Abstract
Recombinant factor VIIa (rFVIIa) is a safe and effective prohemostatic drug for patients with Glanzmann thrombasthenia (GT). However, the mechanism of action of rFVIIa in these patients is still unclear. Although patients with GT are characterized by a complete absence of platelet aggregation to a variety of agonists, it has been shown that GT platelets are able to form aggregates, provided polymerizing fibrin is present. We studied the effect of rFVIIa-mediated fibrin formation on aggregation of αIIbβ3-deficient platelets. When washed platelets from GT patients or platelets from healthy volunteers treated with an arginyl-glycyl-aspartyl–containing peptide were activated with collagen in the presence of rFVIIa and purified coagulation factors X, II, and fibrinogen, complete aggregation occurred after a lag phase. Fibrin generation proceeded via rFVIIa-mediated thrombin generation on the activated platelet surface independently of tissue factor. Electron microscopic analysis of αIIbβ3-independent platelet aggregates showed a densely packed structure suggestive of a true platelet-fibrin interaction and not via trapping of platelets into a fibrin network. Also, rFVIIa-mediated αIIbβ3-independent aggregation was demonstrated under conditions of flow using a collagen-coated surface. In conclusion, the efficacy of rFVIIa in GT patients might be explained by induction of αIIbβ3-independent platelet aggregation, which compensates the lack of αIIbβ3-dependent aggregation.
Introduction
The platelet integrin αIIbβ3 plays a pivotal role in hemostasis since patients with Glanzmann thrombasthenia (GT), who have a qualitative or quantitative defect in this receptor, suffer from a severe bleeding tendency.1 A deficiency of αIIbβ3 on the platelet membrane results in the absence of platelet aggregation in response to agonists such as collagen, adenosine diphosphate (ADP), and epinephrine. Furthermore, platelet–vessel wall interaction is disturbed as αIIbβ3 mediates platelet binding to the subendothelium via collagen-bound von Willebrand factor (VWF), fibrin, vitronectin, and thrombospondin.2
Prophylaxis or treatment of bleeding episodes in patients with GT consists of infusion of platelet concentrates. However, alloimmunization to human leukocyte antigens or to αIIbβ3 might occur, rendering further administration of donor platelets ineffective.
Recombinant factor VIIa (rFVIIa; NovoSeven; Novo Nordisk, Bagsværd, Denmark) has been shown to be an alternative to platelet transfusion for patients with GT.3,4 Originally developed for treatment of patients with inhibitor-complicated hemophilia,5 rFVIIa is thought to enhance thrombin generation at the site of vessel wall damage. In vitro experiments showed that enhancement of thrombin generation by rFVIIa can proceed via tissue factor (TF)–dependent6-9 or -independent10-12 pathways, and it has been postulated that both mechanisms are operative in vivo.13 The consequences of enhanced thrombin generation for the composition and stability of the hemostatic plug may be multifactorial. Enhancement of platelet activation,7 fibrin generation,14 and thrombin-activatable fibrinolysis inhibitor (TAFI) activation6 have been shown using in vitro models. Furthermore, changes in rate and amount of thrombin generation have been shown to influence the biochemical and physical characteristics of the fibrin network, resulting in enhanced stability and resistance to breakdown by the fibrinolytic system.15,16
Little is known about the mechanism of action of rFVIIa in patients with GT. In a previous publication we showed that TF-independent thrombin generation via rFVIIa substantially enhanced platelet deposition to subendothelial components or collagen.10 It was hypothesized that enhancement of platelet deposition at the site of injury would facilitate further thrombin and fibrin deposition mediated by an increased exposure of a catalytic anionic phospholipid surface, and enhanced fibrin deposition might compensate for the lack of platelet aggregation. Experimental evidence for this hypothesis came from a study performed by Galan et al,17 who showed enhancement of fibrin deposition and a partial restoration of platelet–vessel wall interaction using whole blood from GT patients, which was perfused over denuded rabbit aorta.
However, a complete lack of platelet-platelet interaction in GT patients, as observed in aggregation experiments in platelet-rich plasma (PRP), might not fully reflect the in vivo defect of these patients. Studies with washed platelets indicate that αIIbβ3-deficient platelets are able to aggregate through polymerizing fibrin. The interaction of αIIbβ3-deficient platelets with polymerizing fibrin and the aggregation of platelets from a patient completely lacking αIIb and β3 was already reported in 1981 and 1989, respectively, but these observations were given little attention.18,19 More recently, Soslau and coworkers20 provided more extensive evidence that αIIbβ3-deficient platelets are indeed able to aggregate through polymerizing fibrin via a mechanism requiring platelet activation mediated by thrombin bound to glycoprotein Ib (GPIb). In these experiments, intact platelet functions in terms of signal transduction were mandatory for αIIbβ3-independent platelet aggregation and an unidentified platelet receptor for polymerizing fibrin was postulated. In contrast, Jarvis et al21 showed that fixed platelets are also able to aggregate through polymerizing fibrin, and therefore these authors concluded that αIIbβ3-independent platelet aggregation is merely a consequence of trapping of platelets into a fibrin network. However, immunoprecipitation studies of αIIbβ3-independent platelet aggregates suggested the existence of one or more specific fibrin receptors on the platelet surface.22
Increased thrombin generation by rFVIIa is thus potentially capable of inducing a αIIbβ3-independent platelet aggregation response through the enhancement of platelet activation and polymeric fibrin formation, which may explain the efficacy of rFVIIa in GT patients.
In this study, the effect of rFVIIa on the aggregation of αIIbβ3-deficient platelets was investigated in a system consisting of isolated platelets and purified coagulation factors. We show that αIIbβ3-deficient platelets, activated by collagen or the thrombin receptor activating peptide (TRAP) in combination with pharmacologic concentrations of rFVIIa, support TF-independent thrombin and fibrin generation resulting in complete aggregation.
Materials and methods
Antibodies and inhibitors
A goat polyclonal antibody against TF was a generous gift from Dr U. Hedner (Novo Nordisk A/S, Måløv, Denmark). The monoclonal antibody 6F1, directed against the α2 subunit of the integrin α2β1 was kindly provided by Dr B. Coller (Mount Sinai Hospital, New York, NY), and a phage antibody against glycoprotein VI (10B12) that inhibits its interaction with collagen was from Dr R. Farndale (University of Cambridge, Cambridge, United Kingdom). Fab fragments of a monoclonal antibody, which specifically inhibits thrombin binding to GPIb (LJIb-10) were a generous gift from Dr Z. M. Ruggeri (The Scripps Research Institute, La Jolla, CA), and a rabbit polyclonal inhibitory antibody against the protease activated receptor-1 (PAR-1) was from Dr D. C. Foster (Cytokine Biology, ZymoGenetics, Seattle, WA). A polyclonal antibody that recognizes both fibrinogen and fibrin was from Dako (Glostrup, Denmark).
Recombinant desulphato hirudin was a generous gift from Dr R. Wallis (Ciba Geigy, Hormsham, United Kingdom). Recombinant annexin A5 was a generous gift from Dr C. P. Reutelingsperger (University of Maastricht, Maastricht, The Netherlands). The fibrin polymerization-inhibiting peptide GPRP (Gly-Pro-Arg-Pro) was from Sigma-Aldrich (St Louis, MO). The P2Y12 antagonist AR-C69931MX was a generous gift from AstraZeneca (Loughborough, United Kingdom). The thromboxane receptor antagonist SQ30741 was a generous gift from Bristol-Meyers-Squibb (Maarssen, The Netherlands).
Platelet preparation
Blood from healthy volunteers, who denied ingestion of aspirin or other nonsteroidal anti-inflammatory drugs for the preceding 10 days, was drawn into one-tenth volume of 3.4% sodium citrate. In some experiments, blood from patients with type I GT was used. Washed platelets were prepared as described previously,10 except that after the final washing step platelets were resuspended in Hepes-Tyrode buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 137 mM NaCl, 2.68 mM KCl, 0.42 mM NaH2PO4, 1.7 mM MgCl2, 5 mM d-glucose). The blood was centrifuged at 200g for 10 minutes at room temperature. The PRP was removed and was subsequently acidified by addition of one-tenth volume of ACD (2.5% trisodium citrate, 1.5% citric acid, and 2% d-glucose). Platelets were spun down (500g, 15 minutes) and the platelet pellet was resuspended in Hepes-Tyrode buffer at pH 6.5. Prostacyclin (10 ng/mL) was added to prevent platelet activation during the subsequent washing step. Platelets were spun down (500g, 15 minutes) and resuspended in Hepes-Tyrode buffer at pH 7.4 to a platelet count of 200 × 109/L (200 000/μL).
Fixed platelets were prepared by resuspending washed platelets in 1.8% paraformaldehyde in Hepes-Tyrode buffer as described.23 After 2 hours, platelets were washed 3 times with Hepes-Tyrode buffer and finally resuspended in the same buffer to obtain a platelet count of 200 × 109/L (200 000/μL).
Aggregation experiments
Platelet aggregation was measured using an optical aggregometer (Chrono-Log Corporation, Haverford, PA).24 Aggregations were performed at 37°C with a sample stir speed of 900 rpm. Aggregation experiments were performed in the presence of the RGD-containing peptide dRGDW (D-arginyl-glycyl-L-aspartyl-L-tryptophane; 200 μM; generously provided by Dr J. Bouchaudon, Rhône Poulenc Rorer, Chemistry Department, Centre de Recherche de Vitry, Vitry sur Seine, France). Platelets were activated by collagen (Horm collagen; Nycomed, Munich, Germany) or the thrombin receptor–activating peptide SFLLRN (TRAP), which was synthesized with a semiautomatic peptide synthesizer (Labortec AG SP650; Bubendorf, Switzerland). Aggregation experiments were performed in presence or absence of a fibrin-generating system consisting of rFVIIa (1.2 μg/mL; a generous gift from Dr U. Hedner), factor X (10 μg/mL, purified from fresh-frozen plasma by immunoaffinity chromatography followed by Q-Sepharose chromatography as described previously25 ), prothrombin (20 ng/mL, purified from fresh frozen plasma according to Koedam et al26 ), and fibrinogen (0.5 mg/mL; Kordia, Leiden, The Netherlands) in the presence of calcium chloride (3 mM).
Prothrombin fragment 1 + 2 and fibrinopeptide A generation during αIIbβ3-independent aggregation
Thrombin and fibrin generation during αIIbβ3-independent aggregation was quantified by measurements of the polypeptides released on activation using the prothrombin fragment 1 + 2 enzyme-linked immunosorbent assay (ELISA) kit (Dade Behring, Marburg, Germany) and the Zymutest fibrinopeptide A (FPA) ELISA kit (Hyphen Biomed, Andrésy, France). At different time points during aggregation, samples were taken and further thrombin generation was prevented by the addition of 50 mM ethylenediaminetetraacetic acid (EDTA) and 5 U/mL hirudin (final concentrations). Subsequently, samples were centrifuged to remove single platelets or small aggregates, and F1 + 2 and FPA were measured in the supernatant by ELISA.
Electron microscopy
Samples (500 μL) were transferred from aggregation cuvettes into tubes containing 4.5 mL of fixative solution (2% paraformaldehyde, 0.2% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4). After washing, the samples were infiltrated in 2.3 M sucrose and frozen in liquid nitrogen. Immunogold labeling was performed on ultrathin cryosections as described previously.27 After immunolabeling, the samples were washed in distilled water, stained for 5 minutes with uranyl-oxalate (pH 7.0), and embedded in a mixture of 1.8% methyl cellulose and 0.3% uranyl acetate at 4°C. Samples were examined using a JEOL 1200 CX electron microscope (Tokyo, Japan).
Collagen-coated surfaces
Collagen type III (Sigma, St Louis, MO) was solubilized in 50 mM acetic acid and sprayed on thermanox coverslips using a retouching airbrush (Badger model 100; Badger Brush Co, Franklin Park, IL) at a density of 30 μg/cm2, as described previously.28 After the spraying procedure, coverslips were blocked for 1 hour at room temperature with 4% human albumin in phosphate-buffered saline (PBS).
Perfusion studies
Perfusions were carried out in a single-pass perfusion chamber as described previously.29 Perfusions experiments were performed with citrated (3.4% sodium citrate 1/10, vol/vol) whole blood to which dRGDW was added 5 minutes before perfusion in the absence or presence of rFVIIa (2 μg/mL). Just before perfusion, calcium chloride (5 mM) was added to the perfusate and subsequently the blood was perfused over a collagen-coated coverslip for 5 minutes at a shear rate of 300 s–1. After perfusion, slides were washed with HEPES buffer (10 mM Hepes, 150 mM NaCl, pH 7.35) and fixed in 0.5% glutaraldehyde in PBS (10 mM phosphate buffer, 150 mM NaCl, pH 7.4). Subsequently, slides were dehydrated in methanol and stained with May-Grünwald and Giemsa as described previously.28 Subsequently, the slides were examined using light microscopy.
Statistical analysis
Statistical analysis was performed using the GraphPad InStat (San Diego, CA) software package. Differences in time to aggregation values were assayed by standard one-way analysis of variance analysis (ANOVA) using the Dunnett test. P values less than .05 were considered statistically significant.
Results
Aggregation of αIIbβ3-deficient or αIIbβ3-inhibited platelets by a combination of collagen and coagulation factors VIIa, X, II, and I
Aggregation studies were performed with washed platelets from 3 unrelated patients with type I GT. When collagen (4 μg/mL) was added to washed thrombasthenic platelets, a small but significant signal corresponding to 10% to 20% aggregation was observed (Figure 1A). Addition of a mixture of coagulation factors VIIa (1.2 μg/mL), X (10 μg/mL), II (20 ng/mL), fibrinogen (0.5 mg/mL), and calcium chloride (3 mM) to αIIbβ3-deficient platelets did not lead to any aggregation in the time frame of the experiment (10 minutes). When collagen was added together with the mixture of coagulation factors, the small aggregation as seen with collagen alone was, after a lag phase, followed by complete aggregation.
Aggregation of αIIbβ3-deficient or αIIbβ3-inhibited platelets by rFVIIa-mediated fibrin formation. Washed platelets from a patient with GT (A) or platelets from healthy volunteers pretreated with dRGDW for 5 minutes (200 μM; B-C) were activated with collagen (4 μg/mL; A-B) or TRAP (15 μM; C), a mixture of factors VIIa (1.2 μg/mL), X (10 μg/mL), II (20 ng/mL), and fibrinogen (0.5 mg/mL), or with collagen or TRAP in combination with the coagulation factors, all in the presence of 3 mM CaCl2. Platelet aggregation was monitored using standard suspension aggregometry at 37°C using a stir speed of 900 rpm. In panels B and C, aggregation of washed platelets with collagen or TRAP in the absence of dRGDW is shown for reference. Panel A is a typical representation of experiments with 3 unrelated GT patients; panels B and C represent a typical representation of more than 10 experiments.
Aggregation of αIIbβ3-deficient or αIIbβ3-inhibited platelets by rFVIIa-mediated fibrin formation. Washed platelets from a patient with GT (A) or platelets from healthy volunteers pretreated with dRGDW for 5 minutes (200 μM; B-C) were activated with collagen (4 μg/mL; A-B) or TRAP (15 μM; C), a mixture of factors VIIa (1.2 μg/mL), X (10 μg/mL), II (20 ng/mL), and fibrinogen (0.5 mg/mL), or with collagen or TRAP in combination with the coagulation factors, all in the presence of 3 mM CaCl2. Platelet aggregation was monitored using standard suspension aggregometry at 37°C using a stir speed of 900 rpm. In panels B and C, aggregation of washed platelets with collagen or TRAP in the absence of dRGDW is shown for reference. Panel A is a typical representation of experiments with 3 unrelated GT patients; panels B and C represent a typical representation of more than 10 experiments.
Subsequently, experiments were performed using washed platelets from healthy volunteers that were pretreated with the αIIbβ3-inhibiting peptide dRGDW (200 μM). As shown in Figure 1B, in this system collagen alone also induced a small signal, the mixture of coagulation factors did not give any response, and the combination resulted in full aggregation after a lag phase. Collagen-induced aggregation of platelets with intact αIIbβ3 is shown for reference. Similar results were observed when TRAP was used instead of collagen as the platelet activator in both the dRGDW system (Figure 1C) and in platelets from GT patients (data not shown).
Aggregation of αIIbβ3-inhibited platelets via rFVIIa is partially dependent on platelet activation via collagen, the formation of thromboxane A2, secretion of ADP, thrombin binding to glycoprotein Ib, and activation of PAR-1
The second wave of aggregation induced by collagen and coagulation factors VIIa, X, II, and I could be completely inhibited by an antibody against the collagen receptors α2β1 (6F1, 20 μg/mL) and was delayed by an antibody against GPVI (10B12, 100 μg/mL), indicating that preactivation of the platelet is required before fibrin formation can proceed (Figure 2). Also, the thromboxane analog SQ30741 (10 μM) and the P2Y12 antagonist AR-C69931MX (1 μM) significantly delayed this second aggregation wave. Furthermore, Fab fragments of a monoclonal antibody against the thrombin binding site on GPIb and a monoclonal antibody against the PAR-1 receptor significantly delayed the aggregation response on addition of collagen and coagulation factors (Figure 2).
Effect of inhibitors of fibrin generation and platelet function on αIIbβ3-independent platelet aggregation induced by collagen and purified coagulation factors VIIa/X/II/I. Aggregation of αIIbβ3-inhibited platelets was induced as described in Figure 1. When collagen alone was added to αIIbβ3-inhibited platelets full aggregation did not occur, when collagen was added together with coagulation factors VIIa/X/II/I full aggregation occurred after approximately 7 minutes (left). In the right part of the graph, aggregation induced by the combination of collagen and coagulation factors in the presence of the compound mentioned at the X-axes is indicated. In the middle part of the graph, the effect of antibodies or antagonists affecting platelet function on aggregation of αIIbβ3-inhibited platelets is depicted; on the right, the effect of compounds affecting coagulation on αIIbβ3-independent aggregation is shown. Antibodies were preincubated with the platelet suspension for 45 minutes at room temperature; the other compounds were added 5 minutes before aggregation was induced. The time to the start of the second wave of aggregation (Figure 1) was determined from the aggregation curves. Data shown are mean ± standard deviation of at least 3 independent experiments. *P < .01, #P < .05 versus collagen plus coagulation factors.
Effect of inhibitors of fibrin generation and platelet function on αIIbβ3-independent platelet aggregation induced by collagen and purified coagulation factors VIIa/X/II/I. Aggregation of αIIbβ3-inhibited platelets was induced as described in Figure 1. When collagen alone was added to αIIbβ3-inhibited platelets full aggregation did not occur, when collagen was added together with coagulation factors VIIa/X/II/I full aggregation occurred after approximately 7 minutes (left). In the right part of the graph, aggregation induced by the combination of collagen and coagulation factors in the presence of the compound mentioned at the X-axes is indicated. In the middle part of the graph, the effect of antibodies or antagonists affecting platelet function on aggregation of αIIbβ3-inhibited platelets is depicted; on the right, the effect of compounds affecting coagulation on αIIbβ3-independent aggregation is shown. Antibodies were preincubated with the platelet suspension for 45 minutes at room temperature; the other compounds were added 5 minutes before aggregation was induced. The time to the start of the second wave of aggregation (Figure 1) was determined from the aggregation curves. Data shown are mean ± standard deviation of at least 3 independent experiments. *P < .01, #P < .05 versus collagen plus coagulation factors.
Aggregation of αIIbβ3-inhibited platelets proceeds via polymerizing fibrin generated independently of tissue factor
Complete aggregation of αIIbβ3-deficient platelets only occurred when a combination of coagulation factors VIIa, X, II, and fibrinogen was added together with collagen or TRAP. When factor VIIa, factor X, or fibrinogen was omitted from the reaction mixture, the aggregation tracing was found identical to that observed when collagen or TRAP was added alone (ie, the second aggregation wave was fully absent, not shown). The lag phase of aggregation dose-dependently decreased with increasing concentrations of rFVIIa and reached a plateau at 1.2 μg/mL. The second wave leading to full aggregation was also fully absent when hirudin (5 U/mL), annexin A5 (50 μg/mL), or the inhibitor of fibrin polymerization GPRP (1.2 mM) were added to the platelet suspension, indicating that the generation of polymerizing fibrin via rFVIIa on a negatively charged surface (ie, the activated platelet) was responsible for aggregation (Figure 2). Preincubation of the platelet suspension with an inhibitory antibody against TF (500 μg/mL) had no effect on the second wave of aggregation. The potency of this antibody was demonstrated using a standard prothrombin time test. The antibody prolonged the prothrombin time from 12 to over 200 seconds.
The generation of thrombin on the activated platelet surface was demonstrated by measurements of prothrombin fragment 1 + 2 (F1 + 2) at different time points during aggregation. As demonstrated in Figure 3A, F1 + 2 generation proceeded progressively in time when both collagen and factors VIIa, X, II, and I were added to the platelet suspension. Addition of collagen alone did not result in the generation of F1 + 2, and F1 + 2 formation did not occur in the absence of platelets. When only the mixture of coagulation factors was added to the platelet suspension, some F1 + 2 generation did occur during the experiments, which presumably reflects the presence of some activated platelets in the washed platelet suspension. The activation state of platelets in whole, citrated blood was compared with that in our washed platelet suspension by flow cytometry analysis of P-selectin expression using a R-phycoerythrin–conjugated monoclonal antibody according to the instructions of the manufacturer (Code no. R7200; Dako). The amount of platelets expressing P-selectin increased from 3.2% ± 0.9% in whole blood to 21.8% ± 5.4% in the washed platelets suspension (mean ± SD, n = 4; P = .02), indeed indicating that the washing procedure slightly activates the platelets. Alternatively, or in addition, the F1 + 2 generation by coagulation factors alone can be explained by presence of minute amounts of activated factor X in the factor X or prothrombin preparation.
Prothrombin and fibrinogen conversion during aggregation of αIIbβ3-inhibited platelets. Washed platelets from healthy volunteers pretreated with dRGDW for 5 minutes (200 μM) were activated with collagen (4 μg/mL), a mixture of factors VIIa (1.2 μg/mL), X (10 μg/mL), II (20 ng/mL), and fibrinogen (0.5 mg/mL), or with collagen in combination with the coagulation factors, all in the presence of 3 mM CaCl2. Incubations took place in aggregometry cuvettes at 37°C using a stir speed of 900 rpm. At different time points after initiation of αIIbβ3-independent aggregation, samples were taken and thrombin generation was quenched by addition of EDTA (50 mM) and hirudin (5 U/mL). After centrifugation, the prothrombin fragment 1 + 2 (A) or the fibrinogen activation peptide FPA (B) were measured in the supernatant by ELISA. F1 + 2 and FPA generation by collagen and coagulation factors VIIa/X/II/I was also measured in the absence of platelets. A representative of 3 independent experiments is shown.
Prothrombin and fibrinogen conversion during aggregation of αIIbβ3-inhibited platelets. Washed platelets from healthy volunteers pretreated with dRGDW for 5 minutes (200 μM) were activated with collagen (4 μg/mL), a mixture of factors VIIa (1.2 μg/mL), X (10 μg/mL), II (20 ng/mL), and fibrinogen (0.5 mg/mL), or with collagen in combination with the coagulation factors, all in the presence of 3 mM CaCl2. Incubations took place in aggregometry cuvettes at 37°C using a stir speed of 900 rpm. At different time points after initiation of αIIbβ3-independent aggregation, samples were taken and thrombin generation was quenched by addition of EDTA (50 mM) and hirudin (5 U/mL). After centrifugation, the prothrombin fragment 1 + 2 (A) or the fibrinogen activation peptide FPA (B) were measured in the supernatant by ELISA. F1 + 2 and FPA generation by collagen and coagulation factors VIIa/X/II/I was also measured in the absence of platelets. A representative of 3 independent experiments is shown.
The conversion of fibrinogen to fibrin was monitored by measurements of the fibrin activation peptide FPA at different time points during aggregation. As shown in Figure 3B, FPA was generated after a lag phase when both collagen and factors VIIa, X, II, and I were added to the platelet suspension. As anticipated from the F1 + 2 experiments, FPA was not generated on addition of collagen alone or in the absence of platelets. Some FPA was generated when only the mixture of coagulation factors was added to the platelets, which was expected, as some thrombin is generated under this condition as described in the previous paragraph.
Aggregates of αIIbβ3-inhibited platelets at the ultrastructural level: fibrin(ogen) is present at some but not all platelet-platelet contact sites
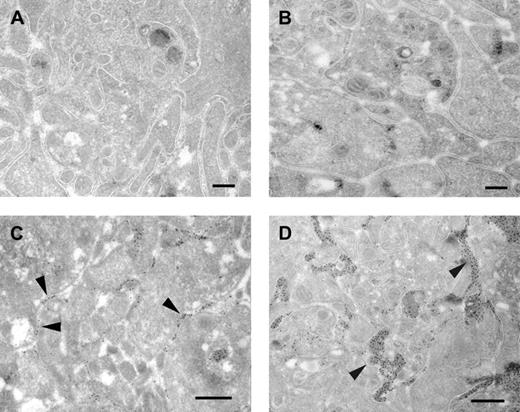
Electron microscopic analysis revealed little differences between an αIIbβ3-dependent and -independent aggregate, in terms of packing of the aggregate. In both cases, a densely packed platelet aggregate, with activated platelets that have released the contents of their alpha and dense granules (Figure 4A-B), was observed. However, important differences were observed when sections of αIIbβ3-dependent and -independent aggregates were labeled with an antibody that recognizes both fibrinogen and fibrin. In the αIIbβ3-dependent aggregate (Figure 4C), fibrinogen was present, as expected, at many platelet-platelet contact sites (arrowheads). In the αIIbβ3-independent aggregate (Figure 4D), fibrin(ogen) was found only at part of the platelet-platelet contact sites (arrowheads). The platelet-platelet contact sites that contained fibrin(ogen) were much wider in the αIIbβ3-independent aggregate compared with the αIIbβ3-dependent aggregate.
Ultrastructural analysis of αIIbβ3-dependent and -independent platelet aggregates. Washed platelets (A,C) or washed platelets pretreated with dRGDW (200 μM; B,D) were stimulated with collagen (A,C) or with collagen and coagulation factors VIIa, X, II, and I in the presence of calcium chloride (B,D) under stirring conditions (900 rpm) at 37°C. After 10 minutes of aggregation, samples were fixed and prepared for electron microscopy as described in “Materials and methods.” Samples were examined directly (A-B) or after immunogold labeling using an antibody against fibrin(ogen) followed by protein A–gold (C-D). Bars indicate 200 nm (A-B) and 500 nm (C-D).
Ultrastructural analysis of αIIbβ3-dependent and -independent platelet aggregates. Washed platelets (A,C) or washed platelets pretreated with dRGDW (200 μM; B,D) were stimulated with collagen (A,C) or with collagen and coagulation factors VIIa, X, II, and I in the presence of calcium chloride (B,D) under stirring conditions (900 rpm) at 37°C. After 10 minutes of aggregation, samples were fixed and prepared for electron microscopy as described in “Materials and methods.” Samples were examined directly (A-B) or after immunogold labeling using an antibody against fibrin(ogen) followed by protein A–gold (C-D). Bars indicate 200 nm (A-B) and 500 nm (C-D).
The αIIbβ3-inhibited washed platelets show microaggregate formation on collagen activation
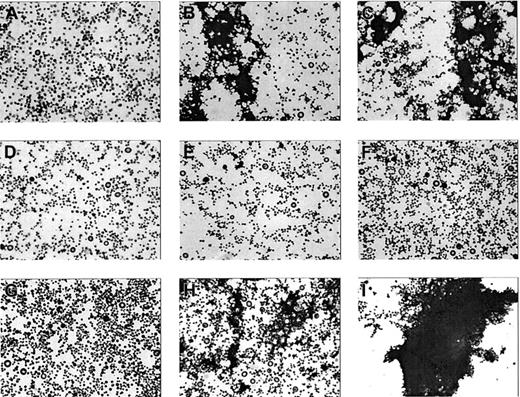
To investigate the nature of the aggregation signal obtained when αIIbβ3-deficient platelets were stimulated with collagen alone (Figure 1A), aliquots of platelet suspensions were taken at different time points during collagen-induced aggregation of αIIbβ3-inhibited platelets and immediately fixed in 3% glutaraldehyde (1:1 vol/vol). The platelets were spun down on a glass coverslip via standard cytospin techniques and stained with May-Grünwald/ Giemsa. Before addition of collagen (Figure 5A) only single platelets were observed, whereas after addition of collagen both single platelets and small aggregates were observed (Figure 5B-C). No differences were observed between time points 2 and 10 minutes, consistent with the aggregation tracing, which remained constant after 2 minutes. When purified coagulation factors VIIa/X/II/I were added to a platelet suspension in the presence of calcium, no microaggregate formation was observed (Figures 5 E-F), consistent with the absence of a response in the aggregometry studies (Figure 1). When collagen and the coagulation factors were added simultaneously, microaggregates were observed after 2 minutes (Figure 5H), whereas a full aggregate was observed after 10 minutes (Figure 5I), which is in accordance with the aggregation tracing shown in Figure 1.
Microaggregate formation on stimulation of washed αIIbβ3-inhibited platelets by collagen. Platelets pretreated with dRGDW (200 μM) were stimulated with collagen (4 μg/mL; A-C) with purified coagulation factors VIIa, X, prothrombin, and fibrinogen (D-F) or with the combination (G-I) in an aggregometry setup as described in Figure 1. At time points 0 (A,D,G), 2 (B,E,H), and 10 minutes (C,F,I) samples were taken, fixed with glutaraldehyde, spun down on a glass coverslip, and stained with May-Grünwald/Giemsa. The coverslips were examined by light microscopy (original magnification, × 400).
Microaggregate formation on stimulation of washed αIIbβ3-inhibited platelets by collagen. Platelets pretreated with dRGDW (200 μM) were stimulated with collagen (4 μg/mL; A-C) with purified coagulation factors VIIa, X, prothrombin, and fibrinogen (D-F) or with the combination (G-I) in an aggregometry setup as described in Figure 1. At time points 0 (A,D,G), 2 (B,E,H), and 10 minutes (C,F,I) samples were taken, fixed with glutaraldehyde, spun down on a glass coverslip, and stained with May-Grünwald/Giemsa. The coverslips were examined by light microscopy (original magnification, × 400).
Fibrin-induced aggregation of fixed platelets is substantially less efficient compared with fibrin-induced aggregation of viable αIIbβ3-inhibited platelets
To further investigate whether αIIbβ3-independent aggregation is indeed a receptor-mediated process or that αIIbβ3-independent aggregation is merely the result of a nonspecific trapping of platelets in a fibrin clot, as suggested previously,21 aggregation experiments were performed in which dRGDW-treated viable platelets were compared with dRGDW-treated fixed platelets. Aggregation was induced by addition of fibrinogen (0.5 mg/mL) and different concentrations of thrombin (Kordia). As shown in Figure 6, the aggregation of viable platelets requires substantially less thrombin compared with the aggregation of fixed platelets, indeed suggesting that αIIbβ3-independent aggregation is, at least in part, a receptor-mediated process.
Fibrin-mediated αIIbβ3-independent aggregation in viable versus fixed platelets. A suspension of washed (A) or fixed platelets (B) was incubated with dRGDW (200 μM) for 5 minutes, and subsequently fibrinogen (0.5 mg/mL) and different concentrations of thrombin (0-0.5 U/mL) were added. Platelet aggregation was monitored using standard suspension aggregometry at 37°C using a stir speed of 900 rpm. A representative aggregation tracing of 3 independent experiments is shown.
Fibrin-mediated αIIbβ3-independent aggregation in viable versus fixed platelets. A suspension of washed (A) or fixed platelets (B) was incubated with dRGDW (200 μM) for 5 minutes, and subsequently fibrinogen (0.5 mg/mL) and different concentrations of thrombin (0-0.5 U/mL) were added. Platelet aggregation was monitored using standard suspension aggregometry at 37°C using a stir speed of 900 rpm. A representative aggregation tracing of 3 independent experiments is shown.
Fibrin-mediated aggregation of αIIbβ3-inhibited platelets induced by rFVIIa under flow conditions in a whole blood system
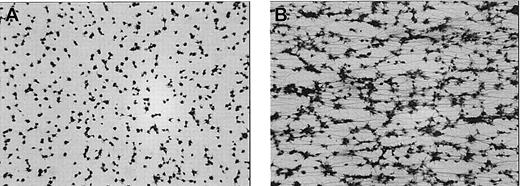
Subsequently, it was investigated whether αIIbβ3-independent aggregation also occurs under flow conditions. Therefore, citrated whole blood was incubated for 5 minutes with dRGDW at 37°C in presence or absence of rFVIIa (2 μg/mL). Subsequently, calcium chloride (5 mM, final concentration) was added, and the blood was perfused immediately over a collagen-coated surface. As shown in Figure 7A, perfusion of recalcified, αIIbβ3-inhibited whole blood resulted in adhesion of platelets to the collagen surface. No platelet-platelet contacts or fibrin were observed. In contrast, when rFVIIa was added, massive fibrin deposition occurred, and platelet aggregates were observed (Figure 7B).
Aggregation of αIIbβ3-inhibited platelets under flow conditions induced by rFVIIa. Citrated whole blood was incubated with dRGDW (200 μM) in absence (A) or presence (B) of rFVIIa (2 μg/mL) for 5 minutes at 37°C. After addition of calcium chloride (5 mM), the blood was immediately perfused over a coverslip coated with collagen type III at a shear rate of 300 s–1 for 5 minutes at 37°C using a single-pass perfusion chamber. After perfusion, coverslips were fixed and stained with May-Grünwald/Giemsa and examined by light microscopy (original magnification × 400). Representative images of 3 independent experiments are shown.
Aggregation of αIIbβ3-inhibited platelets under flow conditions induced by rFVIIa. Citrated whole blood was incubated with dRGDW (200 μM) in absence (A) or presence (B) of rFVIIa (2 μg/mL) for 5 minutes at 37°C. After addition of calcium chloride (5 mM), the blood was immediately perfused over a coverslip coated with collagen type III at a shear rate of 300 s–1 for 5 minutes at 37°C using a single-pass perfusion chamber. After perfusion, coverslips were fixed and stained with May-Grünwald/Giemsa and examined by light microscopy (original magnification × 400). Representative images of 3 independent experiments are shown.
Discussion
In this study we have shown that fibrin generated via recombinant factor VIIa restores collagen or TRAP-induced aggregation of αIIbβ3-deficient platelets. The αIIbβ3-independent aggregation of platelets, induced by rFVIIa-mediated fibrin formation compensates for the defective αIIbβ3-dependent aggregation and might thereby, in part, explain the efficacy of rFVIIa in patients with GT.
In accordance with previous results,18-20 we propose a platelet aggregation pathway independent of αIIbβ3, which is mediated by polymerizing fibrin and a yet unidentified platelet receptor. Although this alternative platelet aggregation pathway has been previously recognized, it has never been extensively investigated, most likely because most studies on platelet aggregation are performed in citrated PRP or in a washed platelet suspension, as a result of which fibrin formation cannot occur.
Using the experimental evidence presented in this paper we postulate the following sequence of events leading to aggregation. (1) Platelets are activated by collagen or TRAP resulting in the exposure of procoagulant phospholipid surface. Activation of αIIbβ3-deficient platelets results in the formation of microaggregates via a yet unknown mechanism, observed as a small but significant signal in the aggregometer. (2) High-dose rFVIIa subsequently binds to the activated platelet, which has been shown to occur previously.10,11 (3) Platelet-bound rFVIIa activates FX, and activated FX subsequently combines with platelet-derived FV and converts prothrombin into thrombin. (4) Thrombin converts fibrinogen into fibrin, which binds to a yet unidentified receptor on the platelet surface, leading to complete aggregation.
The herein described αIIbβ3-independent platelet aggregation is not the result of random trapping of platelets in a fibrin network as previously suggested.21 Instead, several indications point to a receptor-mediated platelet-fibrin interaction. Firstly, fibrin-induced aggregates are densely packed, suggesting a close interaction. Furthermore, studies in which thrombin plus fibrinogen-induced aggregation of αIIbβ3-inhibited viable platelets are compared with fixed platelets showed that the process is substantially less efficient when fixed platelets are used. In other words, the trapping of platelets requires much more fibrin compared with the receptor-mediated process. Finally, platelet activation (via collagen or TRAP) and subsequent intracellular signal transduction pathways via thrombin binding to GPIb, activation of PAR-1, formation of thromboxane A2, and secretion of ADP are in part required for αIIbβ3-independent aggregation, suggesting that the postulated receptor requires either activation or translocation to the cell surface before it is able to bind fibrin. The identity of the postulated fibrin receptor remains unknown but this will be investigated in further studies.
Although αIIbβ3-independent aggregation by rFVIIa-mediated fibrin formation seems a rather inefficient process, as indicated by the slower aggregation response compared with normal platelets (Figure 1), the occurrence of the lag phase in the αIIbβ3-deficient situation is caused by the experimental setup chosen. Analogous to a previous study,10 we used a low concentration of prothrombin to ensure that thrombin generation is limited, as no inhibitors of thrombin generation are present in our system. As the generation of massive amounts of fibrin would lead to receptor-independent aggregation as discussed in the previous paragraph, we chose not to change the prothrombin concentration. However, when the prothrombin concentration was increased, the lag phase dose-dependently decreased, indicating that indeed this lag phase is the consequence of our experimental setup.
In our experimental system containing a suspension of washed αIIbβ3-deficient platelets, a small amount of aggregation (10%-20%) already takes place on addition of collagen or TRAP in the absence of fibrin generation. Light microscopic analysis of the αIIbβ3-deficient platelet suspension after stimulation with collagen indeed demonstrated the formation of microaggregates, which were also observed using electron microscopic analysis (data not shown). The mechanism by which this partial aggregation occurs is unknown but is presumably not of physiologic relevance, as it only occurs in a washed platelet suspension and not in PRP (data not shown).
In a previous study we have shown that rFVIIa enhances platelet deposition on collagen and on endothelial cell–derived matrix.10 Together with the results described in this paper, we propose the following model, by which rFVIIa induces hemostasis in GT patients. Administration of rFVIIa to a patient with GT results in enhancement of thrombin generation at the site of vascular damage. This augmented thrombin generation results in an increased number of (activated) platelets deposited at the site of injury and, consequently, an increase in available procoagulant surface. A more extensive procoagulant surface facilitates and enhances further thrombin formation, leading to feedback enhancement of platelet activation and deposition and enhancement of fibrin formation. The enhanced formation of fibrin results in enhancement of αIIbβ3-independent aggregation, which may, in part compensate for the defective αIIbβ3-dependent aggregation. It is, however, conceivable that other mechanisms contribute to rFVIIa-mediated induction of hemostasis in GT patients. Possible other mechanisms by which rFVIIa might induce a more stable hemostatic plug include enhancement of fibrin deposition,17 alterations in fibrin structure,30 enhancement of the activation of TAFI,6 and enhancement of FXIII activation. Analysis of hemostatic plugs obtained in vivo (eg, in animal models) might be helpful in identifying the prominent mechanism of action of rFVIIa in GT patients.
Prepublished online as Blood First Edition Paper, October 30, 2003; DOI 10.1182/blood-2003-07-2287.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr U. Hedner for the generous gift of rFVIIa and the anti-TF antibody, Dr J. Bouchaudon for dRGWD, Dr R. Wallis for desulphato hirudin, and Dr C. Reutelingsperger for annexin A5. Furthermore, we would like to thank Drs B. Coller, R. Farndale, Z. Ruggeri, and D. Foster for their gifts of antibodies against α2β1, GPVI, the thrombin binding site on GPIb, and PAR-1, respectively. Also, AstraZeneca and Bristol-Meyers-Squibb Co are acknowledged for the P2Y12 and thromboxane receptor antagonists, respectively. Finally, we are grateful to Drs M. Peters (Academic Medical Centre, Amsterdam, The Netherlands), H .K. Nieuwenhuis (University Medical Centre Utrecht, The Netherlands), and I. Novakova (University Medical Center Nijmegen, The Netherlands) for their kind assistance with the patient studies.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal