 days longer than 131I anti-MHCII alone (P < .01). Despite the CD38 antigen being expressed on B cells at similar levels to CD19 (Table 1), enhancement in therapy with 131I-labeled anti-MHCII was seen with anti-CD19 mAb, but not with the anti-CD38 mAb, where the level of tumor protection observed was similar to that achieved with 131I anti-MHCII alone.
Anti-CD19 improves survival in combination with 131I-labeled anti-MHCII in the BCL1 lymphoma model. BALB/c mice were inoculated with 105 cells by intravenous injection on day 0 and treated 10 days later with18.5 MBq 131I-labeled anti-MHCII plus unlabeled anti-Id, anti-CD38, or anti-CD19 mAb (500 μg of each intravenously). The above experiment is representative of 1 of 3 identical experiments and shows a significant survival improvement when the anti-Id or anti-CD19 was added to 18.5 MBq 131I anti-MHCII over 18.5 MBq 131I anti-MHCII alone (P < .01). No improvement was observed following addition of anti-CD38 mAb.
Anti-CD19 improves survival in combination with 131I-labeled anti-MHCII in the BCL1 lymphoma model. BALB/c mice were inoculated with 105 cells by intravenous injection on day 0 and treated 10 days later with18.5 MBq 131I-labeled anti-MHCII plus unlabeled anti-Id, anti-CD38, or anti-CD19 mAb (500 μg of each intravenously). The above experiment is representative of 1 of 3 identical experiments and shows a significant survival improvement when the anti-Id or anti-CD19 was added to 18.5 MBq 131I anti-MHCII over 18.5 MBq 131I anti-MHCII alone (P < .01). No improvement was observed following addition of anti-CD38 mAb.
Anti-Id and anti-CD19 mAbs induce direct cell surface–mediated signaling
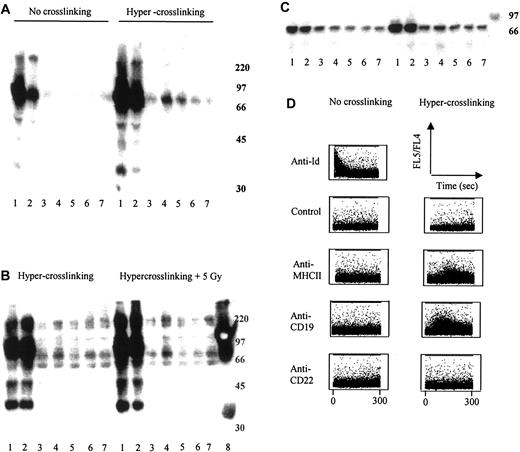
Finally, we investigated whether the clearance of tumor in vivo might be linked to the ability of some mAbs to induce cell surface signaling. We initially assessed signal transduction by measuring the up-regulation of cellular protein tyrosine phosphorylation (PTP) using Western blot analysis. After stimulating BCL1 tumor cells with a panel of B-cell–binding mAbs in the presence or absence of a second hyper–cross-linking polyclonal Ab, it was clear that mAbs directed to the IgM heavy chain or Id regions of the B-cell receptor (BCR) induce profound up-regulation of PTP on proteins of approximately 72 kDa (Figure 8A). Anti-CD19 and to a lesser extent anti-CD22 mAbs also induced some increase in PTP on the proteins of same molecular weight, but only after hyper–cross-linking.23 In contrast, no demonstrable increase in PTP was seen for anti-MHCII, anti-CD38, or an isotype-matched control irrelevant mAb in the presence or absence of hyper–cross-linking. As we had seen such a significant therapeutic improvement using these signaling mAbs with the addition of targeted irradiation in vivo, we repeated the experiment with the addition of 5 Gy EBRT. Irradiation appears to significantly increase the amount of PTP we saw on the Western blot over and above that seen with hyper–cross-linking mAbs alone (Figure 8B).
Measurement of intracellular signaling. (A) Western blot analysis of protein tyrosine phosphorylation was performed using fresh BCL1 cells as described in “Materials and methods.” Lane 1 indicates anti-μ (IgM heavy chain); 2, anti-Id; 3, anti-MHCII; 4, anti-CD19; 5, anti-CD22; 6, anti-CD38; 7, control; and 8, molecular weight marker. Anti-μ and anti-Id as single mAb demonstrate intracellular signaling through the surface Ig receptor (BCR) as measured by the induction of cellular protein tyrosine phosphorylation. Hyper–cross-linking of primary mAb with sheep antirat IgG demonstrates signal transduction in anti-CD19 and anti-CD22 as well as via the BCR. (B) Addition of radiation enhances the level of signaling observed through these molecules. (C) Immunoprecipitation of phosphorylated proteins using 4G10, followed by immunoblotting with anti-Syk mAb, revealed that the approximately 70-kDa phosphorylated protein is likely to be Syk. (D) Measurement of intracellular calcium flux in lymphoma cells after hyper–cross-linking mAb. πBCL1 lymphoma cells were loaded with Indo-1-AM and then incubated with either mAb alone (2 μg/mL) or mAb plus an appropriate secondary polyclonal anti-IgG (20 μg/mL). Profiles of FL5/FL4 are shown versus time. Control or irrelevant IgG was a nonbinding anti-Id mAb (to the A31 lymphoma) in both sets of experiments.
Measurement of intracellular signaling. (A) Western blot analysis of protein tyrosine phosphorylation was performed using fresh BCL1 cells as described in “Materials and methods.” Lane 1 indicates anti-μ (IgM heavy chain); 2, anti-Id; 3, anti-MHCII; 4, anti-CD19; 5, anti-CD22; 6, anti-CD38; 7, control; and 8, molecular weight marker. Anti-μ and anti-Id as single mAb demonstrate intracellular signaling through the surface Ig receptor (BCR) as measured by the induction of cellular protein tyrosine phosphorylation. Hyper–cross-linking of primary mAb with sheep antirat IgG demonstrates signal transduction in anti-CD19 and anti-CD22 as well as via the BCR. (B) Addition of radiation enhances the level of signaling observed through these molecules. (C) Immunoprecipitation of phosphorylated proteins using 4G10, followed by immunoblotting with anti-Syk mAb, revealed that the approximately 70-kDa phosphorylated protein is likely to be Syk. (D) Measurement of intracellular calcium flux in lymphoma cells after hyper–cross-linking mAb. πBCL1 lymphoma cells were loaded with Indo-1-AM and then incubated with either mAb alone (2 μg/mL) or mAb plus an appropriate secondary polyclonal anti-IgG (20 μg/mL). Profiles of FL5/FL4 are shown versus time. Control or irrelevant IgG was a nonbinding anti-Id mAb (to the A31 lymphoma) in both sets of experiments.
Syk is known to be one of the key initiators of the complex signaling cascades triggered following BCR ligation and has a molecular weight of 72 kDa.31 We therefore hypothesized that the protein seen on our Western blot analyses might be phosphorylated Syk, and we performed immunoprecipitations using a Syk-specific antibody. The tyrosine-phosphorylated proteins immunoprecipitated by the anti-Syk antibody were identical to the approximately 72-kDa proteins detected using the phosphotyrosine-specific antibody. Therefore, phosphorylation of Syk appears to correlate with therapeutic responsiveness (Figure 8C).
Intracellular-free calcium flux is another well-documented measure of intracellular signaling30 and so we therefore measured intracellular calcium levels in πBCL1 cells (a variant of BCL1 that can be grown in vitro)20 shortly after treatment with the same panel of mAbs. Figure 8D reveals profiles of FL5/FL4 versus time, with an increase in the ratio of FL5/FL4 indicating a rise in intracellular-free calcium levels. These data show that anti-Id induces profound calcium flux in the absence of hyper–cross-linking, but as with the PTP experiments above, hyper–cross-linking is required for the induction of calcium flux with anti-CD19. In this assay, hyper–cross-linked anti-MHCII mAb also induces a degree of calcium flux that is not seen with either the anti-CD22 or the control mAb (Figure 8D).
Discussion
In this study we have investigated the mechanisms operating in RIT of B-cell lymphoma in two syngeneic models and have attempted to define the contributions of “targeted” irradiation and the intrinsic mAb effector mechanisms to the long-term clearance of tumor in vivo. We have shown that the successful clearance of tumor in RIT can be provided by using two different mAbs working in combination, whereby one mAb is more effective at targeting the radiation to tumor and the second mAb initiates intracellular signaling. Finally we have shown that a radiation dose response exists for RIT of B-cell lymphoma in the presence of a signaling mAb.
We have previously shown the importance of mAb specificity to the therapeutic outcome of RIT in the BCL1 tumor model.16 In the current study we have expanded that initial work and using MIRD dose calculations have determined the total radiation dose delivered by a panel of B-cell antigen–targeting mAbs in vivo. Although the anti-MHCII and anti-Id mAbs bound to BCL1 tumor at similar levels,16 the anti-MHCII mAb delivered approximately 3 to 6 times more irradiation to the spleen. This observation appears to be explained by the fact that anti-MHCII mAb has a longer half-life and remains on the surface of tumor cells in vivo, while the anti-Id is cleared rapidly.16 The rapid clearance of anti-Id mAb and poor delivery of radiation to tumor by anti-Id, we believe, is due to rapid endocytosis and dehalogenation of 131I, in keeping with previously published work on anti-μ mAb.32,33
The requirement for individual patient dosimetry and its role in predicting response to RIT is currently a controversial issue in clinical RIT.2,34,35 Our studies indicate that there is a poor correlation between the radiation dose delivered by RIT and the therapeutic outcome. Even accurate radiation dosimetry appears inadequate at predicting the tumor response. Thus we found a similar level of performance for the three different radioimmunoconjugates 131I-labeled anti-CD19, anti-Id, and anti-MHCII mAbs in our B-cell lymphoma models, despite widely different radiation dose delivery to tumor-bearing organs. This may be explained by the fact that the tumor cell kill induced by the mAb is not quantified by such radiation dosimetry. We therefore conclude that for the radiolabeled anti-CD19 and anti-Id mAbs there appears to be two components to the therapy, namely mAb effector mechanism and targeted radiotherapy, with only the targeted radiation being measured by the dosimetry.
Although the lymphoma models used in this study have different growth patterns from human lymphomas, the mAbs used in this work all target antigens where equivalents have been tested in clinical trials.6,15,36-38 Indeed, the 131I–Lym-1, which has been extensively investigated in clinical trials,11,15 has very similar characteristics to the 131I anti-MHCII used in this study. We believe that we have made an important observation and proof of principle here and that our results using therapeutically active mAbs demonstrate that RIT is more than simply targeted radiation. Furthermore, this study may help to clarify why clinical RIT tumor dosimetry using radiolabeled anti-CD20 mAb in B-cell lymphoma frequently fails to correlate with the clinical responses seen, as the tumor dosimetry is unable to quantify the anti-CD20 mAb effector mechanisms.
From these experiments, it appears that larger doses of targeted 131I are best delivered by a mAb directed against a highly expressed target antigen that fails to modulate upon binding, such as the MHCII complex. However, it is clear from this study that radiation alone, whether targeted using 131I-labeled anti-MHCII mAbs or delivered by EBRT, is inadequate in producing long-term clearance of tumor. For RIT or “systemic radiotherapy,” no improvement in survival was seen as the radiation dose was increased above 9.25 MBq 131I anti-MHCII. However, when external EBRT or 131I-labeled anti-MHCII mAb was combined with a signaling mAb, significant improvements in the therapy were seen, resulting in long-term survivors in the anti-Id plus 131I-labeled anti-MHCII mAb groups. Interestingly, under these circumstances in the presence of anti-Id mAb, we found that a radiation dose response existed for targeted radiation. We found this dose response to be reproducible in two different B-cell lymphoma models, underlying the potential importance of this observation.
This study suggests that the substantial increases in therapeutic effect seen with the addition of anti-Id and anti-CD19 to targeted radiotherapy occurs because these mAbs initiate intracellular signal transduction. Previous work from our group has shown that the anti-Id and anti-CD19 performed relatively poorly in complement-dependent cytotoxicity (CDC) and Ab-dependent cellular cytotoxicity (ADCC) assays but these mAbs were the most therapeutically active.17 In contrast, the anti-MHCII mAb was the most potent in the CDC and ADCC assays but provided no protection in vivo. These data argue that Fc-dependent effector mechanisms as measured in these assays cannot themselves explain the therapeutic activity of mAbs. However, other in vitro assays revealed that mAbs directed to the BCR complex were particularly active at inducing growth arrest of tumor cell lines, presumably through intracellular signaling.17 We now provide direct evidence that anti-Id and anti-CD19 mAbs, but not other mAb specificities (CD38, MHCII), initiate intracellular signaling shown by both up-regulation of protein tyrosine phosphorylation and calcium flux. It is well documented that signals transmitted by the BCR control the fate of normal and neoplastic B cells and that mAbs directed to this receptor can induce apoptotic cell death.39,40 Furthermore it is well established that signals from the BCR can be modulated by coreceptors such as CD1941 and that anti-CD19 mAbs are capable of transmitting growth inhibitory/apoptotic signals, when sufficiently cross-linked.42 Presumably therefore, the signals induced by these 2 mAbs are similar and are generated through a shared pathway. Our Western blot analysis and subsequent immunoprecipitation suggests that the protein tyrosine kinase Syk is involved in this signal transduction. Phosphorylation of Syk is one of the key steps in the complex signaling cascades activated following BCR ligation and is an important trigger for intracellular calcium flux.31 This is again in keeping with our data, where there is a good correlation between the degree of calcium mobilization observed and the extent of Syk phosphorylation. Moreover, the strength of intracellular signal obtained appears to be proportional to the amount of BCR cross-linking.43 We observed that anti-μ and anti-Id mAb signaling was potentiated after hyper–cross-linking and that potent signaling through CD19 was only observed after such treatment. Evidence suggests that the function of hyper–cross-linking in vivo is likely to be fulfilled by Fc-receptor–bearing cells, such as macrophages and natural killer (NK) cells, which are able to bind and aggregate the Fc domains of mAbs.44 Thus, our data supports the notion that the therapeutic efficacy of anti-Id and anti-CD19 mAb is related to the signaling activity of these reagents and that the response seen is proportional to the strength of intracellular signal delivered. These results appear entirely compatible with the clinical results published by the Stanford group using anti-Id mAb in the treatment of follicular lymphoma, where the clinical response appeared to correlate with the degree of signal transduction.29
This study does not exclude the possibility that other host immune effector mechanisms, such as T cells, may act as an important component of clinical RIT. However, in our models, the long-term clearance of tumor by radiation and anti-Id does not appear to involve a T-cell response. Indeed, the number of CD4+ and CD8+ T cells is actually lower in mice treated with EBRT and anti-Id than in untreated controls. From previous studies, we know that the tumor itself can stimulate an increase in CD4+ and CD8+ cells.45 This decrease may therefore reflect a reduction in the amount of tumor (and therefore antigen) present due to the potent cytotoxic activity of the combination therapy, as well as the direct immunosuppressive effect of the EBRT itself.
In conclusion, we have demonstrated that the biodistribution of a single radiolabeled mAb does not predict tumor response when a significant biologic contribution is derived from a signaling mAb. Furthermore, we have shown that successful eradication of lymphoma in RIT consists of targeted irradiation and mAb signaling. Long-term clearance of tumor in vivo can be achieved by combining a therapeutically active signaling mAb that is itself poor at delivering radiation with a mAb that is a more effective vector for targeting radiation to tumor. Importantly, this tumor eradication is achieved with minimal and reversible hematologic toxicity. Our results confirm that RIT is much more than targeted radiation and provide a scientific rationale to support the use of selecting combinations of mAbs in RIT rather than using a single mAb. This study also suggests that a radiation dose response may exist for RIT in B-cell lymphoma, when the targeted radiation is augmented by the presence of a signaling mAb. We have demonstrated for the first time in vivo that this combination approach is effective in RIT and we believe that these provocative data indicate that this type of approach could be readily translated to the clinic.
Prepublished online as Blood First Edition Paper, October 23, 2003; DOI 10.1182/blood-2003-06-2037.
Supported by the Cancer Research UK, Tenovus Cancer Charity, and Wessex Cancer Trust.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank all the members of the Tenovus Cancer Laboratory, who provided expert technical support. In particular we thank Richard Reid, Maureen Power, and Dr Ruth French for invaluable technical assistance, and colleagues within the Cancer Sciences Division of Southampton University for their helpful comments about the manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal