Abstract
Although immunosuppression has long been recognized in Hodgkin lymphoma (HL), the underlying basis for the lack of an effective immune response against the tumor remains unclear. The aim was to test our hypothesis that regulatory T cells dominate involved lymph nodes. The approach was to assay CD4+ T-cell function in HL-infiltrating lymphocytes (HLILs) and paired peripheral blood mononuclear cells (PBMCs) of 24 patients. Strikingly, unlike PBMCs, HLILs were anergic to stimulation with mitogen, primary, or recall antigens, mounting no proliferative responses and only rare T-helper 1 (Th1) or Th2 cytokine responses. Mixing paired HLILs and PBMCs showed the anergic effect was dominant and suppressed PBMC responses. Furthermore, flow cytometry demonstrated that HLILs contained large populations of both interleukin-10 (IL-10)–secreting T-regulatory 1 (Tr1) and CD4+CD25+ regulatory T cells. We found evidence for 3 mechanisms of action implicated in the suppressive functions of regulatory T cells: the inhibition of PBMCs by HLILs was ameliorated by neutralizing IL-10, by preventing cell-to-cell contact, and by blocking anti–cytotoxic T lymphocyte–associated antigen 4 (anti–CTLA-4). Thus, HLILs are highly enriched for regulatory T cells, which induce a profoundly immunosuppressive environment and so provide an explanation for the ineffective immune clearance of Hodgkin-Reed Sternberg cells.
Introduction
A distinguishing feature of Hodgkin lymphoma (HL) is the paucity of malignant Hodgkin-Reed Sternberg (HRS) cells. They usually compose only a minor proportion of the tumor, with the remainder mainly inflammatory lymphocytes. Although the composition of this infiltrate is heterogeneous, most are CD4+ lymphocytes. Despite numerous data concerning their immunophenotypes and secreted cytokines and chemokines,1 the pathogenic roles of these Hodgkin lymphoma–infiltrating lymphocytes (HLILs) remain poorly understood. Here, we investigate the possibility that an important role is to suppress effector immune responses, which might contribute to tumor persistence.
Immunosuppression has long been known to be associated with HL: Dorothy Reed demonstrated attenuated type I hypersensitivity reactions to intradermal purified protein derivative (PPD) as early as 1902.2 Since then, a variety of indicators of poor immune function have been documented, including poor graft rejection,3,4 a predisposition to graft-versus-host disease after blood transfusion,5 and poor mitogenic responses of peripheral blood mononuclear cells (PBMCs).6 During the 1970s, at least part of this immunosuppression was ascribed to suppressor cells,7 but at that time such cells were poorly characterized.
In recent years, major advances have been made in understanding the phenotypes and functions of T-cell subsets. It is now realized that the initial dichotomy8 of CD4+ cells into T-helper 1 (Th1) and Th2 is overly simplistic. In particular, recently described subsets termed regulatory cells are currently the subjects of intense interest.9,10 These cells inhibit effector immune responses and are important in the control of responses to foreign antigens, including responses in transplantation,11 as well as in protection against autoimmune disease. Currently, at least 2 forms of regulatory cells have been described, although the relationship between these is uncertain. One form inhibits through the secretion of immunosuppressive cytokines: this form includes interleukin 10 (IL-10)–producing T-regulatory 1 (Tr1) cells,12 which are responsible for a number of inhibitory phenomena, and transforming growth factor–β (TGF-β)–producing Th3 cells, which mediate some forms of oral tolerance.13 The other form comprises CD4+ T cells that mediate suppression in many systems, not via secretion of cytokines, but through mechanisms dependent on cell-cell contact.14 A key feature of these CD4+ T cells is constitutive expression of CD25, the α-chain of the IL-2 receptor, although the receptor is nonfunctional. In addition to having roles in autoimmune diseases and transplantation immunology, regulatory T cells may also inhibit effective antitumor immune responses.15
Supporting evidence for the hypothesis that regulatory cells are important in the pathogenesis of HL comes from a reinterpretation of previously published data concerning HLILs. It is widely believed that the inflammatory infiltrate of HL comprises mainly activated Th2-like lymphocytes.1,16 However, while there is ample evidence that HLILs have few Th1 characteristics, Th2 features are equally sparse.1,16,17 Thus, very few HLILs produce the key Th2 cytokines IL-4 or IL-13.1,17-19 Although IL-10, which was originally classified as a Th2 cytokine, is present,1,18,19 in the absence of IL-4 it is now recognized as a marker of Tr1 cells. Furthermore, the lack of protective response to antigen expressed by HRS cells is consistent with the activity of regulatory T cells in affected nodes. For example, monoclonal infection with Epstein-Barr virus (EBV) is found in the HRS cells of 20% to 40% of Western cases of HL,20-22 but the expression of viral antigens does not stimulate an effective antitumor immune response,23,24 which may be related to induction of regulatory T cells.25
To test the hypothesis that regulatory T-cell activity is important in HL, we characterized CD4+ T-cell responses and phenotypes in HLILs. Such activity could mediate the immunosuppression associated with HL and contribute to immune evasion by HRS cells.
Patients, materials, and methods
Patients
Consecutive patients with suspected HL were recruited from Aberdeen Royal Infirmary (Aberdeen, United Kingdom), which is the sole specialty hospital serving the relatively stable population of Grampian (catchment population, 570 000). The study received local ethical approval, and informed consent was obtained from all patients. Twenty-four patients had confirmed HL and yielded sufficient cells for this study. A control group comprised 3 patients with no malignant process identified, together with 2 additional patients who had predominantly normal nodal architecture, but also minor infiltration with breast cancer or anaplastic lymphoma. In addition, a further control group consisted of 20 healthy EBV-seropositive donors, whose PBMCs were sampled for responses to antigen and mitogen. Patient characteristics are described in Table 1. All samples were obtained before any treatment for HL was given. The EBV status of HL nodes was assessed by immunostaining fixed sections of diagnostic node with a mixture of antibodies against EBV-expressed latent membrane protein 1 (CS1–4; Novocastra Laboratories, Newcastle, United Kingdom).
Clinical details of patients with HL and controls
Subject* . | Age, y . | Sex . | Histology . | Site . | Stage . | EBV status . | Hb concentration, g/L . | WBC count, × 109/L . | Lymphocyte count, × 109/L . | Albumin concentration, g/L . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 22 | F | NS | C | 2a | + | 119 | 4.3 | 1.1 | 45 |
| 2 | 28 | F | NS | C | 2a | + | 129 | 12.4 | 1.6 | 41 |
| 3 | 29 | M | NS | C | 2b | - | 135 | 14.1 | 1.4 | 49 |
| 4 | 26 | M | NS | C | 2b | - | 105 | 18.3 | 1.5 | 43 |
| 5 | 32 | F | NS | C | 2b | + | 94 | 43.0 | 2.3 | 41 |
| 6 | 62 | M | MC | C | 4b | + | 140 | 4.9 | 0.8 | 42 |
| 7 | 44 | M | U† | A | 2b | - | 128 | 10.9 | 1.6 | 41 |
| 8 | 37 | F | NS | C | 2b | - | 107 | 11.2 | 1.5 | 44 |
| 9 | 21 | M | NS | C | 2a | - | 137 | 12.0 | 1.8 | 43 |
| 10 | 24 | M | MC | C | 1a | + | 134 | 7.6 | 2.4 | 49 |
| 11 | 22 | F | NS | C | 2b | - | 128 | 13.0 | 2.9 | 47 |
| 12 | 48 | M | LP | A | 1a | - | 148 | 4.6 | 1.3 | 41 |
| 13 | 25 | M | NS | C | 2b | + | 118 | 6.9 | 1.4 | 36 |
| 14 | 33 | M | NS | C | 2a | + | 134 | 6.7 | 0.6 | 40 |
| 15 | 30 | F | NS† | I | 1a | - | 153 | 9.1 | 2.1 | 50 |
| 16 | 24 | F | NS | C | 2a | - | 131 | 9.0 | 1.3 | 42 |
| 17 | 19 | F | NS | C | 2a | - | 131 | 11.9 | 2.1 | 42 |
| 18 | 41 | F | MC | C | 1a | + | 136 | 6.5 | 1.5 | 47 |
| 19 | 21 | M | NS | A | 4b† | - | 134 | 7.7 | 1.3 | 46 |
| 20 | 54 | M | NS | C | 1a† | + | 117 | 5.6 | 1.2 | 39 |
| 21 | 20 | F | NS | C | 2a | - | 131 | 7.1 | 1.1 | 42 |
| 22 | 38 | M | MC | C | 2b | + | 150 | 8.5 | 2.0 | 47 |
| 23 | 20 | F | NS | C | 2a | - | 115 | 13.7 | 2.2 | 41 |
| 24 | 27 | F | NS | C | 2a | - | 111 | 12.6 | 1.1 | 43 |
| C1 | 17 | F | Reactive | C | NA | nt | 130 | 9.0 | 3.3 | 41 |
| C2 | 33 | F | Reactive | C | NA | nt | 154 | 6.1 | 1.4 | 49 |
| C3 | 20 | M | PT | A | NA | nt | 150 | 7.2 | 2.3 | 51 |
| C4 | 53 | F | Breast cancer | C | NA | nt | 143 | 9.7 | 1.6 | 46 |
| C5 | 17 | F | A-NHL | C | NA | nt | 108 | 6.3 | 0.6 | 41 |
Subject* . | Age, y . | Sex . | Histology . | Site . | Stage . | EBV status . | Hb concentration, g/L . | WBC count, × 109/L . | Lymphocyte count, × 109/L . | Albumin concentration, g/L . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 22 | F | NS | C | 2a | + | 119 | 4.3 | 1.1 | 45 |
| 2 | 28 | F | NS | C | 2a | + | 129 | 12.4 | 1.6 | 41 |
| 3 | 29 | M | NS | C | 2b | - | 135 | 14.1 | 1.4 | 49 |
| 4 | 26 | M | NS | C | 2b | - | 105 | 18.3 | 1.5 | 43 |
| 5 | 32 | F | NS | C | 2b | + | 94 | 43.0 | 2.3 | 41 |
| 6 | 62 | M | MC | C | 4b | + | 140 | 4.9 | 0.8 | 42 |
| 7 | 44 | M | U† | A | 2b | - | 128 | 10.9 | 1.6 | 41 |
| 8 | 37 | F | NS | C | 2b | - | 107 | 11.2 | 1.5 | 44 |
| 9 | 21 | M | NS | C | 2a | - | 137 | 12.0 | 1.8 | 43 |
| 10 | 24 | M | MC | C | 1a | + | 134 | 7.6 | 2.4 | 49 |
| 11 | 22 | F | NS | C | 2b | - | 128 | 13.0 | 2.9 | 47 |
| 12 | 48 | M | LP | A | 1a | - | 148 | 4.6 | 1.3 | 41 |
| 13 | 25 | M | NS | C | 2b | + | 118 | 6.9 | 1.4 | 36 |
| 14 | 33 | M | NS | C | 2a | + | 134 | 6.7 | 0.6 | 40 |
| 15 | 30 | F | NS† | I | 1a | - | 153 | 9.1 | 2.1 | 50 |
| 16 | 24 | F | NS | C | 2a | - | 131 | 9.0 | 1.3 | 42 |
| 17 | 19 | F | NS | C | 2a | - | 131 | 11.9 | 2.1 | 42 |
| 18 | 41 | F | MC | C | 1a | + | 136 | 6.5 | 1.5 | 47 |
| 19 | 21 | M | NS | A | 4b† | - | 134 | 7.7 | 1.3 | 46 |
| 20 | 54 | M | NS | C | 1a† | + | 117 | 5.6 | 1.2 | 39 |
| 21 | 20 | F | NS | C | 2a | - | 131 | 7.1 | 1.1 | 42 |
| 22 | 38 | M | MC | C | 2b | + | 150 | 8.5 | 2.0 | 47 |
| 23 | 20 | F | NS | C | 2a | - | 115 | 13.7 | 2.2 | 41 |
| 24 | 27 | F | NS | C | 2a | - | 111 | 12.6 | 1.1 | 43 |
| C1 | 17 | F | Reactive | C | NA | nt | 130 | 9.0 | 3.3 | 41 |
| C2 | 33 | F | Reactive | C | NA | nt | 154 | 6.1 | 1.4 | 49 |
| C3 | 20 | M | PT | A | NA | nt | 150 | 7.2 | 2.3 | 51 |
| C4 | 53 | F | Breast cancer | C | NA | nt | 143 | 9.7 | 1.6 | 46 |
| C5 | 17 | F | A-NHL | C | NA | nt | 108 | 6.3 | 0.6 | 41 |
Parameters relevant to the Hasenclever prognostic index26 as well as histologic classification and EBV status as judged by latent membrane protein 1 (LMP1) expression are given. Stage is according to the Ann-Arbor classification.
Hb indicates hemoglobin; WBC, white blood cell; NS, nodular sclerosing; C, cervical; MC, mixed cellularity; U, unclassifiable showing elements of MC and NS; A, axillary; LP, lymphocyte predominant; I,inguinal; NA, not applicable; nt, not tested; PT, progressive transformation of germinal centers; A-NHL, anaplastic non-Hodgkin lymphoma.
The numbers in this column refer to the identifiers in Figures 2, 5, 6 and 7, where regulatory activity is demonstrated.
Indicates a relapse.
Antigens and mitogen
The control recall antigen mycobacterial PPD (Statens Seruminstitut, Copenhagen, Denmark), the T-cell mitogen concanavalin A (ConA; Sigma, Poole, United Kingdom), and the primary antigen keyhole limpet hemocyanin (KLH; Calbiochem-Behring, San Diego, CA) were each used to stimulate cultures at 10 μg/mL. PPD readily provokes recall T-cell responses in vitro,27 since most British citizens have been immunized with bacille Calmette-Guérin.
Cell proliferation and cytokine assays
As described elsewhere,28 PBMCs were separated from fresh blood samples by density gradient centrifugation. Single-cell suspensions of HL and control node lymphocytes were prepared from nodes immediately after surgical removal, by gentle, repetitive scraping of immobilized nodal surface with the use of a sharp scalpel with no enzymatic digestion. Cells were cultured in 1 mL volume at a cell concentration of 1.25 × 106/mL. Cellular proliferation was estimated from the incorporation of 3H-thymidine in triplicate 100-μL aliquots taken from the wells 5 days after stimulation, when recall T-cell responses are maximal.27 Proliferation results are presented as the mean counts per minute ± standard deviation (SD) of triplicate wells. Differences in counts between cultures on the same plate greater than 3-fold are interpreted as significant, and positive responses are defined as more than 3 times the background in unstimulated wells.28 Production of the Th cytokines interferon-γ (IFN-γ), IL-4, and IL-10 was assessed in duplicate 100-μL aliquots taken 5 days after stimulation of the cultures, with the use of a sensitive cellular enzyme-linked immunosorbent assay (ELISA).28 Differences in levels between cultures on the same plate greater than 2-fold are interpreted as significant, and positive responses are defined as more than 2 times the background in unstimulated wells.28
Characterization of responding cells
Immunophenotyping by flow cytometry was performed on unstimulated HLILs and PBMCs within 24 hours of preparation. Secretion of protein was inhibited by the addition of 0.05 μM Brefeldin A (Sigma) 8 to 12 hours prior to cell staining. Cells were then thoroughly washed before staining with antihuman CD4 fluorescein isothiocyanate and anti-CD25 phycoerythrin–cyanin 5.1 (both Beckman Coulter, High Wycombe, United Kingdom). After fixation and permeabilization (Intraprep; Beckman Coulter), cells were stained with antihuman IL-10–phycoerythrin and antihuman cytotoxic T-lymphocyte–associated antigen (CTLA)–4-biotin (both BD Pharmingen, Oxford, United Kingdom). Finally, streptavidin-phycoerythrin-Texas Red (Beckman Coulter) was added to allow detection of CTLA-4. Stained cells were analyzed by means of an EPICS XL cytometer (Beckman Coulter) and Expo32 analysis software (Applied Cytometry Systems, Sheffield, United Kingdom).
Mechanisms of regulatory T-cell function
To determine the mechanisms of regulatory T-cell function, as before,29 neutralizing antihuman IL-10 antibody (BD Pharmingen) at 1 ng/mL, or antihuman CTLA-4 F(ab)2 at 0.5 ng/mL (Ancell, Bayport, MN), was added to cultures. Alternatively, sterile transwell filters (0.4 μm polycarbonate membrane tissue-culture inserts) (Nunc, Roskilde, Denmark) were used to prevent cell-to-cell contact between putative regulatory and effector populations.
Statistical analysis
Statistical analyses were performed by means of SigmaStat 2.0 (Jandel Scientific, San Rafael, CA). Comparisons of control responses were performed by means of the Mann-Whitney rank sum or Kruskal-Wallis tests. HL patient flow cytometry data were analyzed by means of one-way analysis of variance and/or Kruskal-Wallis or t tests.
Results
Hodgkin lymphoma–infiltrating lymphocytes are hyporesponsive to mitogen and antigens
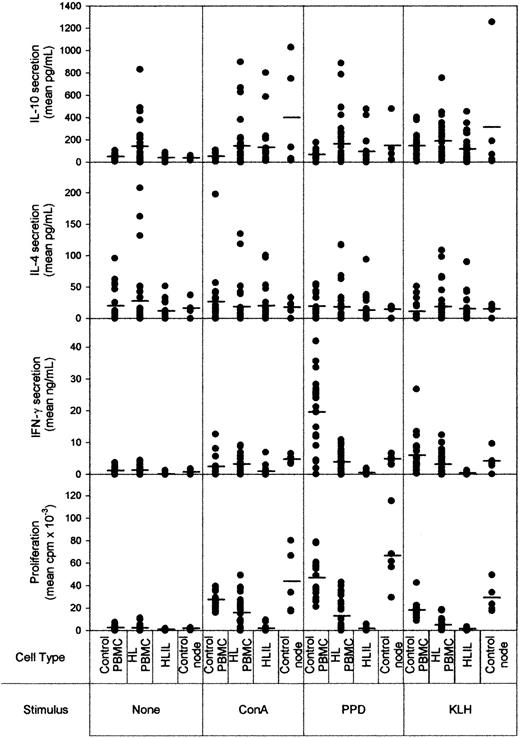
Given the lack of an effective immune response against HRS cells, we hypothesized that HLILs would be suppressed. As a first step in testing this proposal, HLILs were isolated from 24 patients and tested for responses to the mitogen ConA, the recall antigen PPD, and the primary antigen KLH after 5 days' stimulation. To represent the main types of Th cell responses, we assayed cellular proliferation, the Th1 cytokine IFN-γ, the Th2 cytokine IL-4, and the Tr1 cytokine IL-10. Figure 1 compares the responses of HLILs with paired PBMCs from HL patients (n = 24), cells obtained from control nodes (n = 5), and PBMCs from healthy volunteers (n = 20).
Proliferative and cytokine responses to mitogen and antigens by lymphocytes from HL patients and controls. The y-axis shows cytokine (IL-10, IL-4, and IFN-γ) and proliferative responses measured after 5 days' culture. Cells from healthy volunteer PBMCs (n = 20), HL patient PBMCs (n = 24), HLILs (n = 24), or control nodes (n = 45) were either left unstimuated or stimulated with mitogen (ConA), recall antigen (PPD), or primary antigen (KLH). The mean of the replicates for each patient is shown: the solid line on each plot shows the median responses obtained for each patient group.
Proliferative and cytokine responses to mitogen and antigens by lymphocytes from HL patients and controls. The y-axis shows cytokine (IL-10, IL-4, and IFN-γ) and proliferative responses measured after 5 days' culture. Cells from healthy volunteer PBMCs (n = 20), HL patient PBMCs (n = 24), HLILs (n = 24), or control nodes (n = 45) were either left unstimuated or stimulated with mitogen (ConA), recall antigen (PPD), or primary antigen (KLH). The mean of the replicates for each patient is shown: the solid line on each plot shows the median responses obtained for each patient group.
Strikingly, it can be seen there was no proliferative response to any stimulus by any HL patient sample, indicating a substantial defect in the ability of HLILs to mount immune responses. In contrast, control lymphocytes proliferated when stimulated with ConA, PPD, or KLH (P = .001 for all comparisons of HL versus controls; Kruskal-Wallis, Dunn method). Although HLILs from some patients were able to mount IFN-γ responses above the levels in unstimulated wells, secretion of this Th1 cytokine was low compared with that seen in lymphocytes from control cultures (P < .001 for all stimuli; Kruskal-Wallis, Dunn method). When the proliferation and IFN-γ data are taken into consideration, HLILs are severely constrained in their ability to mount Th1-type responses. HLILs were also analyzed on the basis of LMP1 status, but there were no significant differences between the proliferative and cytokine responses mounted by LMP1+ and LMP1– HLILs for any of the stimuli (P > .05 for all responses and stimuli; Mann-Whitney rank sum).
If HLILs contain large quantities of activated Th2 cells, as previously believed, then such cells would be expected to respond to stimulation by secreting the Th2 cytokine IL-4. Although occasional samples mounted significant responses, we observed no significant overall difference in the levels of IL-4 secreted from HLILs when stimulated as compared with either background levels (P = .31; Kruskal-Wallis) or samples from control cultures (P = .1; Kruskal-Wallis).
Although IL-10 was initially classed as a Th2 cytokine, in the absence of IL-4 it is now recognized as characteristic of Tr1 cells. IL-10 could be measured in all node cultures, and there was a significant difference above background when HLILs were stimulated with ConA (P < .05; Kruskal-Wallis, Dunn method). However, there were no significant differences in responses between HL and non-HL nodes (ConA, P = .111; PPD, P = .203; KLH, P = .323: Mann-Whitney rank sum).
HLILs suppress PBMC activation
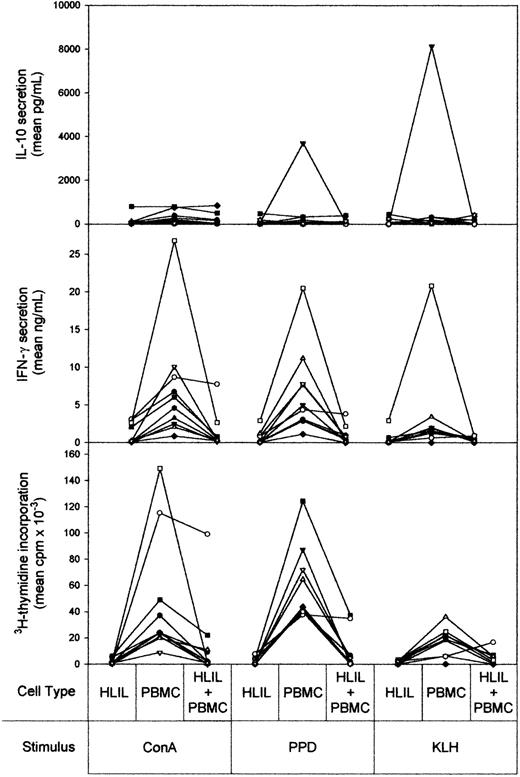
As responses from HL patient PBMCs retain responsiveness to antigens and mitogen, obtaining paired blood and node samples from 10 patients enabled us to test whether the apparently anergic HLILs were able to exert suppressive effects.
Paired cultures of HLILs and PBMCs, either alone or mixed in a 1:1 ratio, were stimulated with mitogen, recall antigen, or primary antigen. Figure 2 shows the cytokine and proliferative responses obtained. As demonstrated above, HLILs alone were anergic to all 3 of the stimuli, with no significant proliferative or cytokine responses measured. In contrast, PBMC samples alone responded to stimulation by the mitogen ConA and the recall antigen PPD with significant proliferation and IFN-γ secretion. PBMC responses to the primary antigen KLH were common but less marked. IL-10 production by HLILs and PBMCs was similar, except for samples from patient 4, whose PBMCs responded to the 2 antigens by secreting high levels of the cytokine, a response previously shown to be associated with the cyclosporin treatment received by this patient.30 IL-4 responses by HLILs and PBMCs were low and similar in all patients tested (data not shown).
Anergic HLILs dominantly suppress autologous PBMC responses. The vertical axis shows cytokine (IL-10 and IFN-γ) and proliferative responses measured after 5 days' stimulation. Separate cultures of HLILs and autologous PBMCs, and cultures containing a 1:1 mixture of both cell types, were stimulated with mitogen (ConA), recall antigen (PPD), or primary antigen (KLH). The results for samples from individual patients are linked by solid lines. Each patient (n = 10) is identified by a symbol: •, HL1; ▪, HL2; ▴, HL3; ▾, HL4; ♦, HL5; ⬡, HL6; ○, HL7; □, HL8; ▵, HL9; ▿, HL10.
Anergic HLILs dominantly suppress autologous PBMC responses. The vertical axis shows cytokine (IL-10 and IFN-γ) and proliferative responses measured after 5 days' stimulation. Separate cultures of HLILs and autologous PBMCs, and cultures containing a 1:1 mixture of both cell types, were stimulated with mitogen (ConA), recall antigen (PPD), or primary antigen (KLH). The results for samples from individual patients are linked by solid lines. Each patient (n = 10) is identified by a symbol: •, HL1; ▪, HL2; ▴, HL3; ▾, HL4; ♦, HL5; ⬡, HL6; ○, HL7; □, HL8; ▵, HL9; ▿, HL10.
In the key experiments, when the 2 cell types were cultured together, the HLIL anergy was found to be dominant over PBMC activation by mitogen, recall antigen, or primary antigen. Thus, proliferative and IFN-γ responses by PBMCs from 9 of 10 patients were profoundly suppressed by HLILs to levels of anergy similar to those exhibited to HLILs alone. The one pair of samples in which this suppression was not observed was obtained from a patient with an early relapse. As expected, IL-4 and IL-10 secretion in the mixed cultures was similar to that observed from the individual cell types.
Identification of regulatory populations in HLILs
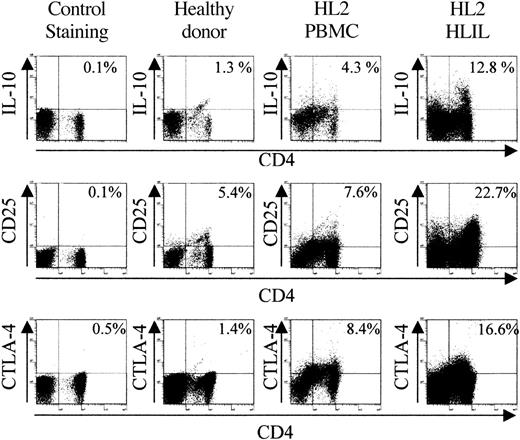
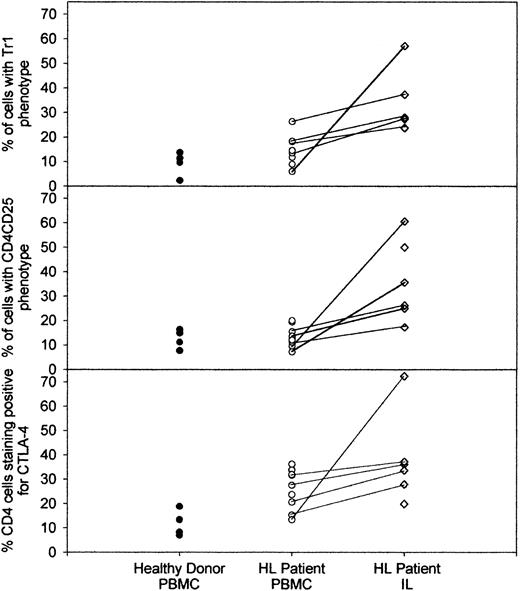
Having demonstrated the suppressive properties of anergic HLILs, we next determined whether these cells contain populations with the phenotype of regulatory T cells. HLILs (n = 6) and PBMCs from HL patients (n = 9) and healthy donors (n = 4) were stained for expression of CD4, CD25, and intracellular IL-10. This allowed identification of Tr1 (CD4+IL-10+) cells and CD4+CD25+ suppressor cells. In addition, samples were stained for intracellular CTLA-4, which is an inhibitory costimulatory molecule expressed by many regulatory populations.29,31 Figure 3 illustrates flow cytometric data obtained when PBMCs from a healthy donor are compared with PBMCs and HLILs from a representative HL patient; Figure 4 summarizes the analyses of all donors and patients tested. The most striking observation is that CD4+ cells expressing IL-10, CD25, or CTLA-4 are highly enriched in HLILs. Thus, the number of cells with a Tr1 phenotype is much higher in HLILs (mean, 32.9%; SD, 12.9%) compared with HL PBMCs (14.5% ± 5.9%; P < .05; analysis of variance [ANOVA]/Kruskal-Wallis) or control donor PBMCs (9.3% ± 4.9%; P < .05; ANOVA/Kruskal-Wallis). Although the mean number of CD4+IL-10+ cells was higher in PBMCs from HL patients than from healthy donors, this was not statistically significant (P = .15; t test).
Comparison of regulatory cell marker expression in healthy donor PBMCs and HL patient PBMCs, and HLILs. Staining for representative examples of healthy donor PBMCs (n = 4) and HL patient PBMCs (n = 9) and HLILs (n = 6). Plots show the percentage of lymphocytes staining positive for CD4 and IL-10, or CD4 and CD25, or the percentage of CD4+ lymphocytes staining positive for the costimulatory molecule CTLA-4. Cells were stained within 24 hours of purification and were unstimulated prior to staining. The control staining plots show the percentage of lymphocytes staining positive for CD4 and isotype control antibodies.
Comparison of regulatory cell marker expression in healthy donor PBMCs and HL patient PBMCs, and HLILs. Staining for representative examples of healthy donor PBMCs (n = 4) and HL patient PBMCs (n = 9) and HLILs (n = 6). Plots show the percentage of lymphocytes staining positive for CD4 and IL-10, or CD4 and CD25, or the percentage of CD4+ lymphocytes staining positive for the costimulatory molecule CTLA-4. Cells were stained within 24 hours of purification and were unstimulated prior to staining. The control staining plots show the percentage of lymphocytes staining positive for CD4 and isotype control antibodies.
Summary of percentages of regulatory T cells in healthy donor and HL patient PBMCs and HLILs. A comparison of the percentage of healthy donor PBMCs (n = 4) and HL patient PBMCs (n = 9) and HLILs (n = 6) that stained positive for either CD4 and IL-10 (Tr1 phenotype), CD4 and CD25, or CD4 and CTLA-4. The percentage of cells with each phenotype is shown on the vertical axis, and the source of cells is shown on the horizontal axis. Where PBMC and HLIL samples were obtained from the same patient, these linked samples are indicated by the solid lines. Cells were stained within 24 hours of purification and were unstimulated prior to staining.
Summary of percentages of regulatory T cells in healthy donor and HL patient PBMCs and HLILs. A comparison of the percentage of healthy donor PBMCs (n = 4) and HL patient PBMCs (n = 9) and HLILs (n = 6) that stained positive for either CD4 and IL-10 (Tr1 phenotype), CD4 and CD25, or CD4 and CTLA-4. The percentage of cells with each phenotype is shown on the vertical axis, and the source of cells is shown on the horizontal axis. Where PBMC and HLIL samples were obtained from the same patient, these linked samples are indicated by the solid lines. Cells were stained within 24 hours of purification and were unstimulated prior to staining.
Similarly, the number of cells with a CD4+CD25+ suppressor phenotype is enriched in HLILs (mean, 35.8%; SD, 16.6%) compared with HL PBMCs (mean, 13.5% ± 4.2%; P < .05; ANOVA/Kruskal-Wallis) or control donor PBMCs (mean, 12.5% ± 3.9%; P < .05; ANOVA/Kruskal-Wallis). CD4+CD25+ cells were only slightly more frequent in HL than in healthy donor PBMCs (P = .71; t test). It is not clear whether Tr1 and CD4+CD25+ cells are distinct lineages: we therefore analyzed the degree to which CD4+CD25+ cells in the HLILs secreted IL-10. In all HLIL samples, only a minority (range, 2.9%-15%) of CD4+CD25+ secreted IL-10 (data not shown), suggesting that Tr1 cells and CD4+CD25+ suppressor cells form distinct populations.
The number of CD4+ cells expressing CTLA-4 is much higher in both HLILs (mean, 37.9%; SD, 18.1%) and HL PBMC samples (mean, 26.0% ± 8.2%) compared with control donor PBMCs (mean, 11.8% ± 5.4%; P < .05; ANOVA/Kruskal-Wallis). Both HLILs and HL PBMCs contained significantly more CTLA-4–staining cells than healthy donor PBMCs (P = .01, P = .025, respectively; t test). In addition, all patient node and blood samples displayed stronger expression of CTLA-4 than healthy donor PBMCs.
Overall, the flow cytometric analysis reveals that, in every patient examined, more than half of CD4+ HLILs exhibit regulatory phenotypes.
Mechanism of suppression
A number of different mechanisms of suppression have been reported to be used by T-regulatory cells. We therefore ascertained which of 3 potential mechanisms of suppression, IL-10 secretion,9,12 cell-cell contact,10,14 and CTLA-4 engagement,31 was used by the regulatory T cells we had identified in the HLILs.
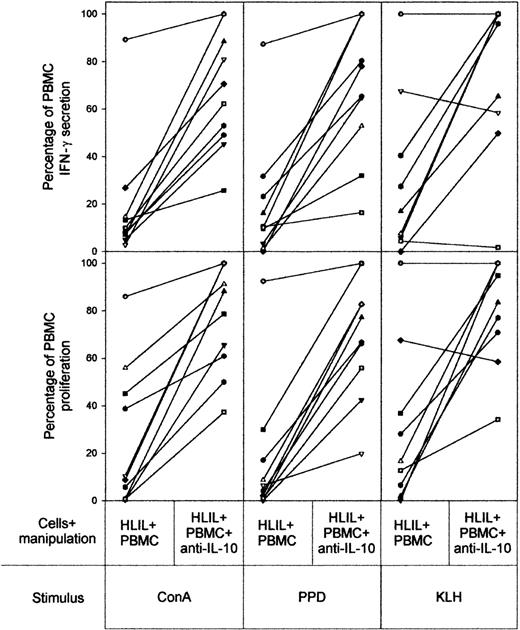
Neutralizing antibody specific for IL-10 was used to block the inhibitory effects of Tr1 cells and was added to cocultures of HLILs and paired PBMCs. Figure 5 summarizes the results of these experiments from the 10 HL patients whose HLILs and PBMCs were obtained in sufficient numbers. In all cases, addition of anti–IL-10 allowed recovery of HLIL-suppressed proliferative and IFN-γ PBMC responses to ConA and PPD, although the extent of reversal varied widely among patients. Recovery of suppression to KLH stimulation was also seen in 8 of 10 examples. We conclude that IL-10 is an important mediator of suppression by HLILs.
Contribution of IL-10 to HLIL-mediated suppression of PBMC responses. The effects of addition of neutralizing anti–IL-10 antibody to 1:1 mixtures of HLILs and paired PBMC cultures stimulated with mitogen (ConA), recall antigen (PPD), or primary antigen (KLH). The vertical axis indicates IFN-γ secretion and proliferative responses expressed as a percentage of responses obtained from PBMCs cultured alone (up to 100% maximum). The results for samples from individual patients are linked by solid lines. Each patient (n = 10) is identified by a symbol as in Figure 2.
Contribution of IL-10 to HLIL-mediated suppression of PBMC responses. The effects of addition of neutralizing anti–IL-10 antibody to 1:1 mixtures of HLILs and paired PBMC cultures stimulated with mitogen (ConA), recall antigen (PPD), or primary antigen (KLH). The vertical axis indicates IFN-γ secretion and proliferative responses expressed as a percentage of responses obtained from PBMCs cultured alone (up to 100% maximum). The results for samples from individual patients are linked by solid lines. Each patient (n = 10) is identified by a symbol as in Figure 2.
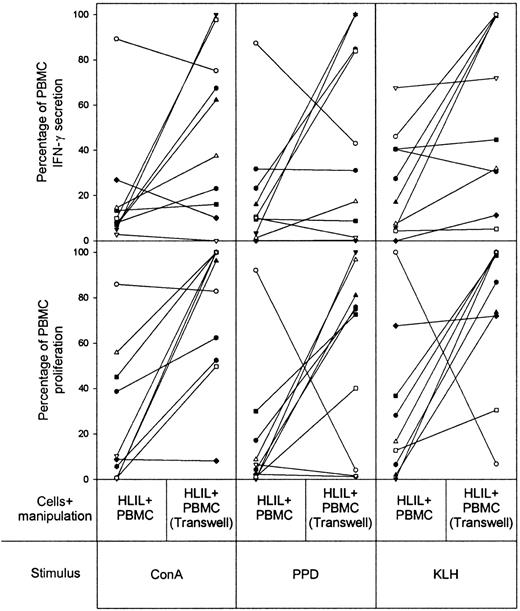
To inhibit a second potential suppressive mechanism, cell-to-cell contact between HLILs and paired PBMCs was prevented by means of transwell tissue-culture inserts, which allow exchange of only soluble factors between the 2 cell populations. It can be seen (Figure 6) that this separation alleviates the suppression of proliferative and IFN-γ responses to all 3 stimuli in most cases, but a strong effect is not observed as frequently as with IL-10 blockade.
Contribution of cell-to-cell contact to HLIL-mediated suppression of PBMC responses. The effects of incubating HLILs adjacent to paired PBMCs but separated from direct contact by transwell tissue-culture inserts. Cultures were stimulated with mitogen (ConA), recall antigen (PPD), or primary antigen (KLH). The vertical axis indicates IFN-γ secretion and proliferative responses expressed as a percentage of responses obtained from PBMCs cultured alone (up to 100% maximum). The results for samples from individual patients are linked by solid lines. Each patient (n = 10) is identified by a symbol as in Figure 2.
Contribution of cell-to-cell contact to HLIL-mediated suppression of PBMC responses. The effects of incubating HLILs adjacent to paired PBMCs but separated from direct contact by transwell tissue-culture inserts. Cultures were stimulated with mitogen (ConA), recall antigen (PPD), or primary antigen (KLH). The vertical axis indicates IFN-γ secretion and proliferative responses expressed as a percentage of responses obtained from PBMCs cultured alone (up to 100% maximum). The results for samples from individual patients are linked by solid lines. Each patient (n = 10) is identified by a symbol as in Figure 2.
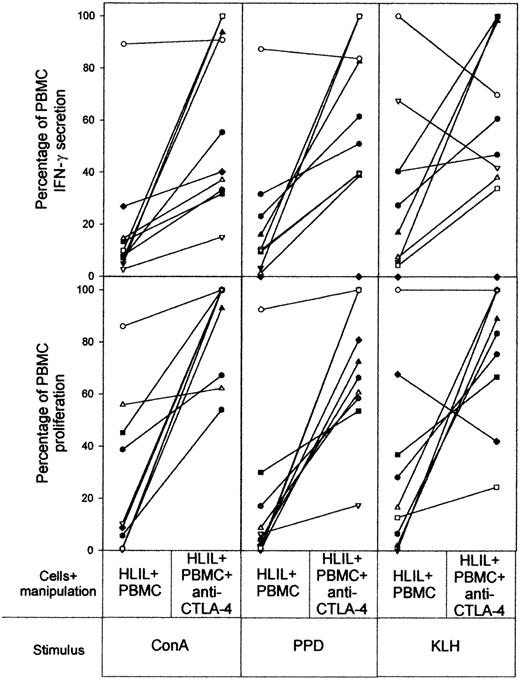
Finally, to block ligation of the inhibitory costimulatory molecule CTLA-4, anti–CTLA-4 F(ab)2 was added to cocultures of HLILs and paired PBMCs. Figure 7 demonstrates this blockade reversed suppression by HLILs in most cases, but again to varying extents.
Contribution of CTLA-4 to HLIL-mediated suppression of PBMC responses. The effects of blocking CTLA-4 binding by the addition of anti–CTLA-4 F(ab)2 antibody to 1:1 mixtures of HLIL and paired PBMC cultures stimulated with mitogen (ConA), recall antigen (PPD), or primary antigen (KLH). The vertical axis indicates IFN-γ secretion and proliferative responses expressed as a percentage of responses obtained from PBMCs cultured alone (up to 100% maximum). The results for samples from individual patients are linked by solid lines. Each patient (n = 10) is identified by a symbol as in Figure 2.
Contribution of CTLA-4 to HLIL-mediated suppression of PBMC responses. The effects of blocking CTLA-4 binding by the addition of anti–CTLA-4 F(ab)2 antibody to 1:1 mixtures of HLIL and paired PBMC cultures stimulated with mitogen (ConA), recall antigen (PPD), or primary antigen (KLH). The vertical axis indicates IFN-γ secretion and proliferative responses expressed as a percentage of responses obtained from PBMCs cultured alone (up to 100% maximum). The results for samples from individual patients are linked by solid lines. Each patient (n = 10) is identified by a symbol as in Figure 2.
Overall, these data demonstrate that all 3 mechanisms that were examined can play a role in the regulatory activity of HLILs. However, there is clearly variability in the strength and form of regulatory T-cell activity among different patients, and examination of the data in Figures 5, 6, 7 indicates certain patterns. In particular, in one case (HL7), the HLILs consistently demonstrated very little suppression of PBMC responses, which, in contrast to all the other patients, were exacerbated rather than reversed by prevention of cell-cell contact. Of the remaining patients whose HLILs were suppressive, in some cases (HL3, HL4, HL6, HL7, and HL9), the inhibition was susceptible to disruption of all 3 mechanisms, while in the others (HL1, HL2, HL5, HL8, and HL10) only some maneuvers, most commonly IL-10 neutralization, were effective.
Discussion
The main results reported here are that the lymphocytes from nodes affected by Hodgkin lymphoma not only are anergic, but also can profoundly inhibit Th cell responses. Immunophenotyping demonstrated that IL-10–secreting and CD4+CD25+ cells form substantial parts of the infiltrates. Thus, regulatory cells are the dominant T-cell population in HLILs, a conclusion that has important implications for understanding the pathogenesis of HL.
It has previously been generally accepted that HLILs are predominantly Th2 in character.1,16 However, a critical reexamination of the published evidence reveals little evidence that HLILs are capable of secreting the definitive Th2 cytokines IL-4 or IL-13.16-19,32 Although IL-10 has been frequently detected17,18 and is known to be secreted by Th2 cells, in the absence of IL-4 it is now more properly considered a Tr1 cytokine. HLILs have also been considered to display an activated phenotype, largely because they commonly express CD25. However, HLILs often fail to express functional IL-2 receptor because they lack the β-chain33 ; this instead suggests the presence of CD4+CD25+ regulatory cells. The frequent expression of CTLA-4 is also consistent with the presence of regulatory T cells, as this costimulatory molecule is expressed on both Tr1 and CD4+CD25+ suppressor T cells.15,29,34 Taken together, the published immunophenotyping data are actually more consistent with HLILs containing regulatory T cells, rather than Th2 cells, and our data support this reinterpretation. Although we cannot exclude the possibility that the inhibitory effects are mediated by HRS cells, the relative paucity of neoplastic cells, together with the demonstration that IL-10–secreting and CD4+CD25+ cells form substantial parts of the infiltrates, indicate that regulatory cells are the dominant T-cell population in HLILs.
Different forms of regulatory T cells have been recognized. In the context of HL, our data demonstrate that both Tr1 cells producing IL-10 and CD4+CD25+ cells are present in large numbers in involved nodes, with phenotypic analysis showing only limited (less than 15%) overlap between the 2 populations. The functional experiments, demonstrating mechanisms of suppression, are also consistent with the activity of both Tr1 and CD4+CD25+ cells in HLILs: inhibition of PBMC responses could be partially reversed either by neutralizing IL-10, by preventing cell-to-cell contact with HLILs, or by blocking CTLA-4 ligation. However, regulatory T cells are a rapidly developing area of study, and the different subtypes, together with their interrelationships and mechanisms of action, are still incompletely understood. Thus, when different subsets of regulatory T cells are analyzed, the mechanisms of action are not necessarily distinct. For example, CD4+CD25+ cells, generally held to suppress by cell-to-cell contact, have been described as inhibiting by IL-10 and/or TGF-β secretion,31 and, conversely, Tr1-mediated suppression may be mediated by cell-to-cell contact in addition to the more characteristic cytokine production.35 Although the presence of TGF-β–producing regulatory T cells13 was not determined in this study, previously published data demonstrate that up to 61% of primary HL samples contain T lymphocytes secreting TGF-β.36 Taken together with our current results, this indicates that the majority of CD4+ HLILs are regulatory T cells.
HL patients varied in the degree to which different mechanisms of suppression contributed to HLIL-mediated inhibition of PBMC responses. From the examination of these data (Figures 5, 6, 7), we can divide the patients into 3 categories. In the first group, all 3 mechanisms tested contributed to suppression by HLILs (patients HL3, HL4, HL6, and HL9). In the second category, HLIL suppressive activity was readily apparent, but not all mechanisms appeared to be relevant (patients HL1, HL2, HL5, HL8, and HL10). Within this group, the most common mediator of suppression was IL-10, although in some of these patients cell-to-cell contact or CTLA-4 engagement was as important or more so. Finally, in one patient (HL7), we saw little suppression of PBMC responses by HLILs. However, in the absence of cell-to-cell contact, suppressive activity was revealed; thus, regulatory T cells are present in this patient but rendered ineffective by an undetermined mechanism. The reasons for this variation among groups remain unclear, since analysis by HL subtype, EBV status, age, or percentage of regulatory T cells did not yield any significant correlation. However, these patterns of suppression are likely to reflect important differences in pathogenic mechanisms; patient 7, whose suppressive activity was atypical, was also unusual in experiencing a very early relapse (at 5 weeks).
Our description of regulatory T-cell activity in HLILs may explain reports from the very early literature of suppressor cells being overrepresented.7 The concentration of regulatory T cells that we have identified in the infiltrate would be expected to exert profound local immunosuppressive effects and therefore protect HRS cells from immunological surveillance and clearance. Regulatory T cells may also play a role in the maintenance of other tumors. For example, depletion of CD25+ T cells in murine models allows the development of immune responses against privileged tumor antigens.37 Furthermore, the regulatory cell–associated molecule CTLA-4 has been indicated to contribute to the prevention of immune responses against a number of murine and human tumors.38 Although regulatory T cells have been detected in other human tumors,39,40 the number and activity of regulatory T cells in HL are exceptional.
The recognition of the importance of regulatory T cells in HL pathogenesis will allow the rational design of more effective treatments. For instance, adoptive transfer of cytotoxic lymphocytes41 may be more effective if combined with measures to overcome this regulatory cell activity. Indeed, evidence from animal models of lymphoma42,43 suggest that conventional chemotherapy- and radiotherapy-based treatments for HL may be effective against regulatory cells, thereby enabling immune clearance of HRS cells. A better understanding of regulatory activity in HL will enable the realization of improvements in therapy.
Prepublished online as Blood First Edition Paper, November 6, 2003; DOI 10.1182/blood-2003-07-2594.
Supported by the Leukaemia Research Fund, the Aberdeen Royal Infirmary Lymphoma Trust Fund, Grampian University Hospitals National Health Service (NHS) Trust (Endowment Research Grants), and the Dawson Trust.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal