Abstract
Antigen-specific CD4+ effector T cells primarily provide help for B-cell antibody responses and CD8+ cytotoxic T-lymphocyte (CTL) responses. We have found an expanded population of HIV-1 p24-specific, T-cell receptor Vbeta17+, CD4+ T lymphocytes, defined by in vitro proliferative and interferon-γ responses to a 15-mer Gag peptide, in the peripheral blood of an individual with long-term nonprogressive HIV-1 infection. Ex vivo, these cells were CCR5+ and CCR7-, consistent with an effector/memory function. Surprisingly, these cells highly expressed several proteins characteristic of cytotoxic lymphocytes, including TIA-1 (T-cell intracellular antigen 1; GMP-17/NKG7), granzymes A and B, CD161 (NKRP-1), and CD244 (C1.7/2B4). Following in vitro peptide stimulation, these cells produced interleukin 2 (IL-2) and intracellular CD40L, suggesting possible helper function, in addition to induction of perforin and cytotoxicity. A subset of cytomegalovirus (CMV)–specific CD4+ T cells in healthy adults similarly expressed these CTL markers and CCR5, ex vivo. Furthermore, this distinct subset of CD4+ T cells was significantly elevated in healthy CMV-seropositive adults, compared with CMV-seronegative individuals. These results suggest that CCR5+ CD4+ CTL may be a major effector mechanism of the immune response to viral infections in humans. Moreover, expression of CCR5 may render them particularly susceptible to cytopathic effects during progressive HIV-1 infection.
Introduction
Antigen-specific CD4+ T lymphocytes are important for optimal B-cell and CD8+ cytotoxic T-lymphocyte adaptive immune responses to viral infection.1,2 However, in the case of HIV-1 infection, antigen-specific helper CD4+ T lymphocytes have proven difficult to detect in the vast majority of untreated individuals and can generally only be found at low levels, as judged by either in vitro lymphoproliferation or intracellular interferon-γ production (reviewed in Picker and Maino3 ). The deficit in these cells occurs early in the course of infection, even when CD4+ T-cell numbers are relatively normal.4,5 Also, HIV peptide-HLA class II tetramers have been difficult to produce, in contrast to the peptide-HLA class I tetramers used to study HIV antigen-specific CD8+ T cells.6 Therefore, little is known about antigen-specific CD4+ helper T cells in progressive HIV-1 infection.
Antiretroviral therapy initiated very early in primary HIV-1 infection (PHI) has been shown to preserve HIV-specific CD4+ T-cell responses.7,8 Preferential infection of HIV-1 antigen-specific CD4+ T cells in vivo has recently been inferred from experiments which used either HIV-1 DNA,9 or recovery of replication competent virus in vitro,10 as a marker of infection in vivo. A possible explanation for these observations is that HIV antigen-specific CD4+ T lymphocytes express the chemokine receptor CCR5 in vivo, which is also the major HIV-1 coreceptor early in infection.11 We have previously found that approximately half of the CD4+ T cells proliferating during PHI express CCR5,12 and it is possible that these cells include newly derived HIV-1 antigen-specific cells. Whether viral antigen-specific human CD4+ memory T cells express CCR5 in vivo, particularly in HIV-1 infection, remains unknown.
Early cell sorting experiments identified CD4+ memory cells in the CD45RO+ subset,13 and more recently have been further functionally subdivided into the CCR7+/CD62L+ proliferative central memory cells and CCR7-negative effector memory cells.14 Changes in other trafficking- and adhesion-related cells surface molecules, including integrins and lymphocyte function antigen 1 (LFA-1), have also been well described.15 Studies conducted in sheep and also in T-cell receptor (TCR)–transgenic mice have shown directly that most of the antigen-experienced CD4+ T cells migrate into peripheral, nonlymphoid tissues,16-18 and similar findings apply to antigen-specific CD8+ memory/effector cells.19,20
Chemokine receptors play a major role in T-cell migration,21 and selective expression of chemokine receptors has been demonstrated on clonal antigen-specific CD4+ T cells, including CXCR3 on T helper 1 (Th1), CCR8 on Th2, and CXCR5 on follicular–helper T cells, respectively.22-25 CCR5 is normally expressed on a small subpopulation of circulating CD4+ T cells with a memory phenotype,26 but it is rapidly down-regulated following TCR stimulation in vitro.27 CCR5 is eventually re-expressed on Th1 clones grown in vitro and at lower levels on Th2 clones21 ; therefore, it is probable that CCR5 is present on at least some antigen-specific CD4+ T cells in vivo. However, the only documented ex vivo expression of CCR5 on antigen-specific T cells in humans is on viral antigen tetramer+ CD8 + T cells.28
In human viral infections, cytomegalovirus (CMV)–specific CD4+ T cells can be readily quantified using the intracellular cytokine (ICC) assay,29 whereas corresponding Epstein-Barr virus (EBV)–, herpes simplex virus (HSV)–, and varicella-zoster virus (VZV)–specific CD4+ T cells have also been reported but at lower frequencies.30-32 These assays allow simultaneous study of cell phenotype and have shown that some CMV-specific CD4+ T cells were CD4+CD8dim T cells33 and also expressed perforin.33,34 Another study using the ICC assay showed that the CCR7-negative effector memory subpopulation, of which about two thirds were CCR5+ ex vivo, contained HIV- and CMV-specific CD4+ T cells,35 suggesting that they expressed CCR5 in vivo.
A small proportion of HIV-infected subjects remain asymptomatic with normal CD4 T-cell counts for more than 10 years. Some of these long-term nonprogessor (LTNP) individuals maintain relatively large in vitro HIV-1 antigen-specific lymphoproliferative responses, particularly to HIV-1 p24.7,36,37 We have previously studied peripheral blood mononuclear cells (PBMCs) from such an individual LTNP with complete natural suppression of HIV-1 replication and described very large CD4+ T-lymphocyte proliferative and interferon-γ responses to HIV-1 p24 protein by using overlapping peptides.38,39 In the present study these CD4+ T-cell responses to individual 15-mer Gag peptides were investigated in more detail. In particular, there was an expansion of TCR V beta (TCRBV) 17+ CD4+ T lymphocytes in the circulation of this LTNP, which contained a large number of effector memory cells specific for a p24 peptide, representing up to 4% of CD4+ T cells. We were able to use TCRBV 17 to identify these cells in the peripheral blood to further investigate their phenotype and found that they expressed the HIV-1 coreceptor, CCR5, as well as several markers characteristic of cytotoxic T lymphocytes. A similar approach was also undertaken to characterize CMV-specific CD4+ memory T cells in healthy adults, immediately ex vivo. A major subset of these CD4+ T cells also exhibited characteristics of cytotoxic T lymphocytes and expressed CCR5 in vivo.
Patients, materials, and methods
Patients
The long-term nonprogressor, subject LTNP-100149, has been described in detail previously.38 His history indicates that he has been infected with HIV-1 for at least 15 years yet exhibits an undetectable load and a stable, normal CD4 cell count. Furthermore, analysis of full-length HIV-1 (9.7 kb) sequences of proviral DNA from this subject's PBMCs showed a complete lack of evolution over at least 8 years, suggesting a complete absence of viral replication during this time.39 It should be noted that LTNP-100149 participated in several clinical trials over this time, including a p24 viruslike particle vaccine trial40 in which he received only 2 of 6 scheduled vaccine doses, because of intolerance of azidothymidine (AZT), administered as part of this trial. We have previously concluded that this treatment was poorly immunogenic and probably did not contribute significantly to his strong CD4+ T-cell responses to HIV-1 gag proteins.38 The HLA type of LTNP-100149 is A1,2; B8,44; DR4,15, and furthermore, he is CCR5Δ32 heterozygous.38
Healthy HIV-uninfected control subjects were recruited from hospital and university staff members. CMV serology was performed by routine diagnostic assay (BioMerieux, Marcy-l'Etoile, France). Approval for these studies was obtained from the institutional review board of St Vincent's and Westmead Hospitals. Written informed consent was provided in accordance with the Declaration of Helsinki.
Antigens
Soluble recombinant HIV-1 p24 was purchased from Protein Sciences (Meriden, CT), stored at -70°C, and used at a final concentration of 1 μg/mL. CMV lysate was from Bio-Whittaker (Walkersville, MD) and used at a final dilution of 1/500.
Overlapping HIV-1 Gag 15-mer peptides derived from the sequence of strain HXB2 were obtained from the National Institutes of Health (NIH) AIDS reference reagents program. We have previously described that 8 of the 122 peptides elicited large CD4+ T-cell responses in PBMCs from LTNP-100149.38 In the current study, responses to 2 of these peptides have been studied in detail. Peptide no. 42 has the sequence SPEVIPMFSALSEGA (NIH catalog no. 5026; residues 33-47 of p24) and peptide no. 106 has the sequence HQMKDCTERQANFLG (NIH catalog no, 5090; residues 44-55 of p7 and 1-3 of p1). Peptide no. 64 has the sequence WMTNNPPIPVGEIYK (NIH catalog no. 5047) and previously elicited a CD8+ cytotoxic T-lymphocyte (CTL) response.38 Peptides were used at a concentration of 2 μg/mL.
Lymphocyte function assays
Lymphoproliferation assays were performed by using 3H-thymidine or carboxyfluorescein succinimidyl ester (CFSE)–labeled PBMCs as previously described.38 PBMCs were cultured in the presence or absence of antigen for 6 days, before overnight addition of 3H-thymidine, and proliferating CFSEdim CD4+ T cells were identified by 4-color flow cytometry after 6 to 7 days.38
Enzyme-linked immunospot (ELISPOT) assays and intracellular cytokine (ICC) assays of interferon-γ production by PBMCs and CD4+ T cells were initially performed as previously described.38 In later experiments, 5-color flow cytometry was used to analyze ICC assays (described in “Flow cytometry”).
Whole blood cultures were performed by mixing 0.25 mL sodium heparin-anticoagulated whole blood with an equal volume of Iscoves modified Dulbecco medium (JRH, Melbourne, Australia) and incubated in the presence or absence of antigens for 40 to 48 hours at 37°Cin5%CO2 in sterile 5-mL loosely capped polystyrene containers (Biolab, Clayton, Australia). At the end of the culture, staining with fluorochrome-labeled antibodies was performed by using the same method as for fresh whole blood, described in “Flow cytometry.”
CTL activity was measured following culture of 5 × 106 PBMCs in 250 μL with individual peptides at 10 μg/mL for 1 hour, then for 8 to 10 days in 5 to 10 mL RPMI with 10% human serum. Interleukin 2 (IL-2; 100 U/mL; provided by the NIH AIDS Reference Reagents Program) was added on days 3 and 6. Cultures were assessed for CTL activity by using autologous B-cell targets labeled with 100 μCi (3.7 MBq) 51Cr and pulsed with peptide (10 μg/mL) for 2 hours, as well as autologous targets infected with recombinant Vaccinia expressing HIV-1 gag (or Escherichia coli lacZ as a control). PBMCs, or purified CD4+ and CD8+ cells, were added to 5000 target cells in 96-well plates, at the indicated effector-to-target ratios, spun at 500g for 3 minutes, and incubated for 4 hours. Released chromium in the supernatant was expressed as the percentage of specific lysis.
Flow cytometry
In initial studies, subsets of CD4+ T lymphocytes fresh peripheral blood were stained for cell surface and intracellular markers and analyzed by 4-color flow cytometry on an EPICS XL flow cytometer (Beckman-Coulter, Hialeah, FL) as previously described.41 Monoclonal antibodies used were CD3-PerCP (peridinin chlorophyll protein), CD11a-FITC (fluorescein isothiocyanate), CD27-FITC, CD28-PE (phycoerythrin), CD38-PE, HLA-DR–FITC, CD57-FITC, CD62L-FITC, CD94-FITC, CD95-PE, CD40L(CD154)–PE, CD158a-FITC, CD161-PE, and CD25-PE and -FITC (Becton Dickinson, San Jose, CA); CXCR4-PE, CCR5-PE and -FITC, CCR7-PE, CD45RA-PE, CD45RO-FITC, CD122-PE, Ki-67–FITC, Bcl-2–FITC, and Perforin-PE (Pharmingen, San Diego, CA); CD4-ECD (phycoerythrin-Texas Red), C1.7(CD244)–PE, TIA-1 (T-cell intracellular antigen 1) (GMP-17)–PE, CD127-PE, CD158b-PE, TCRBV 1-PE, TCRBV 17-PE and -FITC, and TCR zeta chain-PE (Beckman Coulter); and TCR V alpha24-PE (Serotec, Oxford, United Kingdom). For CCR5 analysis, whole blood was processed within 2 hours to avoid spontaneous loss of this marker.42,43 CFSE-stained and cultured PBMCs were analyzed for proliferation by using CD3-PerCP, CD4-ECD, and PE-conjugated monoclonal antibodies as previously described.38 Intracellular staining was performed by using FACSlyse and FACSPermeabilizing Reagents (Becton Dickinson) according to the manufacturer's directions, and they were analyzed as previously described.12
In subsequent experiments, 5- and 6-color flow cytometry was performed on a dual-laser LSR II flow cytometer (Becton Dickinson) using FACSDiva v2.2 software. The sensitivity of this flow cytometer is several-fold improved in the FITC and PE channels than previously obtained with 4-color analysis (data not shown). Additional monoclonal antibodies used were CD3-PerCP-Cy5.5, CD4-PE-Cy7, CD8-APC-Cy7 (Becton Dickinson). A CD3-PerCP-Cy5.5 versus side scatter gate, with a threshold on the PerCP-Cy5.5 channel, was used to identify T lymphocytes, followed by gating on CD4+ T cells. In some experiments, CD8+ staining was used to confirm that CD4+ T cells were not CD4+CD8+ double positive. Generally a minimum of 50 000 events were analyzed. Compensation was set initially by using lymphocytes stained with individual fluorochromes and checked with cells stained simultaneously for CD3-PerCP, CD4-PE-Cy7, CD8-APC-Cy7, CD56-APC, CD19-PE, and CD14-FITC (Becton Dickinson). Further controls included individual tubes in which allophycocyanin (APC), PE, or FITC antibodies were not included. Other monoclonal antibodies used included CD69-APC, CD56-APC, CD25-FITC, and CD161-PE (Becton Dickinson); CCR5-APC, CD45RA-APC, IL-12Rβ-PE, Perforin-FITC, and Granzyme A-FITC (Pharmingen); IL-18R-PE (R&D Systems, Minneapolis, MN) and Granzyme B-APC (Caltag, Burlingame, CA).
Cell separations
CD4+ cells were separated from PBMCs for transmission electron microscopy by positive isolation by using antibody-conjugated magnetic beads (Dynal Biotech, Oslo, Norway) as per manufacturer's instructions. The resulting fractions were more than 98% pure. Similarly, CD4+ and CD8+ cells were separated for CTL assays by using the Detachabead system (Dynal Biotech), and the resulting fractions were more than 95% pure.
CCR5+ and CCR5- CD4+ lymphocytes were purified on a high-speed FACSVantage cell sorter running FACSDiva software (Becton Dickinson). To preserve maximal CCR5 expression on CD4+ T lymphocytes prior to sorting, fresh sodium heparin-anticoagulated whole blood from healthy adults was centrifuged, and buffy coat leukocytes were collected. These cells were immediately stained with CD4-APC and CCR5-PE for 15 minutes at room temperature (RT), followed by lysis of erythrocytes by using 5 to 10 minutes of incubation in NH4Cl buffer at RT, and washed once. Sorted CCR5+ and CCR5- CD4+ cells were plated at 1 × 106/mL in 96-well microtiter plates, in which autologous PBMCs had been already cultured for 1 hour, followed by 3 vigorous washes to remove nonadherent cells, as a source of antigen-presenting cells. Cells were cultured for 6 days in the presence or absence of CMV lysate and analyzed for proliferation by pulsing for 18 hours with 3H-thymidine (as described in “Lymphocyte function assays”).
Transmission electron microscopy (TEM)
TEM was performed on uncultured and unstimulated healthy donor (control) and patient PBMCs and purified CD4+ T lymphocytes. Cells (2 × 106) were fixed in Karnowsky fixative overnight. The cells were postfixed in 2% buffered Osmium tetroxide followed by 2% aqueous uranyl acetate. Following dehydration in graded ethanol series, cell pellets were embedded in Sporr epoxy resin and polymerized at 70°C for 10 hours. Serial sections were cut at 0.5-micron thickness and stained with methylene blue. Ultrathin sections were cut on a Reichort Ultracut E ultramicrotome and stained with 2% uranyl acetate in 50% ethanol followed by Reynold lead citrate. Grids were examined in a Philips CM10 transmission electron microscope operated at 80 Kv.
Statistical analysis
Lymphocyte phenotyping results were expressed as a percentage of CD4 T lymphocytes. Results for each cohort were expressed as medians and interquartile ranges. The Mann-Whitney U test was performed to compare CMV-seropositive and CMV-seronegative subgroups within the healthy adult controls, using Statview v5.0 for Macintosh (Abacus Concepts, Berkeley, CA). A 2-sided P value < .05 was considered statistically significant.
Results
TCR V beta analysis of CD4 responses to HIV-1 p24 protein and 15-mer peptides in PBMCs from LTNP-100149
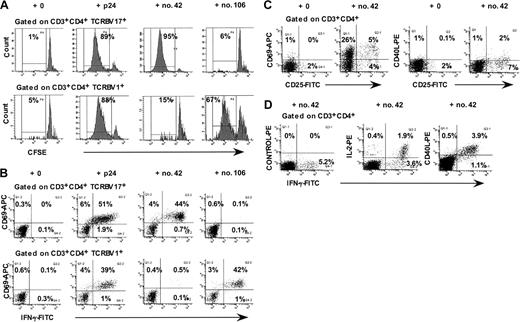
We had previously found that peptides no. 42 and no. 106 elicited large proliferative CD4+ T-cell responses for PBMCs from LTNP-100149, using CFSE-labeling and flow cytometric analysis.38 Figure 1A shows that CD4+ T cells expressing TCR V beta 17 (TCRBV17+) proliferated in response to peptide no. 42 but not to peptide no. 106. Conversely, CD4+ T lymphocytes expressing TCR V beta 1 (TCRBV 1+) proliferated in response to peptide no. 106 but not to peptide no. 42. Using flow cytometric analysis of intracellular interferon γ (IFN-γ) responses to individual peptides, we again found that TCRBV 17+ CD4+ T lymphocytes responded to peptide no. 42 (Figure 1B), whereas TCRBV 1+ CD4+ T lymphocytes responded to peptide no. 106. The results showed that up to 50% of the TCRBV 17+ CD4+ T lymphocytes in peripheral blood from LTNP-100149 responded specifically to peptide no. 42.
Responses of TCRBV 17+ CD4+ T lymphocytes from LTNP-100149 to HIV-1 p24 peptide no. 42. (A) Flow cytometry histograms, gated on CD3+CD4+ TCRBV 17+ and TCRBV1+ lymphocytes, respectively, showing proliferative responses of CFSE-labeled CD4+ T cells in response to p24, peptide no. 42, and peptide no. 106. (B) Flow cytometry histograms, gated on CD3+CD4+ TCRBV 17+ and TCRBV1+ lymphocytes, respectively, showing IFN-γ responses to p24, peptide no. 42, and peptide no. 106 in ICC assays. (C) Flow cytometry histograms, gated on CD3+CD4+ lymphocytes, showing cell surface expression of CD69, CD25, and CD40L after incubation of whole blood for 48 hours with peptide no. 42. (D) Flow cytometry histograms, gated on CD3+CD4+ lymphocytes, showing simultaneous intracellular production of IFN-γ, IL-2, and CD40L in ICC assays. Results are representative of at least 3 independent experiments, on different visits, over more than 1 year.
Responses of TCRBV 17+ CD4+ T lymphocytes from LTNP-100149 to HIV-1 p24 peptide no. 42. (A) Flow cytometry histograms, gated on CD3+CD4+ TCRBV 17+ and TCRBV1+ lymphocytes, respectively, showing proliferative responses of CFSE-labeled CD4+ T cells in response to p24, peptide no. 42, and peptide no. 106. (B) Flow cytometry histograms, gated on CD3+CD4+ TCRBV 17+ and TCRBV1+ lymphocytes, respectively, showing IFN-γ responses to p24, peptide no. 42, and peptide no. 106 in ICC assays. (C) Flow cytometry histograms, gated on CD3+CD4+ lymphocytes, showing cell surface expression of CD69, CD25, and CD40L after incubation of whole blood for 48 hours with peptide no. 42. (D) Flow cytometry histograms, gated on CD3+CD4+ lymphocytes, showing simultaneous intracellular production of IFN-γ, IL-2, and CD40L in ICC assays. Results are representative of at least 3 independent experiments, on different visits, over more than 1 year.
Furthermore, cell surface CD69 and CD25, but not CD40L, were up-regulated on CD4+ T lymphocytes at 40 to 48 hours in response to peptide no. 42 (Figure 1C), prior to commencement of proliferation on day 3. In addition, approximately one third of IFN-γ–producing CD4+ T cells also synthesized IL-2 in response to HIV-1 p24 (Figure 1D). We also detected production of CD40L intracellularly, in the presence of Brefeldin A in the intracellular cytokine assay (Figure 1D). This finding contrasted with the fact that we were consistently unable to detect cell surface CD40L in response to p24 at the same time point (6 hours), whereas CD40L cell surface expression was readily up-regulated in response to polyclonal mitogens, including phytohemagglutinin (PHA) and staphylococcal enterotoxin B (SEB) (data not shown).
Furthermore, the addition of purified blocking monoclonal antibodies to HLA-DR, -DP, and -DQ (10 μg/mL final concentration; kind gift from Prof John Elliott, Edmonton) inhibited IFN-γ and IL-2 production and proliferation in response to peptide no. 42 by an average of 47%, 79%, and 86%, respectively (data not shown).
Immunophenotype of circulating TCRBV 17+ CD4+ T lymphocytes from LTNP-100149
Approximately 12% of CD4+ T lymphocytes in peripheral blood from LTNP-100149 were TCRBV 17+, which is substantially greater than the proportion we have previously observed in healthy adults (median, 6%; range, 3%-7%; n = 12).44 As noted earlier, up to 50% of these circulating TCRBV 17+ CD4+ T lymphocytes in LTNP-100149 produced IFN-γ in response to peptide no. 42. A detailed phenotype of TCRBV 17+ CD4+ T lymphocytes in LTNP-100149 is shown in Table 1.
Expression of T lymphocyte cell surface and intracellular proteins on TCRBV17+ CD4+ T cells from LTNP-100149
Marker . | Designation . | % of CD4+ TCRBV17+ . | Marker . | Designation . | % of CD4+ TCRBV17+ . |
|---|---|---|---|---|---|
| CD45RA | Naive | 7 | CCR5 | HIV coreceptor | 77 |
| CD45RO | Memory | 93 | CXCR4 | HIV coreceptor | 10 |
| CD62L | Trafficking | 36 | CD56 | NK/CTL | < 1 |
| CCR7 | Trafficking | 8 | CD94 | NK/CTL | < 1 |
| CD27 | Differentiation | 17 | CD158a | NK/CTL | < 1 |
| CD28 | Co-stimulation | 98 | CD158b | NK/CTL | < 1 |
| CD38 | Activation | 73 | CD161 | NK/CTL | 88 |
| HLA-DR | Activation | 62 | CD244 (C1.7/2B4) | NK/CTL | 75 |
| CD95 | Apoptosis | 89 | TIA-1 | NK/CTL | 88 |
| CD11a (high) | Adhesion | 89 | Perforin | NK/CTL | 2 |
| CD57 | Differentiation | < 1 | Granzyme A | NK/CTL | 73 |
| CD127 | IL-7Rα | 93 | Granzyme B | NK/CTL | 41 |
| CD25 | IL-2Rα | 2.2 | TCR Vα24 | NKT | < 1 |
| CD122 | IL-2Rβ | < 1 | Ki-67 | Proliferation | 2.4 |
| IL-12Rb | 87 | Bcl-2 dim | Apoptosis | 11 | |
| IL-18R | 91 | CD8 | Suppressor/CTL | 2 | |
| TCR zeta | TCR signaling | 98 |
Marker . | Designation . | % of CD4+ TCRBV17+ . | Marker . | Designation . | % of CD4+ TCRBV17+ . |
|---|---|---|---|---|---|
| CD45RA | Naive | 7 | CCR5 | HIV coreceptor | 77 |
| CD45RO | Memory | 93 | CXCR4 | HIV coreceptor | 10 |
| CD62L | Trafficking | 36 | CD56 | NK/CTL | < 1 |
| CCR7 | Trafficking | 8 | CD94 | NK/CTL | < 1 |
| CD27 | Differentiation | 17 | CD158a | NK/CTL | < 1 |
| CD28 | Co-stimulation | 98 | CD158b | NK/CTL | < 1 |
| CD38 | Activation | 73 | CD161 | NK/CTL | 88 |
| HLA-DR | Activation | 62 | CD244 (C1.7/2B4) | NK/CTL | 75 |
| CD95 | Apoptosis | 89 | TIA-1 | NK/CTL | 88 |
| CD11a (high) | Adhesion | 89 | Perforin | NK/CTL | 2 |
| CD57 | Differentiation | < 1 | Granzyme A | NK/CTL | 73 |
| CD127 | IL-7Rα | 93 | Granzyme B | NK/CTL | 41 |
| CD25 | IL-2Rα | 2.2 | TCR Vα24 | NKT | < 1 |
| CD122 | IL-2Rβ | < 1 | Ki-67 | Proliferation | 2.4 |
| IL-12Rb | 87 | Bcl-2 dim | Apoptosis | 11 | |
| IL-18R | 91 | CD8 | Suppressor/CTL | 2 | |
| TCR zeta | TCR signaling | 98 |
Strikingly, 77% of TCRBV 17+ CD4+ T lymphocytes in LTNP-100149 were CCR5+ (Table 1). Furthermore, 88% of TCRBV 17+ CD4+ T lymphocytes in LTNP-100149 expressed the marker TIA-1 (GMP-17), a component of granules found in cytotoxic T lymphocytes.45 In contrast, TIA-1 is expressed at a much lower level in CD4+ T lymphocytes from healthy adults (median, 7%; range, 2%-18%; n = 1534; and further in this section). Additional markers of CTL were also found, including granzyme A, CD161, and CD244. However, intracellular levels of granzyme B immediately ex vivo were slightly lower, and perforin expression was much lower (Table 1), whereas other markers of natural killer (NK) cells and/or CTLs, including CD56, CD94, CD122, CD158a, CD158b, and TCR V alpha 24, were absent. Receptors for IL-12 and IL-18 were also highly expressed, consistent with a Th1 phenotype (Table 1).
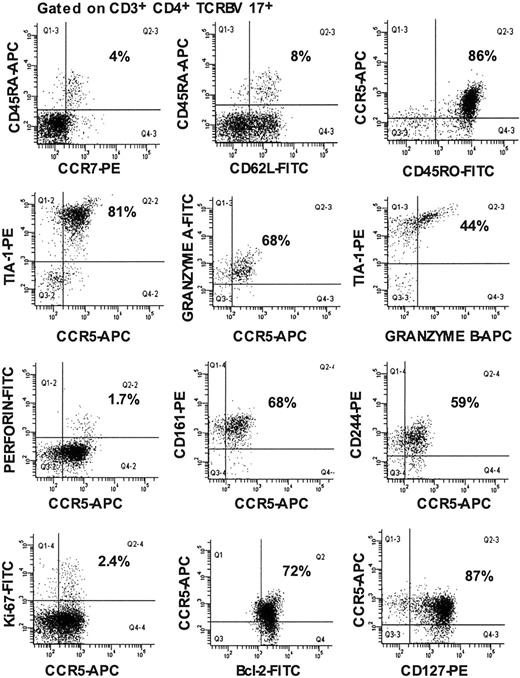
Using 5-color flow cytometry, with increased sensitivity, we found that more than 80% of the TCRBV 17+ CD4+ T lymphocytes in LTNP-100149 were of the CD45RA-CD45RO+, CCR7–memory effector cell phenotype, leaving only a small proportion as phenotypically naive CD45RA+CD62L+ cells (Figure 2). Furthermore, more than 80% of TCRBV 17+ CD4+ T lymphocytes in LTNP-100149 mutually coexpressed CCR5+ and TIA-1+. Similarly, TCRBV 1+ CD4+ T lymphocytes, which contained peptide no. 106-specific cells, were found to highly express CCR5 and granzyme A (data not shown).
Immunophenotyping of TCRBV 17+ CD4+ T lymphocytes from LTNP-100149. Flow cytometry histograms, all gated on CD3+CD4+ TCRBV 17+ lymphocytes, showing coexpression of CCR5 and CTL markers on cells in fresh peripheral blood. Results are representative from at least 3 visits over 9 months. Percentages are of gated populations, as indicated.
Immunophenotyping of TCRBV 17+ CD4+ T lymphocytes from LTNP-100149. Flow cytometry histograms, all gated on CD3+CD4+ TCRBV 17+ lymphocytes, showing coexpression of CCR5 and CTL markers on cells in fresh peripheral blood. Results are representative from at least 3 visits over 9 months. Percentages are of gated populations, as indicated.
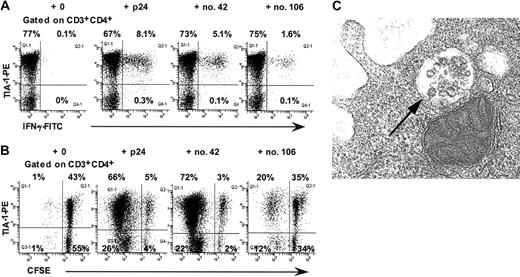
In intracellular cytokine assays, virtually all p24-, peptide no. 42-, and peptide no. 106-specific IFN-γ+ CD4+ T lymphocytes in LTNP-100149 were TIA-1+ (Figure 3A). Taken together with the immunophenotyping results, the intracellular cytokine results demonstrate that peptide no. 42-specific TCRBV 17+ CD4+ T lymphocytes coexpressed CCR5+ and TIA-1+ immediately ex vivo. However, we were unable to demonstrate directly that peptide no. 42-specific IFN-γ+ CD4+ T lymphocytes were CCR5+, because the expression of CCR5 was greatly reduced when cells were incubated in the presence of antigen (data not shown).
Presence of cytotoxic granules in CD4+ T lymphocytes from LTNP-100149. (A) Flow cytometry histograms, gated on CD3+CD4+ lymphocytes, show production of IFN-γ by TIA-1+ CD4+ T cells in response to p24, peptide no. 42, and peptide no. 106 in ICC. (B) Flow cytometry histograms, gated on CD3+CD4+ lymphocytes, show proliferation of CFSE-labeled TIA-1+ CD4+ T cells in response to p24, peptide no. 42, and peptide no. 106. Results are representative of at least 3 experiments over 9 months. (C) TEM showing morphology of granules within some vesicles in purified CD4+ T cells from LTNP-100149. Magnification × 74 000.
Presence of cytotoxic granules in CD4+ T lymphocytes from LTNP-100149. (A) Flow cytometry histograms, gated on CD3+CD4+ lymphocytes, show production of IFN-γ by TIA-1+ CD4+ T cells in response to p24, peptide no. 42, and peptide no. 106 in ICC. (B) Flow cytometry histograms, gated on CD3+CD4+ lymphocytes, show proliferation of CFSE-labeled TIA-1+ CD4+ T cells in response to p24, peptide no. 42, and peptide no. 106. Results are representative of at least 3 experiments over 9 months. (C) TEM showing morphology of granules within some vesicles in purified CD4+ T cells from LTNP-100149. Magnification × 74 000.
Furthermore, when PBMCs were cultured with p24, peptide no. 42, or peptide no. 106, proliferating cells at day 6 were TIA-1+ (Figure 3B). Again, however, CCR5 could not be found on these cells, consistent with down-regulation in the presence of antigen (data not shown).
Cytotoxic granule morphology and cytotoxic activity of circulating CD4+ T lymphocytes from LTNP-100149
The high granule content of peripheral blood lymphocytes from LTNP-100149 was also observed morphologically. On the blood film, 60% of lymphocytes were large granular lymphocytes, characterized by a small nucleus with plentiful weakly basophilic cytoplasm, and prominent azurophilic granules (not shown). The morphology of cytotoxic granules was also studied using TEM of highly purified CD4+ T cells, from LTNP-100149 (Figure 3C). Ultrastructural examination of these granules showed that they were enclosed in a membrane bound structure, were always present close to the plasma membrane, and comprised 25 to 50 granules per vesicle. In addition to these vesicular structures packed with granules, electron dense cores were also seen in the cytoplasm (not shown). Approximately 1 in 4 purified CD4+ T cells from LTNP-100149 appeared to contain such granules, compared with 1 in 100 purified CD4+ T cells from control subjects, in which fewer (10-15) granules were seen per vesicle (not shown).
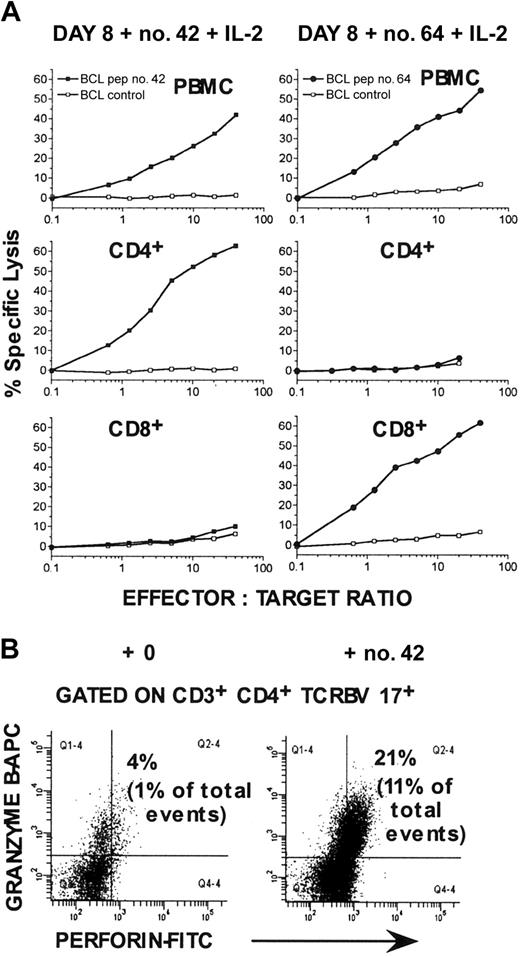
In assays of the cytotoxic activity of PBMCs from LTNP-100149, no significant cytotoxicity was observed immediately ex vivo. However, cytotoxicity was readily detected after culture of PBMCs for 8 to 10 days with individual Gag peptides plus IL-2. (Figure 4A). High purity positive isolation of CD4+ and CD8+ cells before CTL assay demonstrated that peptide no. 42-specific CTLs were confined to the CD4+ T-cell population (Figure 4A). Conversely, purified CD8+ cells lysed peptide no. 64-pulsed target cells, but not peptide no. 42-pulsed target cells after appropriate culture (Figure 4A). Under the same conditions, the relative proportion of TCRBV 17+ CD4+ T lymphocytes containing both granzyme B and perforin was greatly increased (Figure 4B).
Cytotoxic activity of PBMCs from LTNP-100149, stimulated with peptide no. 42. (A) Chromium release assays were performed after incubating PBMCs with peptide no. 42 or peptide no. 64 plus IL-2 for 8 days, against autologous BCL targets labeled with 100 μCi (3.7 MBq) 51Cr and simultaneously pulsed with or without peptide no. 42 or no. 64. CD4+ and CD8+ cells were also purified after culture and assayed against appropriate target cells. Cultured cells were added to target cells at the indicated effector-to-target cell ratios. Results are representative of experiments on at least 2 visits. (B) Flow cytometry histograms, gated on TCRBV 17+ CD3+CD4+ lymphocytes, showing increased expression of granzyme B and perforin after incubation with peptide no. 42 plus IL-2 for 8 days.
Cytotoxic activity of PBMCs from LTNP-100149, stimulated with peptide no. 42. (A) Chromium release assays were performed after incubating PBMCs with peptide no. 42 or peptide no. 64 plus IL-2 for 8 days, against autologous BCL targets labeled with 100 μCi (3.7 MBq) 51Cr and simultaneously pulsed with or without peptide no. 42 or no. 64. CD4+ and CD8+ cells were also purified after culture and assayed against appropriate target cells. Cultured cells were added to target cells at the indicated effector-to-target cell ratios. Results are representative of experiments on at least 2 visits. (B) Flow cytometry histograms, gated on TCRBV 17+ CD3+CD4+ lymphocytes, showing increased expression of granzyme B and perforin after incubation with peptide no. 42 plus IL-2 for 8 days.
CMV-specific CD4+ T lymphocytes in HIV-uninfected controls
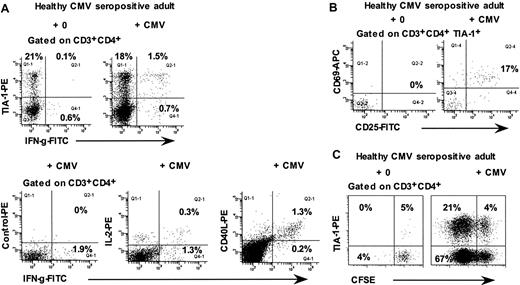
Because previous results33,34 had suggested that some CMV-specific CD4+ T cells were also cytotoxic T lymphocytes, we used the ICC assay to investigate the phenotype of CMV-specific CD4+ T cells in 5 healthy CMV-seropositive adults. The results show that approximately 60% to 70% of interferon-γ–producing CD4+ T cells were TIA-1+ (Figure 5A). Conversely, after 48 hours culture of whole blood with CMV lysate, approximately 20% of TIA-1+ CD4+ T cells expressed both CD69+ and CD25+, consistent with antigen-specific activation (Figure 5B). Also, in 6-day cultures of CFSE-labeled PBMCs with CMV lysate, TIA-1+ cells were found among the proliferating CD4+ T cells but constituted a minority of these cells (Figure 5C; range, 3%-30% of proliferating CD4+ T lymphocytes from 3 different individuals).
Responses of CD4+ T lymphocytes from CMV-seropositive healthy adults to CMV lysate. (A) Flow cytometry histograms, gated on CD3+CD4+ lymphocytes, showing intracellular IFN-γ production by the TIA-1+ subset in response to CMV lysate. (B) Flow cytometry histograms, gated on TIA-1+ CD3+CD4+ lymphocytes, showing expression of CD69 and CD25 after incubation of whole blood for 48 hours with CMV lysate. (C) Flow cytometry histograms, gated on CD3+CD4+ lymphocytes, showing proliferation of TIA-1+ cells in response to CMV lysate. Results are representative of 3 to 4 experiments.
Responses of CD4+ T lymphocytes from CMV-seropositive healthy adults to CMV lysate. (A) Flow cytometry histograms, gated on CD3+CD4+ lymphocytes, showing intracellular IFN-γ production by the TIA-1+ subset in response to CMV lysate. (B) Flow cytometry histograms, gated on TIA-1+ CD3+CD4+ lymphocytes, showing expression of CD69 and CD25 after incubation of whole blood for 48 hours with CMV lysate. (C) Flow cytometry histograms, gated on CD3+CD4+ lymphocytes, showing proliferation of TIA-1+ cells in response to CMV lysate. Results are representative of 3 to 4 experiments.
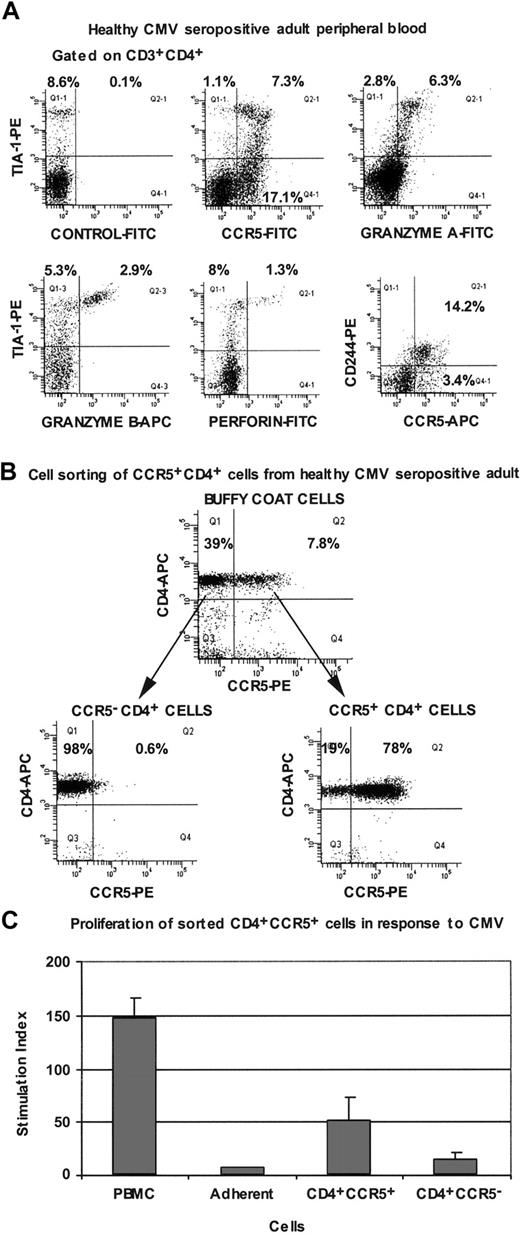
In peripheral blood from healthy CMV-seropositive adults, we also observed a subset of CD4+ T cells which were CCR5+, TIA-1+, granzyme A+, and CD244+, with lower expression of granzyme B and perforin (Figure 6A).
Immunophenotyping of peripheral blood CD4+ T lymphocytes from CMV-seropositive healthy adults. (A) Flow cytometry histograms are all gated on CD3+CD4+ lymphocytes and are representative of results obtained for at least 4 different individuals. (B) Flow cytometry histograms, gated on lymphocytes, showing cell sorting of CCR5+ and CCR5- subpopulations of CD4+ cells from buffy coats. (C) Proliferation of PBMCs and purified CCR5+ and CCR5- CD4+ T lymphocytes from CMV-seropositive healthy adults, in response to CMV lysate. Error bars show SD for duplicates, and cpm in unstimulated cultures ranged from 600 to 1200. Results are representative of 3 independent experiments.
Immunophenotyping of peripheral blood CD4+ T lymphocytes from CMV-seropositive healthy adults. (A) Flow cytometry histograms are all gated on CD3+CD4+ lymphocytes and are representative of results obtained for at least 4 different individuals. (B) Flow cytometry histograms, gated on lymphocytes, showing cell sorting of CCR5+ and CCR5- subpopulations of CD4+ cells from buffy coats. (C) Proliferation of PBMCs and purified CCR5+ and CCR5- CD4+ T lymphocytes from CMV-seropositive healthy adults, in response to CMV lysate. Error bars show SD for duplicates, and cpm in unstimulated cultures ranged from 600 to 1200. Results are representative of 3 independent experiments.
Because CCR5 expression was greatly reduced when either whole blood or PBMCs were cultured with antigen (see “Immunophenotype of circulating TCRBV 17+ CD4+ T lymphocytes from LTNP-100149”), CD4+ T cells from HIV-uninfected, CMV-seropositive individuals were first sorted on the basis of CCR5 expression (Figure 6B), and then separated populations were cultured in the presence of autologous adherent cells and CMV lysate. Proliferation measured after 7 days showed that the CCR5+ CD4+ cells contained CMV-specific memory cells (Figure 6C).
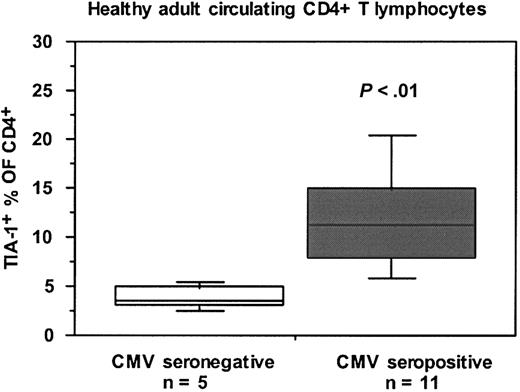
TIA-1+ CD4 T lymphocytes are elevated in CMV-seropositive individuals
We noted a wide variation in the proportion of CD4+ T lymphocytes which were TIA-1+ between different HIV-uninfected individuals. Figure 7 shows that the proportion of CD4+ T lymphocytes which were TIA-1+ was significantly higher in CMV-seropositive, HIV-uninfected control subjects compared with CMV-seronegative, HIV-uninfected control subjects. It should be noted that LTNP-100149 is CMV seropositive but exhibits only a modest proliferative response to CMV lysate in vitro.38
Proportions of TIA-1+ subset of CD4+ T lymphocytes in CMV-seropositive and CMV-seronegative healthy adults. Box plots show median, interquartile range, and 10th and 90th percentiles for each cohort (error bars). The number of subjects in each subgroup is shown, and the P value was determined by Mann-Whitney nonparametric comparison of the 2 subgroups.
Proportions of TIA-1+ subset of CD4+ T lymphocytes in CMV-seropositive and CMV-seronegative healthy adults. Box plots show median, interquartile range, and 10th and 90th percentiles for each cohort (error bars). The number of subjects in each subgroup is shown, and the P value was determined by Mann-Whitney nonparametric comparison of the 2 subgroups.
Discussion
Characterization of a large population of HIV-1 antigen-specific CD4+ T lymphocytes from a unique LTNP has allowed detailed phenotypic analysis of these cells for the first time. Importantly, the results clearly showed that the HIV-1 coreceptor CCR5 was expressed on p24-specific CD4+ T cells. The phenotype of these cells suggests that they represent an expansion of a corresponding subset which we also found in CMV-seropositive healthy adults. The LTNP in this study has exhibited completely undetectable plasma viral load over many years without therapy38 and is highly unusual in terms of the stability of HIV-1 envelope sequences,39 suggesting a virtual absence of viral replication in vivo. Taken together, these results strongly suggest that the HIV antigen-specific CD4+ T lymphocytes would ordinarily have been highly susceptible to HIV-1 infection but have been spared in the case of this LTNP through the absence of viral replication. In support of this conclusion, we have previously shown that isolated CD4+ T cells from LTNP-100149 can be infected in vitro with a CXCR4-tropic laboratory strain and a dual-tropic primary isolate of HIV-1, although there was relatively reduced infection with the CCR5-tropic strain, Bal, suggesting the further possibility of a postentry block to replication.38 Similarly, we have previously found that antigen-specific proliferative responses are present in individuals infected with attenuated nef/LTR-deleted HIV-1,37 and this is associated with increased levels of circulating memory phenotype CD4+ T cells.41
Immune control of HIV-1 replication has been associated with the presence of HIV-1–specific CD4+ proliferative responses in LTNP7 and in patients treated early during PHI.46,47 This has been interpreted mainly in terms of help for CD8 CTLs.48 Genetic studies in mice and humans have shown that CD4 helper T cells express CD154 (CD40L) which interacts with CD40 on B cells to initiate affinity maturation and immunoglobulin class-switching.49 Similarly, CD40L+ CD4 helper T cells license CD40+ antigen-presenting cells (APCs) to help CD8+ CTL,50-52 thus facilitating the expansion of rare antigen-specific naive CD8+ T cells to a relatively large number of memory cells capable of secondary responses.53-55 The HIV-specific CD4+ T cells identified in this study did not exhibit cell surface expression of CD40L, but we were able to detect early production of CD40L by using the ICC assay. We have previously found persisting high-titer neutralizing antibody levels and potent antigen-stimulated antiviral activity in CD8+ T cells in LTNP-100149,38 suggesting robust CD4+ helper activity.
The phenotypic analysis of TCRBV 17+ p24-specific CD4+ T lymphocytes also revealed the expression of markers associated with CTLs, especially the cytotoxic granule protein TIA-1/GMP-17/NKG7,45,56 as well as granzyme A and granzyme B, which are important in CTL function.57,58 Other markers, including CD161 and CD244, are consistent with the CTL phenotype, because these molecules have been demonstrated on NK cells and CD8+ CTLs and reportedly activate cytotoxic function.59,60 However, the HIV-specific CD4+ T cells did not express TCR Vα 24 which defines NKT cells,61 or other markers characteristic of NK cells or CD8+ CTLs. Furthermore, we were able to confirm the presence of granules morphologically and directly demonstrated the development of specific cytotoxicity by CD4+, but not CD8+, cells following culture with peptide plus IL-2.
Several reports have described the in vitro growth of antigen-specific CD4+ T cell clones with cytotoxic activity, from individuals with HIV-1,62,63 EBV,30,64 VZV,65 poliovirus,66 and measles67 infections. Other studies have isolated CD4+ CTL clones from HIV-seronegative individuals immunized with recombinant gp160.68,69 However, there was always the possibility that the cytotoxic activity was the result of prolonged culture in vitro. The current study shows that HIV-specific CD4+ T cells exhibited characteristics of CTL ex vivo, albeit with low perforin expression which could be up-regulated in vitro. Previous studies have also described cytotoxicity mediated by CD4+ T cells via interaction with gp120 on infected cells,70 but this activity did not appear to involve HLA class II presentation nor the perforin/granzyme pathway, as reported here. Our results confirm and extend previous reports which showed that purified CD4+CD8dim PBMCs were able to lyse CMV-infected target cells in vitro,33 as well as our previous description of expression of perforin in some CMV-specific CD4+ T cells.34 It must be noted that the antigen-specific CD4+ T cells from LTNP-100149 were clearly CD8- and inhibited by blocking of HLA class II. Overall, therefore, it is possible that inducible cytotoxicity is a major function of these cells in vivo.
The generation of antigen-specific CCR5+ CD4+ CTLs may be most evident during primary viral infection. We previously found that the CCR5+ subpopulation of peripheral blood CD4+ T cells was proportionally slightly increased in PHI and greatly increased during acute EBV infection, because of a high proliferation rate.12,43 In another study, we reported that an unusual subpopulation of CD4+ T lymphocytes which expressed perforin was also increased in acute and chronic HIV-1 infection, as well as in acute EBV infection.34 The results described here suggest that these 2 subpopulations of CD4+ T cells coincide. However, our previous results,34 as well as the current study, suggest that not all granule-containing CD4+ T cells contained perforin in vivo. These results are consistent with previous reports that HIV-antigen tetramer+ CD8+ CTL have low amounts of perforin,71 despite the prevalence of granzyme A.72,73 Nevertheless, we showed in the earlier study34 that cytotoxic activity of freshly isolated CD4+ T cells could be detected in vitro and was inhibited by concanamycin A, consistent with a perforin-mediated mechanism. In preliminary studies, we have found that TIA-1+ cells, as a proportion of CD4+ T cells, are elevated during primary HIV-1 infection and are also greatly elevated during acute EBV infection (data not shown). Therefore, further scrutiny of CCR5+ CD4+ CTLs during primary viral infections is warranted.
The origin of these CD4+ CTLs in LTNP-100149 at such a high level remains unclear, because they do not appear to be proliferating at a high rate. Furthermore, they contain normal intracellular levels of Bcl-2 and highly express the IL-7 receptor, suggesting a stable resting population with relatively low turnover. These results contrast with our earlier studies showing that CCR5+ T lymphocytes have increased turnover during primary12,43 and untreated chronic HIV-1 infection (Zaunders et al, unpublished data, 2001). However, the antigen-specific CD4+ T cells from LTNP-100149 also expressed activation antigens, which is reminiscent of CD4+ and CD8+ antigen-specific T cells found in peripheral tissues in mice.74 LTNP-100149 had at least one other large TCRBV expansion, which was neither HIV-1 nor CMV-specific, and was CD28- (data not shown), suggesting a propensity toward such accumulations in that individual's immune system.
CCR5+ CD4+ T lymphocytes in healthy adults comprised 2 distinct subsets, with either a cytotoxic or noncytotoxic phenotype; both subsets appeared to contain CMV-specific memory cells that were capable of proliferation. Also, the expanded p24-specific CD4+ T cells in LTNP-100149 were CCR7-negative, which would suggest that they were terminally differentiated effector cells rather than central memory CD4+ T lymphocytes.14 Yet these CCR7-negative CD4+ T cells were clearly capable of proliferation in vitro and up-regulated granzyme B and perforin. Furthermore, these cells retained expression of the costimulatory molecule, CD28, which is important in interaction with antigen-presenting cells, but is usually absent in TCRBV expansions.75 How such cells may encounter professional antigen-presenting cells in vivo without the guidance of cell surface CCR721 remains unclear. The possibility exists that what we have seen is an aberrant expansion of CD4+ T cells, but the similarity to a smaller corresponding subset in healthy adults would argue against this.
It has recently been reported that CMV-specific CD4+ T-cell responses were associated with better clinical outcome during primary CMV infection in adults.76 Control of CMV infection during progressive HIV-1 infection is lost with profound decreases in CD4 T-cell counts and is regained following highly active antiretroviral therapy (HAART),77-79 whereas CMV-specific CD8 are relatively unchanged, at least in number,80 and CD8 CTL activity in general may be relatively preserved.81 Furthermore, EBV- and CMV-infected cells express proteins that interfere with HLA class II presentation, in addition to viral effects on class I presentation,82 which may be involved in evasion from the immune system. The results reported here support our previous proposal that CD4+ CTLs contribute to control of viruses which infect HLA class II-bearing cells.34 Interestingly, there has been one report of mutations of HIV-1 within a CD4 epitope, suggesting that there was sufficient pressure to drive the appearance of escape variants.83 It should be noted that the CD4 epitope contained within peptide no. 42 is in a very highly conserved region of p2484 and has been reported as a CD4 epitope recognized by CD4+ T cells from 2 other LTNPs with very high proliferative responses.7
Further studies are required to extend these results to other HIV-infected individuals, particularly during primary infection. The issue of heterogeneity of CCR5+ CD4+ T lymphocytes may also be particularly relevant to HIV-1 infection, because it has recently been reported that a gut-homing
Prepublished online as Blood First Edition Paper, November 26, 2003; DOI 10.1182/blood-2003-08-2765.
Supported by the National Centre in HIV Virology Research (W.B.D.) and partially supported by a grant from the Japan Health Sciences Foundation (A.D.K. and D.A.C.). The National Centre in HIV Epidemiology and Clinical Research is supported by the Commonwealth Department of Health and Ageing through the Australian National Council on AIDS, Hepatitis C and Related Diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the study subject and volunteers for their commitment to this project, as well as the NIH Reference Reagents Program for provision of HIV-1 overlapping Gag peptides, Jenny Bryant for assistance with flow cytometry, and ARCBS nursing staff members for phlebotomy services.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal