Abstract
The prothrombin gene mutation G20210A is a common risk factor for thrombosis and is associated with increased prothrombin levels. However, the mechanism whereby hyperprothrombinemia predisposes to thrombosis remains unclear. Because thrombin is the physiologic activator of TAFI (thrombin activatable fibrinolysis inhibitor), the precursor of an antifibrinolytic carboxypeptidase (TAFIa), we evaluated the influence of hyperprothrombinemia on fibrinolysis. Thirty-two heterozygous carriers of the G20210A mutation and 30 noncarriers were studied. Plasma fibrinolytic factors and TAFI levels were similar in the 2 groups. Mean lysis time of tissue factor–induced plasma clots exposed to 25 ng/mL exogenous tissue-type plasminogen activator (t-PA) was significantly longer in 20210A carriers than in control donors. This difference disappeared on addition of a specific inhibitor of TAFIa. Determination of thrombin and TAFIa activity, generated during clot lysis, revealed that G20210A mutation was associated with a significant enhancement of late thrombin formation and an increase in TAFI activation. Plasma prothrombin level was highly significantly correlated with both clot lysis time and TAFI activation. The addition of purified prothrombin, but not of factors X or VIIa, to normal plasma caused a concentration-dependent, TAFI-mediated inhibition of fibrinolysis. These findings provide a new mechanism that might contribute to the thrombotic risk in prothrombin 20210A carriers.
Introduction
The prothrombin gene mutation G20210A is the second most common genetic disorder associated with venous thrombosis. It is found in 4.6% to 8% of unselected patients with a first episode of deep vein thrombosis, as contrasted to 0.7% to 2.6% in healthy controls, and heterozygote carriers have a 2- to 7-fold increased risk of thrombosis (reviewed in Vicente et al1 ). The presence of the 20210A allele is associated with elevated prothrombin levels, and hyperprothrombinemia has been shown to be an independent risk factor for thrombosis.2 However, the underlying mechanism by which elevated prothrombin promotes thrombosis is still unclear. On the basis of in vitro3 and in vivo4 data, it is surmised that hyperprothrombinemia enhances thrombin generation whenever blood clotting is activated in vivo, thereby promoting excessive fibrin formation. Thrombin plays also an important role in the down-regulation of fibrinolysis by catalyzing the activation of TAFI (thrombin activatable fibrinolysis inhibitor), a plasma precursor of a carboxypeptidase B that inhibits plasmin formation by removing the plasminogen binding sites from partially degraded fibrin.5 This study was designed to evaluate the influence of the G20210A mutation and of hyperprothrombinemia on TAFI-mediated inhibition of fibrinolysis.
Patients, materials, and methods
Patients
Thirty-two carriers of the prothrombin G20210A mutation (all heterozygous) and 30 noncarriers were studied. Blood was collected by vacuum collection tubes (Vacutainer; Becton Dickinson, Meylan, France) containing 1/10 volume of 105 mM trisodium citrate. Citrated blood was centrifuged for 15 minutes at 2500g (room temperature), and the supernatant plasma was collected, snap-frozen in liquid nitrogen, and stored at –70°C until testing, which was performed no later than 6 months from blood collection.
Reagents and laboratory assays
Human prothrombin and factor X were from American Diagnostica, Greenwich, CT (courtesy of Dr D. Santo, Instrumentation Laboratory, Milan, Italy). Recombinant factor VIIa (NovoSeven) was from Novo Nordisk, Bagsværd, Denmark; tissue-type plasminogen activator (t-PA) was from Boehringer Ingelheim, Florence, Italy; potato tuber carboxypeptidase inhibitor (CPI) and hippuryl-Arg were from Sigma, Milan, Italy; thromboplastin (Recombiplastin) was from Instrumentation Laboratory; phospholipid vesicles (Unichrom) were purchased from Cabru, Peregallo di Lesmo, Milan, Italy. TAFI-depleted plasma6 was a generous gift of Prof B. N. Bouma, Utrecht, The Netherlands. Plasma levels of t-PA were assayed by commercially available enzyme-linked immunosorbent assay (ELISA; Imulyse t-PA; Biopool, Umea, Sweden). The activity levels of plasminogen activatory inhibitor 1 (PAI-1; Coatest PAI), α2-PI (plasmin inhibitor test), and plasminogen (Electrachrome Plasminogen) were determined by chromogenic assays (Instrumentation Laboratory). Total TAFI levels were assayed with a recently described ELISA that is based on monoclonal antibodies that are independent from the TAFI genotypes at positions 147 and 325.7 The frequency of TAFI 325 variants (Thr/Ile) in our samples (30 carrier and 30 control donors) was assessed with a genotype-specific ELISA which shows no reactivity toward the Ile-325 isoform.7 The genotype was deduced from the ratio between the TAFI antigen levels recorded with the genotype-specific and with the genotype-independent ELISAs. A ratio of 0 corresponds to Ile/Ile, a ratio between 0.45 and 0.65 to Thr/Ile, and a ratio higher than 0.8 to Thr/Thr. If the ratio fell between 0.66 and 0.79, the genotype was considered uncertain (Thr/Ile or Thr/Thr). Prothrombin levels were measured by using S2238 (Instrumentation Laboratory) as chromogenic substrate and Echis Carinatus (Sigma) as activator.8 Detection of the G20210A mutation in the prothrombin gene was performed as described by Poort et al.2
Clot lysis
The lysis of a tissue factor–induced plasma clot exposed to a physiologic concentration of exogenous t-PA was studied by using a turbidimetric assay9 modified as follows. Citrated plasma (100 μL), 10 μL phospholipid vesicles, 5 μL t-PA (25 ng/mL, final concentration), 60 μL buffer, 5 μL thromboplastin (1/1000 final dilution, unless otherwise stated), and 50 μL 0.04 M CaCl2 were added to a microplate well. The plate was then incubated at 37°C, and the changes in optical density (OD) at 405 nm were monitored every 10 seconds for the first 15 minutes (to evaluate clotting time) and every 5 minutes thereafter up to 3 hours. Clotting time was defined as the time to reach the midpoint of clear-to-maximum turbid transition, whereas clot lysis time was defined as the time from clot formation to the midpoint of the maximum turbid-to-clear transition.9 To evaluate the influence of TAFI activation on fibrinolysis, the clot lysis experiment was performed in the presence of CPI (50 μg/mL), an inhibitor that specifically quenches TAFIa activity.10 The shortening of the lysis time caused by CPI is a measure of the antifibrinolytic activity of TAFIa and is expressed as CPI sensitivity ratio (hereafter referred to as CPI ratio)—that is, the ratio between lysis time in the absence of CPI and lysis time in the presence of CPI. In some experiments, pooled plasma from donors not carrying the G20210A mutation was supplemented with purified prothrombin, factor X, or factor VIIa. In these cases the required volume of clotting factor solution was added directly to the microplate well, and the volume of buffer was reduced accordingly to maintain a constant total volume.
Assay of TAFI activation
TAFIa generated during in vitro fibrinolysis was assayed as CPI-sensitive arginine carboxypeptidase (Cp) activity by using hippuryl-Arg as the substrate.11 In these experiments the clot lysis mixtures were prepared in 2.5-mL tubes and consisted of defibrinated instead of normal plasma to avoid clot formation. Defibrinated plasma was prepared by ancrod treatment as reported.12 The amount of TAFIa generated during the clot lysis experiments is expressed as the percentage of total TAFI present in pooled normal plasma by comparison to a calibration curve constructed by testing dilutions of normal plasma after complete activation of TAFI.12
Assay of thrombin generation
The profile of thrombin generation, under conditions similar to those used for clot lysis assay, was determined essentially as previously reported.11 A clot lysis mixture, consisting of defibrinated plasma, was prepared in a 5-mL tube (final volume 1 mL) and incubated at 37°C. At predetermined intervals, a 50-μL aliquot was taken and transferred to a prewarmed tube containing 100 μL human fibrinogen (6 mg/mL) dissolved in citrate-Tris (tris(hydroxymethyl)aminomethane) buffer (0.38% sodium citrate). The clotting time was determined by the manual (tilt tube) technique, and thrombin activity was calculated by reference to a calibration curve constructed with purified human thrombin (Sigma).
Statistical analysis
All analyses were carried out by using the GraphPad Prism software (San Diego, CA). Unless otherwise stated, data are expressed as mean ± SD. The differences between groups were assessed by the Mann-Whitney test; correlations between variables were evaluated by the Pearson correlation coefficient.
Results
Prothrombin levels in 20210A carriers were 124% ± 18.3% as compared with 97% ± 13.5% in noncarriers (P < .0001). No difference in the plasma levels of TAFI or other relevant fibrinolytic factors was found between the 2 groups (Table 1). Moreover, the frequency of TAFI Ile-325 isoform, which has been reported to display a higher antifibrinolytic activity than Thr-325,13 was similar in carrier and control donors, as assessed by a genotype-specific ELISA (Ile/Ile, 3.3% versus 10%; Thr/Ile, 43% versus 37%; Thr/Thr, 43% verus 40%; uncertain, 10% versus 13%).
Plasma levels of fibrinolytic factors and TAFI in carriers of prothrombin G20210A mutation
Fibrinolytic factors and TAFI . | Noncarriers . | Carriers . |
|---|---|---|
| Plasminogen, % | 95.8 ± 12.2 | 93.3 ± 9.7 |
| α2-plasmin inhibitor, % | 102.0 ± 13.1 | 98.2 ± 11.8 |
| t-PA, ng/mL | 4.8 ± 2.6 | 5.1 ± 2.9 |
| PAI-1, U/mL | 15.7 ± 5.1 | 14.2 ± 4.6 |
| TAFI, μg/mL | 15.2 ± 8.3 | 14.8 ± 6.3 |
Fibrinolytic factors and TAFI . | Noncarriers . | Carriers . |
|---|---|---|
| Plasminogen, % | 95.8 ± 12.2 | 93.3 ± 9.7 |
| α2-plasmin inhibitor, % | 102.0 ± 13.1 | 98.2 ± 11.8 |
| t-PA, ng/mL | 4.8 ± 2.6 | 5.1 ± 2.9 |
| PAI-1, U/mL | 15.7 ± 5.1 | 14.2 ± 4.6 |
| TAFI, μg/mL | 15.2 ± 8.3 | 14.8 ± 6.3 |
For noncarriers, n = 30; for carriers, n = 32.
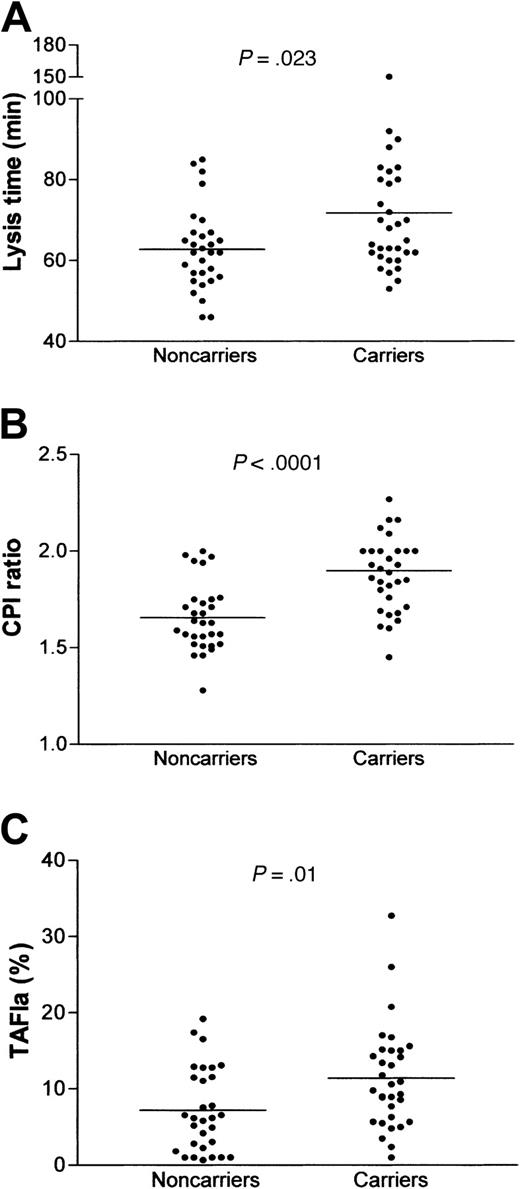
The lysis time of plasma clots exposed to exogenous t-PA was significantly prolonged in prothrombin 20210A carriers as compared with control donors (71.8 ± 17.9 versus 62.7 ± 10.1 minutes, P = .023; Figure 1A), whereas the coagulation time, recorded during the same clot lysis experiments, was similar in the 2 groups (297 ± 39 seconds and 276 ± 42 seconds in 20210A carrier and control donors, respectively). No difference in clot lysis was observed among samples with different TAFI 325-genotype (not shown). To see whether impaired fibrinolysis in hyperprothombinemic samples was due to up-regulation of the TAFI pathway, the clot lysis experiments were performed in the presence of CPI, a specific inhibitor of TAFIa. Figure 1B illustrates the fibrinolytic changes induced by CPI, expressed as the ratio between the lysis times in the absence and in the presence of CPI (CPI ratio), and shows that the inhibition of TAFIa caused a significantly more pronounced profibrinolytic effect in prothrombin 20210A carriers than in control donors (CPI ratio, 1.9 ± 0.22 versus 1.66 ± 0.18, P < .0001). Remarkably, the difference in clot lysis time between the 2 groups was no longer detectable on addition of CPI (37.8 ± 6.3 minutes versus 38.1 ± 9.2 minutes), suggesting that enhancement of TAFI activation was the main cause of reduced fibrinolysis in prothrombin 20210A carriers. This finding is further supported by the significantly higher CPI-sensitive carboxypeptidase activity detected in these latter samples 30 minutes after the start of the clot lysis experiments (11.4% ± 6.7% versus 7.2% ± 5.5%, P = .01; Figure 1C) and by the strong correlation between TAFIa activity and lysis time (r = 0.414, P = .0008).
Fibrinolytic capacity and TAFIa generation in 20210A carriers. Fibrinolysis was studied by a turbidimetric assay using a tissue factor–induced plasma clot exposed to 25 ng/mL exogenous t-PA. (A), lysis time; (B), fibrinolytic response to CPI (50 μg/mL) expressed as CPI ratio (lysis time in the absence of CPI divided by the lysis time in the presence of CPI); (C), TAFIa generated in the clot lysis mixture 30 minutes after the start of the experiment. TAFIa activity was measured as CPI-sensitive carboxypeptidase activity and is expressed as the percentage of total TAFI activity of pooled normal plasma. The horizontal bars represent the means of the groups. The differences were assessed by the Mann-Whitney test.
Fibrinolytic capacity and TAFIa generation in 20210A carriers. Fibrinolysis was studied by a turbidimetric assay using a tissue factor–induced plasma clot exposed to 25 ng/mL exogenous t-PA. (A), lysis time; (B), fibrinolytic response to CPI (50 μg/mL) expressed as CPI ratio (lysis time in the absence of CPI divided by the lysis time in the presence of CPI); (C), TAFIa generated in the clot lysis mixture 30 minutes after the start of the experiment. TAFIa activity was measured as CPI-sensitive carboxypeptidase activity and is expressed as the percentage of total TAFI activity of pooled normal plasma. The horizontal bars represent the means of the groups. The differences were assessed by the Mann-Whitney test.
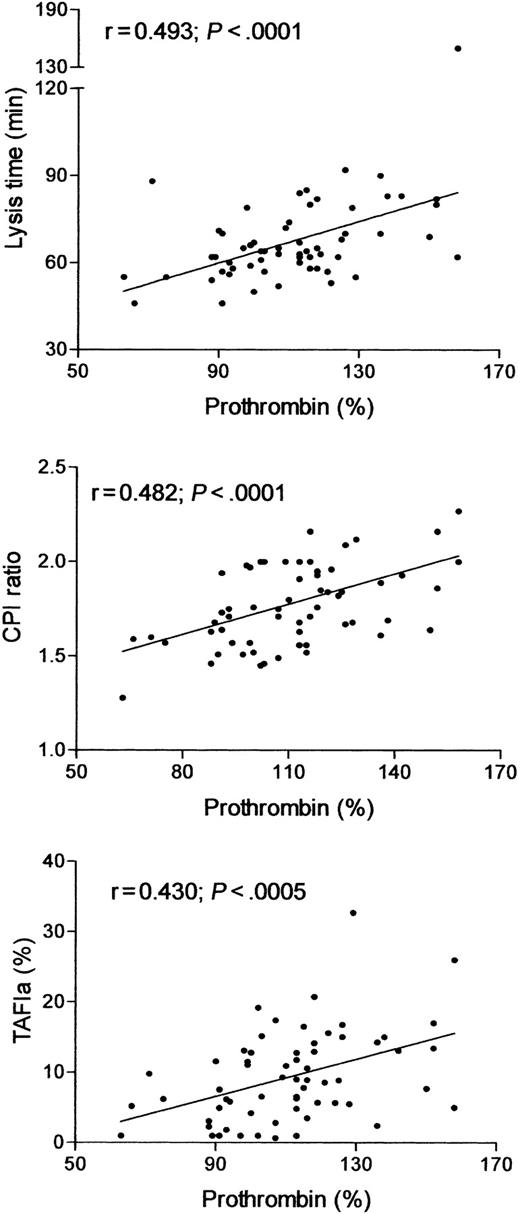
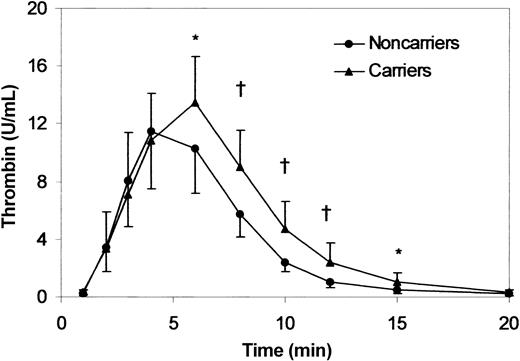
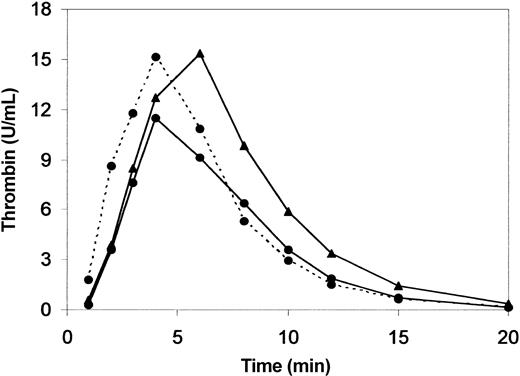
The relation between TAFI-dependent inhibition of fibrinolysis and increased prothrombin levels is suggested by the strong correlation between prothrombin concentration and clot lysis time, CPI ratio and TAFIa activity generated during the fibrinolysis experiments (Figure 2). In view of the pivotal role of thrombin in the activation of TAFI,14 we evaluated the profile of thrombin generation during clot lysis. As shown in Figure 3, the initial rate of thrombin formation was similar in 20210A carrier and control donors, and peak thrombin activity was slightly but not significantly higher in the former. In contrast, both the time to peak and thrombin activity at late intervals (from 6 to 15 minutes) were significantly higher in prothrombin 20210A carriers.
Correlation of prothrombin activity with lysis time (top), CPI ratio (middle), and TAFIa activity generated during clot lysis (bottom).
Correlation of prothrombin activity with lysis time (top), CPI ratio (middle), and TAFIa activity generated during clot lysis (bottom).
Thrombin generation in 20210A carriers. Thrombin generation during clot lysis was determined in 10 prothrombin 20210A carriers (prothrombin activity, 130% ± 19.5%) and in 10 control donors (prothrombin activity, 95% ± 15.4%). Thrombin activity was measured by clotting assay on aliquots taken from the clot lysis mixture at the indicated intervals. Results are the mean ± SD. * indicates P < .05; †, P < .01. Times to peak were 5.9 and 4.5 minutes in 20210A carriers and control donors, respectively (P < .025).
Thrombin generation in 20210A carriers. Thrombin generation during clot lysis was determined in 10 prothrombin 20210A carriers (prothrombin activity, 130% ± 19.5%) and in 10 control donors (prothrombin activity, 95% ± 15.4%). Thrombin activity was measured by clotting assay on aliquots taken from the clot lysis mixture at the indicated intervals. Results are the mean ± SD. * indicates P < .05; †, P < .01. Times to peak were 5.9 and 4.5 minutes in 20210A carriers and control donors, respectively (P < .025).
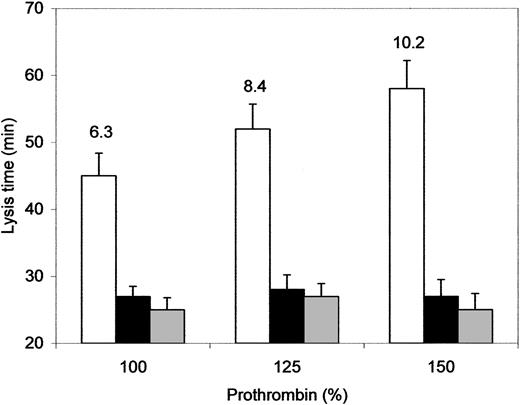
To directly assess the influence of hyperprothrombinemia on fibrinolysis we added purified human prothrombin to pooled normal plasma to raise the prothrombin level to 125% and 150%. As shown in Figure 4, the lysis time of prothrombin-enriched plasma clots was prolonged proportionally to the amount of added prothrombin. This antifibrinolytic effect was abolished by CPI and was no longer detectable when prothrombin was added to TAFI-depleted plasma, indicating that prothrombin supplementation inhibited fibrinolysis through a TAFI-mediated mechanism. Accordingly, TAFI-dependent carboxypeptidase activity generated during clot lysis increased fairly proportionally to prothrombin level (Figure 4). No effect on fibrinolysis was observed when normal plasma was supplemented with factor X (up to 200%) or with factor VIIa (up to 60 U/mL) or when thromboplastin concentration was increased by 10-fold (not shown). Determination of thrombin activity during clot lysis confirmed that prothrombin supplementation was associated with an enhancement of thrombin activity at late intervals (Figure 5). In contrast, the thrombin generation curve recorded in a sample in which coagulation was triggered by a 10-fold higher thromboplastin concentration was characterized by a selective increase of the initial rate of thrombin formation. Neither factor X nor factor VIIa addition influenced thrombin generation to any noticeable extent (not shown).
Effect of prothrombin supplementation on fibrinolysis and TAFI activation. Normal pooled plasma (NP, □) and TAFI-depleted plasma (▦) were supplemented with purified human prothrombin to obtain the indicated plasma concentration of total prothrombin. Fibrinolysis was studied by a turbidimetric assay using a tissue factor–induced plasma clot exposed to 25 ng/mL exogenous t-PA. NP was also tested in the presence of CPI (50 μg/mL, ▪). The numbers on NP bars indicate the amount of TAFIa, expressed as percentage of total TAFI, recorded at 30 minutes from the start of the experiment. Data are the mean ± SD of 3 experiments.
Effect of prothrombin supplementation on fibrinolysis and TAFI activation. Normal pooled plasma (NP, □) and TAFI-depleted plasma (▦) were supplemented with purified human prothrombin to obtain the indicated plasma concentration of total prothrombin. Fibrinolysis was studied by a turbidimetric assay using a tissue factor–induced plasma clot exposed to 25 ng/mL exogenous t-PA. NP was also tested in the presence of CPI (50 μg/mL, ▪). The numbers on NP bars indicate the amount of TAFIa, expressed as percentage of total TAFI, recorded at 30 minutes from the start of the experiment. Data are the mean ± SD of 3 experiments.
Effect of prothrombin supplementation on thrombin generation. Thrombin generation during clot lysis was determined in samples consisting of normal plasma (100% prothrombin, •) or plasma supplemented with purified prothrombin to obtain a total prothrombin concentration of 150% (▴). Thrombin activity was measured by clotting assay on aliquots taken from the clot lysis mixture at the indicated intervals. The dotted line refers to normal plasma in which coagulation was triggered by a 10-fold higher concentration of thromboplastin. Results are the mean of 3 experiments. SDs were omitted for clarity.
Effect of prothrombin supplementation on thrombin generation. Thrombin generation during clot lysis was determined in samples consisting of normal plasma (100% prothrombin, •) or plasma supplemented with purified prothrombin to obtain a total prothrombin concentration of 150% (▴). Thrombin activity was measured by clotting assay on aliquots taken from the clot lysis mixture at the indicated intervals. The dotted line refers to normal plasma in which coagulation was triggered by a 10-fold higher concentration of thromboplastin. Results are the mean of 3 experiments. SDs were omitted for clarity.
Discussion
The data reported here indicate that carriers of the prothrombin G20210A mutation have a reduced fibrinolytic capacity because of an enhancement of the TAFI-mediated antifibrinolytic pathway. The fibrinolytic model used in this study consists of a tissue factor–induced plasma clot exposed to physiologic concentrations of t-PA and is thought to better mimic the in vivo conditions than the classical fibrinolytic assays.6 Using this model we found that the lysis time of clots made from G20210A plasma was significantly longer than that of control plasma. This result could not be ascribed to a difference in plasma levels of fibrinolytic factors known to influence in vitro fibrinolysis, including TAFI, or to a higher frequency of the TAFI Ile-325 isoform, which is characterized by an extended functional half-life.13 The reduced fibrinolytic capacity in 20210A carriers was largely, if not totally, due to an up-regulation of TAFI activation because greater amounts of TAFIa activity were recorded during the clot lysis experiments and because the difference in lysis time between control donors and 20210A carriers disappeared on addition of the TAFIa inhibitor. The causal relationship between elevated prothrombin and impaired fibrinolysis is suggested by the highly significant correlation between prothrombin levels and each of the studied fibrinolytic parameters (clot lysis time, CPI ratio, TAFIa generation) and by the dose-dependent, TAFI-mediated, antifibrinolytic effect induced by the addition of purified prothrombin to normal pooled plasma to obtain a prothrombin level comparable to that found in 20210A carriers. This finding adds to those of Broze and Higuchi15 who demonstrated that the increase in prothrombin concentration (from < 1% to 50%) leads to a progressive lengthening of lysis time. Notably, neither the addition of factor X or factor VIIa nor the activation of coagulation by a 10-fold higher thromboplastin concentration induced any detectable change in fibrinolysis time.
Thrombin is the main activator of TAFI,14 and there is evidence that prothrombin G20210A mutation is associated with an enhancement of thrombin generation.3,4 Therefore, we evaluated thrombin generation under conditions similar to those used for clot lysis assay and found that the most relevant difference between prothrombin 20210A carrier and control donors was a significant increase in the amount of “late” thrombin generated in the former. This behavior could be fully reproduced by supplementing control plasma with purified prothrombin, indicating that the enhancement of thrombin formation occurred because of hyperprothrombinemia. This finding is a plausible explanation for the observed TAFI-mediated down-regulation of fibrinolysis in 20210A carriers. Indeed, there is compelling evidence that, when coagulation is induced by low amounts of tissue factor, TAFI activation is largely dependent on the second burst of prothrombin activation occurring after a clot is formed by a FXI-dependent positive feedback.16-19 This evidence is further supported by the observation that the enhancement of the initial rate of thrombin generation induced by a 10-fold higher thromboplastin concentration does not inhibit fibrinolysis as also shown by others.20
Sustained thrombin formation associated with prothrombin G20210A mutation might dampen fibrinolysis by other mechanisms such as the increase of factor XIII activation, resulting in a hyperstabilized clot,21 or the formation of a clot with an altered fibrin structure (reduced mass-to-length ratio).22 It is unlikely, however, that these TAFI-independent mechanisms contributed significantly to the inhibition of fibrinolysis under our experimental conditions. First, the difference in clot lysis rate between hyperprothrombinemic samples (either 20210A carriers or prothrombinenriched plasma) and control samples disappeared when the assay was carried out in the presence of a specific TAFIa inhibitor. Second, the antifibrinolytic effect induced by prothrombin addition was no longer detectable if plasma was depleted of TAFI. It is conceivable, therefore, that the increase in prothrombin concentration above normal levels impairs fibrinolysis by up-regulating the second burst of thrombin generation, thereby enhancing the activation of TAFI.
The relevance of TAFI in the regulation of fibrinolysis is suggested by several studies in experimental animals.23-26 Moreover, there is evidence that the up-regulation of the TAFI pathway may predispose to venous thrombosis. Indeed, increased levels of TAFI, which have been shown to inhibit plasma fibrinolysis,27 have been found to be a risk factor for thrombosis.28 In addition, it has been proposed that the higher incidence of venous thrombosis in subjects with elevated FXI or with APC resistance might be due, at least in part, to an increase in TAFI activation.29,30 Our finding of up-regulated, TAFI-dependent, inhibition of fibrinolysis in subjects with high prothrombin levels provides an additional mechanism for the increased thrombotic risk in carriers of the prothrombin G20210A mutation.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-06-2169.
Supported by a grant from Ministero della Università e della Ricerca Scientifica e Tecnologica.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr A. Gils and Prof P. Declerck, Laboratory for Pharmaceutical Biology and Phytopharmacology, Katholieke Universiteit Leuven, Belgium, for performing the TAFI antigen assays and for helping in the identification of TAFI genotypes.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal