Abstract
Killer immunoglobulin–like receptors (KIRs) regulate cell activity of natural killer (NK) cells and some T cells. The predominant ligand for inhibitory KIRs is HLA-C, which subdivides into 2 groups based on the specificity of inhibitory KIRs. The ligands for activatory KIRs are unknown. Following hematopoietic stem cell transplantation (HSCT), recipient tissues may not express a ligand for KIRs present within the graft, and the combination of donor KIR and recipient HLA-C types could influence outcome. HLA and KIR genotypes were determined in 220 donor-recipient pairs from HLA-matched sibling HSCTs performed for myeloid (n = 112) and lymphoid (n = 108) diseases. In HSCTs performed for myeloid disease, overall survival was worse in patients homozygous for group 2 HLA-C (C2) than in patients who carried a group 1 HLA-C (C1) allele (P < .005). Moreover, this effect is seen only when the donor additionally carries the activating KIR gene KIR2DS2 (P = .045). No effect was seen in patients with lymphoid disease. Thus, in HLA-matched sibling HSCT for myeloid leukemia, patients homozygous for C2 alleles receiving a graft from a donor carrying the KIR gene KIR2DS2 have a significantly reduced chance of survival.
Introduction
Killer immunoglobulin–like receptors (KIRs) are members of the immunoglobulin superfamily expressed on the surface of natural killer (NK) cells and a subset of T cells.1 They are encoded by highly polymorphic genes located on chromosome 19q13.4 in a region known as the leukocyte receptor cluster (LRC).2,3 KIRs are present in 2 main forms, delivering either an inhibitory or an activating signal following ligation. Inhibitory receptors possess a cytoplasmic tail bearing immunoreceptor tyrosine-based inhibitory motifs (ITIMs),4 whereas activating KIRs lack a cytoplasmic tail, requiring an adaptor protein (DAP12) to generate intracellular signals via activating motifs (ITAMs) on the adaptor protein.5
The natural ligands of several KIRs have been identified as class I HLA molecules that, in contrast to KIRs, are expressed by most nucleated cells.6 HLA-C is the predominant ligand for most inhibitory KIRs. The KIR-HLA interaction is sensitive to a genetic polymorphism at position 80 in the HLA-C α-helix.7 Group 1 HLA-C molecules (referred to as C1) possess an asparagine residue at position 80, whereas group 2 HLA-C molecules (referred to as C2) have a lysine at the corresponding position. The KIR2DL1 receptors recognize C2 molecules as their natural ligand, whereas the KIR2DL2/3 receptors recognize C1 molecules.8 In a similar fashion, HLA-B alleles may be subdivided into 2 groups based on the polymorphism that determines either the Bw4 or Bw6 motifs. KIR3DLl has been demonstrated to bind Bw4 as its natural ligand,9 but no KIR has yet been shown to bind to the Bw6 motif.10
The activating KIRs have striking homology with their inhibitory counterparts in the extracellular domains, but debate remains as to whether or not HLA class I molecules are their natural ligand. Although they have been demonstrated to mediate cell killing through recognition of class I HLA, their affinity for class I molecules is much weaker than that of the inhibitory receptors.11 Alternative natural ligands might include non–major histocompatibility complex (MHC) molecules, virally encoded MHC class I homologs or other foreign proteins. Recent work has demonstrated that a murine analog of activatory KIRs, Ly49H, recognizes the gene product m157 from murine cytomegalovirus (MCMV) and that inheritance of Ly49H is the major determinant of susceptibility or resistance to MCMV infection in different mouse strains.12
Up to 12 KIR genes are expressed, and a number of KIR haplotypes have been determined that are primarily characterized by variation in the number of activating KIR genes. Approximately 50% of the population have a single activating receptor (KIR2DS4), whereas the rest carry genes for between 2 and 5, including the genes KIR2DS1 or KIR2DS2.13 As a result of this combination of diversity in KIR gene number, variable KIR specificity, and independent segregation of HLA genes, it is possible that individuals will inherit KIR genes for which the corresponding ligand is not expressed. In the setting of hemopoietic stem cell transplantation (HSCT), the possibility of biologically significant KIR/HLA-C mismatches is potentially important. Dependent on KIR genotype, the donor graft may contain cells expressing inhibitory KIRs that fail to interact with a ligand on recipient tissues. Theoretically, this could generate an unopposed alloreactive response in cells lacking appropriate inhibitory receptors, resulting in graft versus host disease (GVHD), graft versus leukemia (GVL), or both. This disparity is more likely with increasing degrees of HLA mismatch between donor and recipient as seen with unrelated donor (UD) or haploidentical HSCT.14,15 Indeed, a positive outcome of KIR/HLA disparity has been demonstrated in the setting of haploidentical HSCT.16,17 Extremely low rates of leukemia relapse and a paradoxical reduction in GVHD were noted when the haploidentical graft possessed KIRs for which the recipient had no ligand. Furthermore, alloreactive NK clones that killed recipient leukemic blasts in vitro were isolated from the recipient following the transplant. Notably, this effect was seen only in patients with myeloid leukemia but not in those with lymphoid disease. A recent study has examined the same question in transplants from unrelated donors where HLA-C mismatches commonly occur.18 Again it was found that KIR ligand incompatibility, defined by the absence of a KIR ligand in the recipient that is present in the donor, could lead to a favorable outcome.
The potential clinical significance of activating KIRs is now receiving attention. Martin et al19 indicated that activating KIRs may have a role in the pathogenesis of psoriatic arthritis (PsA). In particular, it was demonstrated that the presence of an activating KIR in the absence of the ligand for its inhibitory counterpart significantly increased the risk for PsA.
Most HSCT patients receive their graft from an HLA-identical sibling donor. In this context, the chances of a KIR/HLA disparity remains (as their loci are on separate chromosomes) but is reduced in comparison with haploidentical or UD transplants. Based on the study by Martin et al,19 we hypothesized that, in HLA-matched sibling HSCTs, the presence of activating KIRs (in the donor) could significantly affect the outcome of HSCT if the ligand for the inhibitory counterpart were absent in the recipient (along the lines of the findings in PsA). In this study we have examined the clinical significance of donor KIR genotype and recipient HLA-C genotype in this group. These transplants are particularly important to investigate, as they allow the evaluation of the influence of KIR types without the confounding effects of HLA mismatch.
In transplants for myeloid leukemia, we report the novel observation that when patients do not carry the genes for C1 molecules (the ligands for the inhibitory KIR2DL2/2DL3), survival is impaired following HSCT. Moreover, this effect is seen only when the donor additionally carries the activatory KIR gene KIR2DS2. Indeed, only 1 of 10 patients who lacked C1 alleles and received a transplant from a donor carrying KIR2DS2 survived beyond 4 years. No such effect was seen in patients with lymphoid malignancy.
Patients, materials, and methods
Selection of patients
Two-hundred and twenty transplants performed between January 1994 and April 2002 were analyzed. Patients received HLA-matched sibling allografts for myeloid leukemia (acute myeloid leukemia [AML], chronic myeloid leukemia [CML], and myelodysplastic syndrome [MDS]) or lymphoid disease (acute lymphoblastic leukemia [ALL], chronic lymphocytic leukemia [CLL], or non-Hodgkin lymphoma [NHL]) at Birmingham Children's Hospital, Birmingham Heartlands Hospital or Queen Elizabeth Hospital, Birmingham, United Kingdom. Patient characteristics are given in Table 1. Clinical information was obtained by reviewing data submitted to transplant registries and the use of medical records. A control population for KIR gene frequencies was composed of 126 new blood donors from the same geographical area as the patients. Ethics approval for this study was obtained from South Birmingham Local Research Ethics Committee.
Patient characteristics
. | Lymphoid disease* . | Myeloid leukemia† . |
|---|---|---|
| Male:female | 68:40 | 64:48 |
| Mean age, y (range) | 33.1 (2-60) | 38.4 (4-59) |
| Disease | ||
| ALL, CR1 | 29 | NA |
| ALL, CR2 | 25 | NA |
| NHL, CR1 | 4 | NA |
| NHL, CR2 | 3 | NA |
| NHL, CR3+ | 6 | NA |
| NHL, progressive | 30 | NA |
| CLL, CR1 | 1 | NA |
| CLL, PR | 8 | NA |
| CLL, progressive | 2 | NA |
| AML, CR1 | NA | 28 |
| AML, CR2+ | NA | 8 |
| AML progressive | NA | 16 |
| CML, CP1 | NA | 43 |
| CML, CP2 | NA | 3 |
| CML, accelerated phase | NA | 3 |
| MDS, CR1 | NA | 2 |
| MDS, untreated | NA | 9 |
| TBI/cyclophosphamide or TBI/etoposide conditioning | 61 | 101 |
| Reduced intensity conditioning | 47 | 11 |
| Cyclosporin only as GVHD prophylaxis | 56 | 15 |
| Cyclosporin and methotrexate as GVHD prophylaxis | 52 | 97 |
. | Lymphoid disease* . | Myeloid leukemia† . |
|---|---|---|
| Male:female | 68:40 | 64:48 |
| Mean age, y (range) | 33.1 (2-60) | 38.4 (4-59) |
| Disease | ||
| ALL, CR1 | 29 | NA |
| ALL, CR2 | 25 | NA |
| NHL, CR1 | 4 | NA |
| NHL, CR2 | 3 | NA |
| NHL, CR3+ | 6 | NA |
| NHL, progressive | 30 | NA |
| CLL, CR1 | 1 | NA |
| CLL, PR | 8 | NA |
| CLL, progressive | 2 | NA |
| AML, CR1 | NA | 28 |
| AML, CR2+ | NA | 8 |
| AML progressive | NA | 16 |
| CML, CP1 | NA | 43 |
| CML, CP2 | NA | 3 |
| CML, accelerated phase | NA | 3 |
| MDS, CR1 | NA | 2 |
| MDS, untreated | NA | 9 |
| TBI/cyclophosphamide or TBI/etoposide conditioning | 61 | 101 |
| Reduced intensity conditioning | 47 | 11 |
| Cyclosporin only as GVHD prophylaxis | 56 | 15 |
| Cyclosporin and methotrexate as GVHD prophylaxis | 52 | 97 |
CR indicates complete remission; PR, partial remission; CP, chronic phase; and NA, not applicable.
n = 108.
n = 112.
Patient and donor DNA was prepared from peripheral blood mononuclear cells and used for HLA typing prior to transplantation and stored at –20°C. KIR gene typing was performed retrospectively but without knowledge of outcome.
HLAtyping
HLA-A, B, and C type was determined by polymerase chain reaction–sequence-specific primers (PCR-SSP) using a regularly updated version of the HLA phototype method.20 HLA-C alleles were characterized to the level that allowed assignment of KIR HLA-C groups 1 and 2 on the basis of the codon determining amino acid position 80.
KIRtyping
KIR genotype was determined by PCR-SSP using a modified set of primers based on those published by Uhrberg et al.21 Our modifications take account of the recent marked increase in KIR sequences available, and all reactions were validated using cell lines from the 10th International Histocompatibility workshop.22 PCR was performed in a total reaction volume of 13 μL, consisting of 67 mM Tris [tris(hydroxymethyl)aminomethane] base pH 8.8, 16.6 mM ammonium sulfate, 2 mM magnesium chloride, 0.01% (vol/vol) Tween 20, 200 μM of each deoxyribonucleoside triphosphate (dNTP), 0.2-0.75 μM KIR primer, 0.l5 μM control primer (hGH), between 0.1 and 0.2 μg genomic DNA and 0.125 units Taq polymerase (Bioline, London, United Kingdom). Genomic PCR analysis was performed under the following conditions: initial denaturation at 95°C for 120 seconds, then 10 cycles of 95°C for 10 seconds, and 65°C for 60 seconds, then 20 cycles of 95°C for l0 seconds, 61°C for 50 seconds, and 72°C for 30 seconds. Products were visualized on 1.5% agarose gel prestained with ethidium bromide.
Statistics
Data were collected on the frequency and severity of acute GVHD, disease relapse, infection, death, and cause of death and allowed calculation of disease-free survival (DFS) and overall survival (OS). The χ2 or Fisher exact test was used to compare differences between groups for KIR frequencies and GVHD. The probability of survival was estimated with the use of the product-limit method of Kaplan and Meier with death (any cause) defined as the event. The probabilities between groups were compared with the use of the log-rank statistic and calculated using SPSS for Windows (SPSS, Woking, United Kingdom) and EPI INFO 2000 software (http://www.cdc.gov/epiinfo/). Relative linkage disequilibrium was calculated using the method of Baur and Danilovs.23
Results
Patient characteristics
Details of the 220 patients are shown in Table 1. They were divided into lymphoid and myeloid groups, as previous studies had indicated that the KIR effect is more pronounced in patients with myeloid disease.16-18 Patients in the lymphoid disease group had a slightly lower mean age and were more likely to have undergone transplantation using a reduced-intensity regime. The KIR gene frequencies detected in the donor and recipient populations for both disease groups are given in Table 2. These are in line with frequencies published elsewhere24-28 and did not differ significantly from controls. There was no significant difference between the 3 transplant centers as measured by transplant outcome (overall survival and disease-free survival, data not shown). In addition, neither conditioning regime nor GVHD prophylaxis regime had a significant effect on outcome.
KIR gene frequencies as detected by PCR-SSP for all patients and recipients and a control panel of healthy blood donors
. | Lymphoid disease, %* . | . | Myeloid leukemia, %† . | . | . | ||
|---|---|---|---|---|---|---|---|
| KIR . | Donor . | Recipient . | Donor . | Recipient . | Controls, %‡ . | ||
| 2DL1 | 94 | 97 | 91 | 91 | 93 | ||
| 2DL2 | 42 | 43 | 50 | 49 | 52 | ||
| 2DL3 | 92 | 96 | 90 | 91 | 93 | ||
| 2DL4 | 100 | 100 | 100 | 100 | 100 | ||
| 2DL5 | 43 | 43 | 41 | 43 | 40 | ||
| 3DL1 | 93 | 93 | 91 | 90 | 94 | ||
| 3DL2 | 100 | 100 | 100 | 100 | 100 | ||
| 2DS1 | 39 | 39 | 38 | 37 | 45 | ||
| 2DS2 | 42 | 42 | 51 | 52 | 55 | ||
| 2DS3 | 27 | 23 | 25 | 26 | 25 | ||
| 2DS4 | 91 | 91 | 90 | 82 | 87 | ||
| 2DS5 | 31 | 27 | 28 | 27 | 33 | ||
| 3DS1 | 32 | 34 | 28 | 30 | 42 | ||
. | Lymphoid disease, %* . | . | Myeloid leukemia, %† . | . | . | ||
|---|---|---|---|---|---|---|---|
| KIR . | Donor . | Recipient . | Donor . | Recipient . | Controls, %‡ . | ||
| 2DL1 | 94 | 97 | 91 | 91 | 93 | ||
| 2DL2 | 42 | 43 | 50 | 49 | 52 | ||
| 2DL3 | 92 | 96 | 90 | 91 | 93 | ||
| 2DL4 | 100 | 100 | 100 | 100 | 100 | ||
| 2DL5 | 43 | 43 | 41 | 43 | 40 | ||
| 3DL1 | 93 | 93 | 91 | 90 | 94 | ||
| 3DL2 | 100 | 100 | 100 | 100 | 100 | ||
| 2DS1 | 39 | 39 | 38 | 37 | 45 | ||
| 2DS2 | 42 | 42 | 51 | 52 | 55 | ||
| 2DS3 | 27 | 23 | 25 | 26 | 25 | ||
| 2DS4 | 91 | 91 | 90 | 82 | 87 | ||
| 2DS5 | 31 | 27 | 28 | 27 | 33 | ||
| 3DS1 | 32 | 34 | 28 | 30 | 42 | ||
n = 108.
n = 112.
n = 126.
Influence of HLA-C group of the patient on survival
Five-year overall survival was not significantly different between the 2 patient groups (52.5% and 54.5% for myeloid and lymphoid groups, respectively, Figure 1). Both groups were analyzed to see if the presence or absence of any individual KIR gene was associated with overall survival, but no such association was seen (data not shown). Outcome as determined by the HLA-C group of the recipient was then analyzed. No significant differences were found in the lymphoid patients (P = .64). However, a marked difference in survival was found when recipients in the myeloid group were examined on the basis of the presence or absence of the KIR ligand C1 in the recipient (Figure 2). In those patients where no C1 was present (ie, C2 homozygous, n = 22), 4-year OS was 31.6%, compared with 56.1% in those patients where C1 alleles were present in either homozygous or heterozygous form (n = 90; P < .005). No such effect was seen for the presence or absence of the KIR ligand C2.
Kaplan-Meier plot for OS following HLA-identical sibling HSCT for lymphoid disease (n = 108) and myeloid disease (n = 112).
Kaplan-Meier plot for OS following HLA-identical sibling HSCT for lymphoid disease (n = 108) and myeloid disease (n = 112).
Recipient HLA-C genotype has a significant effect on OS following HLA-identical sibling HSCT for myeloid disease. Kaplan-Meier plot of OS following HLA-identical sibling HSCT for myeloid disease. HLA-C group 1 (C1; n = 90), and HLA-C group 2 (C2; n = 22).
Recipient HLA-C genotype has a significant effect on OS following HLA-identical sibling HSCT for myeloid disease. Kaplan-Meier plot of OS following HLA-identical sibling HSCT for myeloid disease. HLA-C group 1 (C1; n = 90), and HLA-C group 2 (C2; n = 22).
The influence of donor KIR gene repertoire
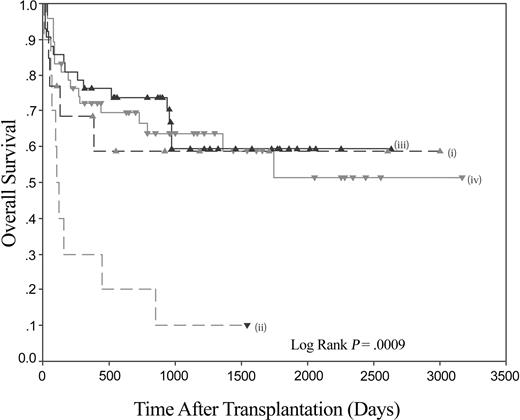
Thus, patients with myeloid malignancy have markedly impaired survival following stem cell transplantation (SCT) if they lack the KIR ligand C1. The receptor for C1 molecules is KIR2DL2/3, and the homologous activating KIR is KIR2DS2. Using the model of psoriatic arthritis,19 the data were therefore analyzed to see if the presence of KIR2DS2 in the donor had an influence on the impaired outcome of patients lacking C1 alleles. The combination of the absence of C1 in the recipient and the presence of KIR2DS2 in the donor resulted in an overall survival of 10% at the maximal follow-up of 1543 days. When compared to other recipient C1/donor KIR2DS2 combinations (ie, C1 pos/KIR2DS2 pos, C1 pos/KIR2DS2 neg, and C1 neg/KIR2DS2 neg), it can be seen that the poor survival rate of C1 neg/KIR2DS2 pos is significantly different (Figure 3). A pairwise comparison shows that the C1 neg/KIR2DS2 pos group of interest is significantly different to each of the 3 combinations (versus C1 pos/KIR2DS2 pos P = .0003, versus C1 pos/KIR2DS2 neg P = .0001, and versus C1 neg/KIR2DS2 neg P = .045). The last comparison is important as it demonstrates that in the subgroup of patients homozygous for C2, the KIR status of the donor has a significant effect on survival (Figure 4). No significant differences were found between any of the other pairs analyzed. No significant differences in the rates of acute GVHD of grade higher than II were found.
The combination of recipient HLA-C and donor KIR genotypes can significantly affect OS following HLA-identical sibling HSCT for myeloid disease. Kaplan-Meier plot of overall survival following HLA-identical sibling HSCT for myeloid disease. (i) Recipient C1 negative/donor KIR2DS2 negative (n = 12); (ii) recipient C1 negative/donor KIR2DS2 positive (n = 10); (iii) recipient C1 positive/donor KIR2DS2 negative (n = 43); and (iv) recipient C1 positive/donor KIR2DS2 positive (n = 47).
The combination of recipient HLA-C and donor KIR genotypes can significantly affect OS following HLA-identical sibling HSCT for myeloid disease. Kaplan-Meier plot of overall survival following HLA-identical sibling HSCT for myeloid disease. (i) Recipient C1 negative/donor KIR2DS2 negative (n = 12); (ii) recipient C1 negative/donor KIR2DS2 positive (n = 10); (iii) recipient C1 positive/donor KIR2DS2 negative (n = 43); and (iv) recipient C1 positive/donor KIR2DS2 positive (n = 47).
Overall survival following HLA-identical sibling HSCT for myeloid disease is significantly reduced in recipients homozygous for C2 if the donor is positive for KIR2DS2. Kaplan-Meier plot demonstrating the effect of overall survival following HLA-identical sibling HSCT for myeloid disease. (i) KIR2DS2 absent (n = 12), and (ii) KIR2DS2 present (n = 10).
Overall survival following HLA-identical sibling HSCT for myeloid disease is significantly reduced in recipients homozygous for C2 if the donor is positive for KIR2DS2. Kaplan-Meier plot demonstrating the effect of overall survival following HLA-identical sibling HSCT for myeloid disease. (i) KIR2DS2 absent (n = 12), and (ii) KIR2DS2 present (n = 10).
A similar analysis was performed for KIR2DS1 and the presence/absence of C2 alleles. No significant additional effect of the KIR was found when looking at overall survival or acute GVHD.
Causes of mortality
In the C1 neg/KIR2DS2 pos group, 6 of 9 deaths were due to relapse and the remaining 3 due to infection. Patients in the 3 other groups died from a variety of causes including relapse, infection, GVHD, or pulmonary complications with no single cause predominating. The differences in relapse rates between groups did not reach statistical significance (data not shown).
Discussion
In this study we have examined whether the interaction between donor KIR and patient HLA-C genotypes has an impact on overall survival following an HLA-identical sibling stem-cell allograft for myeloid and lymphoid disease. We analyzed sibling pairs as they are matched for HLA-C, whereas transplants between an unrelated donor and recipient, particularly those typed before more accurate genotyping methods became available, are often mismatched at the HLA-C locus. Although others have analyzed the significance of HLA-C allele mismatch in SCT29,30 or HLA-C differences as a cause for NK alloreactivity,16,17 this is the first study to demonstrate recipient HLA-C groups (as defined by their interaction with inhibitory KIRs) as risk factors in the absence of a mismatch with the donor. In patients with myeloid disease, the first novel finding is that the HLA-C group of the recipient influences overall survival. This was not apparent in patients with lymphoid disease. Patients with myeloid disease who were homozygous for C2 alleles had a significantly worse overall survival than those who were either homozygous or heterozygous for a C1 allele, and this finding was highly significant (P < .005). The frequency of C2 alleles in most populations is between 30% and 40%, and the frequency of homozygous individuals should therefore range from 9% to 16%. The percentage in our patient group (15.4%) is in keeping with this estimate.
Transplants where the patient is homozygous for C2 alleles can be further divided into 2 groups on the basis of the KIR2DS2 status of their donor, with the presence of KIR2DS2 in the donor being associated with a further reduction in survival. In contrast, when the KIR2DS2 gene is absent, the overall survival is not significantly different from those recipients who possess C1 alleles. Indeed, the epistatic interaction between recipient C2 and donor KIR2DS2 accounts completely for the observation that C2 homozygous recipients have a poorer outcome.
These results demonstrate a significant clinical role of an HLA-KIR interaction in HLA-identical sibling donor HSCT, although the biologic basis of this is uncertain. The activatory gene KIR2DS2 has a high degree of sequence homology within the extracellular domains with its inhibitory counterpart KIR2DL2/3 (reviewed in Vilches and Parham31 ), whose natural ligands are C1 alleles. Impaired clinical outcome was seen only when the activating genes were present in donors and the patients lacked the ligand (C1) for the inhibitory receptor KIR2DL2/3. There is highly significant linkage disequilibrium in the inheritance of the inhibitory KIR gene KIR2DL2 and KIR2DS2,24-28 and this is confirmed in our study (P < .00001 for relative linkage disequilibrium for myeloid disease group, lymphoid disease group, and controls). KIR2DL2 and KIR2DL3 are currently thought to be alleles. All donors in this study possessed a gene for KIR2DL2 and/or KIR2DL3, and this confirms that the findings we have made relate only to the activating KIR KIR2DS2 and not to the inhibitory homolog KIR2DL2/3. KIR2DL2 and KIR2DS2 are invariably expressed together on the cell surface of NK cells.32 This suggests that it is the NK or T cells expressing an activating KIR in the absence of an inhibitory signal that result in the clinical observations, as the absence of the activating KIR2DS2 does not in itself compromise clinical outcome. If the effector cells are NK cells, this would be somewhat contrary to current thinking, which suggests that all NK cells express at least one inhibitory KIR for self (the “at least one” theory). However, it is possible for a subject to have a combination of KIRs and class I HLA such that there are no possible interactions between KIR and HLA. It is assumed that an inhibitory function in such individuals is performed by lectin-type receptors of the NKG2 family (discussed in Vilches and Parham31 ). Alternatively, T cells may be the effector cells that result in the clinical outcome described. Much less is known about the comparative expression of inhibitory and activating KIRs on T-cell subsets, and there is currently no data on T-cell expression of KIRs in the posttransplant period.
Disease relapse and infection were identified as the causes of death in C2 homozygous patients receiving cells from donors carrying KIR2DS2 genes, but there was no increase in graft versus host disease. These observations suggest that the graft versus leukemia effect and immune reconstitution may be compromised in this group. In a previous analysis of 75 donor-recipient pairs it was shown that donor KIR repertoire could influence outcome (as determined by aGVHD).33 In the sibling transplants, no GVHD was seen when the recipient KIR genotype was included in the donor KIR genotype. However, in the UD transplants, 100% of recipients developed aGVHD if the recipient KIR genotype was included in the donor KIR genotype, implying that the donor had an equal or higher number of activating KIRs than the recipient. As with our study, donor-activating KIRs could therefore be linked to clinical outcome. Interestingly, a different KIR, KIR2DS3, was implicated in that study as a potentially high-risk marker for aGVHD, but no attempt was made to relate KIR genotype to overall survival.
The observation that a combination of KIR genotype and HLA-C has such a profound impact on HLA-matched sibling transplant outcome may appear unexpected. However, 2 recent observations have set a precedent in this regard. First, a strikingly similar observation has been made in patients with the T-cell–mediated disease psoriatic arthropathy (PsA).19 Subjects who possess the KIR2DS1 or KIR2DS2 genes carry a genetic risk for the development of PsA but only when HLA ligands for their homologous inhibitory receptors are lacking. Second, an epistatic interaction between KIR3DS1 and HLA-B has been described in HIV-infected individuals.34 It was shown that the absence of particular HLA-B alleles was significantly associated with more rapid progression to AIDS in patients who possessed the gene for KIR3DS1. KIR3DS1 is presumed to be an activatory KIR with an as-yet-undefined ligand. It is an allele of KIR3DL1, the ligands for which are the Bw4 allotypes of HLA-B. These 2 studies demonstrate that the combination of activatory KIR and HLA genotype within an individual predisposes to the development of disease as well as possesses significance in the context of allogeneic transplantation.
It is noteworthy that we saw no influence of KIR or HLA-C genotype in patients with lymphoid malignancy. A similar selective consequence of KIR/HLA mismatch has been reported in patients with leukemia who have undergone haploidentical stem cell transplantation,16,17 a setting where there is frequently a disparity between the HLA-C group of the donor and that of the recipient. Transplants in which patients did not express the appropriate ligands for donor inhibitory KIRs were associated with improved survival due to a reduced incidence of disease relapse and GVHD. Alloreactive NK cells, which were detectable in recipients after transplant, were able to kill recipient leukemic cells in vitro. These effects were seen only in myeloid leukemias rather than lymphoid disease, a finding paralleled in our observations. However, the observation that KIR-HLA-C mismatch is associated with improved clinical outcome in the setting of haploidentical transplantation contrasts with our findings that a mismatch has an adverse effect on outcome. In a retrospective analysis of 175 UD transplants, Davies et al examined the role of KIR ligand incompatibility (as predicted by HLA-C mismatch) on transplant outcome.35 Overall, no differences in survival or aGVHD were seen between transplants with and without KIR ligand incompatibility, although a trend toward increased frequency of grades II-IV aGVHD was seen in those transplants where such incompatibility existed. However, in patients with myeloid malignancy, KIR ligand incompatibility was associated with poor survival, in direct contrast to the findings of Ruggeri et al. More recently, contrary findings in UD transplants have been published,18 showing that in those transplants that were HLA-mismatched, KIR ligand incompatibility did have a favorable effect on outcome, particularly in transplants for myeloid disease. Clearly, more studies need to be done in this area, and the KIR repertoire of the donor needs to be taken into account.
One important difference between haploidentical and sibling donor transplant procedures is in the dose of donor T cells that is administered at the time of transplantation. Haploidentical grafts are heavily T-cell depleted,36 and typically fewer than 104/Kg cells are given with the stem cell graft. NK cells are generated early in the posttransplant period and can remain the predominant lymphoid cell for a prolonged period. In contrast, sibling allografts are generally T-cell replete and would typically contain up to 109 T cells. As KIR molecules are expressed on NK cells and subpopulations of T cells, it is possible that differences in T-cell and NK-cell immune reconstitution are responsible for the contrasting clinical outcome of KIR-HLA-C mismatch in the 2 transplant regimens. In addition, there are significant differences in methodologies between these studies. We have examined donors and recipients for the absence and presence of specific genes. The presence of a gene does not necessarily correlate with expression, although there is evidence that KIR phenotype equates to KIR genotype.37 Ruggeri et al16,17 used antibodies to type the KIR status of cells, but as antibodies specific for individual KIR proteins are not yet available, it is uncertain which particular KIRs are actually expressed. The findings of Davies et al37 and Giebel et al18 were based solely on the knowledge of KIR ligands and not on the KIR type of the donor. Recently, it has been demonstrated that the pattern of KIR expression on NK cells may be unpredictable for many months following HSCT.15 The pattern of KIR expression in T cells after transplant remains to be determined but may have an impact on transplant outcome. Thus, it is apparent that KIRs play an important functional role in transplantation for myeloid disease and that further studies with improved reagents are required to clarify the biologic basis of this relationship.
Our work is the first to demonstrate that the HLA-C group of the recipient (as determined by position 80 of the α-helix) is a significant factor in determining outcome in sibling donor HSCT, when no HLA mismatch between donor and recipient exists. This phenomenon was not seen in patients with lymphoid disease and so may be restricted to patients with myeloid leukemias. In addition, we have found that the poor risk group of patients homozygous for C2 can be separated into normal risk and very poor risk groups on the basis of the KIR genotype of the donor. These findings have implications for the approximately 7% of patients undergoing sibling allograft for myeloid disease who may be predicted to have a very poor clinical outcome. KIR genotyping of potential donors may allow the option of selecting an alternative donor in these situations.
Prepublished online as Blood First Edition Paper, September 22, 2003; DOI 10.1182/blood-2003-02-0438.
Supported by a charitable grant from the Howard Ostins Trust Fund (M.A.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Cathy Porter, Dot Wilcock, and June de la Rue for data collection, and the laboratory staff of the Department of Histocompatibility & Immunogenetics, NBS, Birmingham, for performing HLA typing and providing additional data.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal