Abstract
Stroke is a devastating complication of sickle cell anemia (SCA), affecting up to 30% of children with the disease. Despite the relative frequency of stroke in SCA, few predictors of risk exist. Because stroke in SCA is likely a multifactorial disease, analysis of the combined effect of multiple genetic variants may prove more successful than evaluation of individual candidate genes. We genotyped 230 children with SCA for 104 polymorphisms among 65 candidate vascular genes to identify risk associations with stroke. Patients were phenotyped based on magnetic resonance imaging/angiography (MRI/MRA) findings into large-vessel (LV) versus small-vessel (SV) disease stroke subgroups. Specific polymorphisms in the IL4R 503, TNF (-308), and ADRB2 27 genes were independently associated with stroke susceptibility in the LV stroke subgroup, while variants in the VCAM1 (-1594) and LDLR NcoI genes were associated with SV stroke risk. The combination of TNF (-308)GG homozygosity and the IL4R 503P variant carrier status was associated with a particularly strong predisposition to LV stroke (odds ratio [OR] = 5.5; 95% confidence interval [CI] = 2.3-13.1). We show that several candidate genes may play a role in predisposition to specific stroke subtypes in children with SCA. If confirmed, these results provide a basis for population screening and targeted intervention to prevent stroke in SCA.
Introduction
Stroke is a devastating complication in children with sickle cell anemia (SCA), manifested clinically in 11%1 and as silent cerebral infarction by magnetic resonance imaging (MRI) in another 17% to 22%.2-4 Well-documented risk factors for ischemic stroke in SCA include prior transient ischemic attack (TIA), low steady-state hemoglobin (Hb) levels, high leukocyte counts, hypertension, and a history of acute chest syndrome.1 However, the etiology of stroke in SCA remains unclear. Aside from the absence of lipid deposition and plaque formation, many of the histopathologic findings in cerebrovascular lesions in SCA resemble those found in stroke patients in the general population.5,6 In fact, atherosclerotic stroke is now believed to be a result of chronic inflammation, involving pathways of immune regulation, thrombosis, and cellular adhesion.7,8 These same pathways are likely to contribute to stroke in SCA.
Increasing evidence from epidemiologic, twin, and animal model studies suggest a genetic contribution to the development of stroke in the general population, and multiple genes have been investigated for stroke risk in SCA.9 Driscoll et al recently documented an increased risk of stroke in siblings with SCA.10 Specific β-globin haplotype associations have been shown in some studies, but not others.11,12 Coinherited alpha thalassemia has also been reported to be a protective factor.13,14
We have documented HLA associations with specific stroke subtypes.15 In a recent study of single nucleotide polymorphisms (SNPs) within the VCAM1 gene locus, Taylor et al identified a variant that appears to be protective.16 Other studies have reported no association with prothrombotic polymorphisms.17-22
Because the etiology of stroke is most likely influenced by many genes, each with only modest effects, analysis of the combined effect of multiple genetic variants may prove more successful than evaluation of individual candidate genes. We previously examined the contribution of 36 candidate genes to stroke risk in SCA in a small pilot institutional study of children with and without MRI-documented cerebral infarction.23 To extend these initial results, we surveyed 104 polymorphic markers among 65 candidate atherosclerotic, prothrombotic, and proinflammatory genes in a large, well-characterized population of children enrolled in the Cooperative Study of Sickle Cell Disease (CSSCD).
Patients, materials, and methods
Study patients and design
The CSSCD is a national, multicenter study designed to define the natural history of sickle cell disease (SCD) by following more than 4000 patients with SCD.24 Objectives, eligibility requirements, enrollment procedures, and data collection methods for the CSSCD have been previously reported.24-26 As part of this study, the CSSCD initiated a prospective newborn cohort trial in 1978 into which more than 400 infants were enrolled at birth. These children were followed for more than 13 years and had an average age of 13.1 ± 2.9 years at the end of the study. Children in this cohort underwent brain MRI scanning at age 6 and then every 2 years thereafter, as part of phase 2 of the CSSCD study. Because many children were older than 6 years at the time that brain MRI studies were introduced as part of the study, the mean age for the first MRI study and during follow-up studies was 8.3 years and 12.1 years, respectively.27 Complete MRI histories were available on 266 SCA children from this cohort. Of these, 230 had adequate DNA and were included in this study. Children with SCA (homozygous Hb S) and MRI-documented cerebral infarction (asymptomatic or symptomatic) were included as “case” subjects. Children with MRI evidence of atrophy or isolated cerebral hemorrhage without evidence of preexisting infarction were excluded. MRI-positive patients were subclassified as having large vessel (LV) or small vessel (SV) disease using an algorithm based on MRI/magnetic resonance angiography (MRA) findings, infarct size, and location.15 Because LV disease is the primary cause of clinical stroke, patients with LV disease, with or without SV disease, were classified as LV stroke. Using the definition of cerebrovascular disease previously described by the CSSCD, MRI-positive patients were also characterized by a history of clinical stroke.1 Children from the CSSCD newborn cohort who had a normal MRI at the age of 10 years or older were included as “control” subjects. This minimized the possibility of including in the control group a child who might subsequently develop stroke. Patient identities were blinded and patient samples were collected and stored with a study number previously assigned by the CSSCD. The present study was reviewed and approved by the institutional review board of Children's Hospital Oakland.
MRI scanning
The imaging systems and techniques used at each center for the brain MRI scan and the procedures for review of MRI images have been previously described.2 In most cases, MRI scanning was performed on a 1.5-T MRI scanner. A few centers used a 0.6- or 1.0-T scanner. Criteria for acceptable images were established by the study neuroradiologists and included noncontrast spin-echo T1-weighted pulse sequence (short repetition time [TR], short echo time [TE]) and a T2-weighted axial spin-echo sequence (long TR, long TE) with an intermediate (long TR, short TE) axial and coronal T2 image. All MRIs were reviewed centrally by 3 neuroradiologists blinded to patient clinical history and genotyping results. There were 2 neuroradiologists who read each MRI scan independently and recorded interpretations on a standardized form. If the interpretations differed, consensus was reached in discussion with the third neuroradiologist. This study included results from all MRI scans performed on eligible patients.
Multilocus genotyping assay
A total of 104 polymorphisms among 65 genes were examined, representing various pathways implicated in the development of vascular disease (Table 1).
Candidate gene polymorphisms assessed
Locus . | Marker . | rs no. . |
|---|---|---|
| Lipid metabolism | ||
| ADRB3 | W64R | 4994 |
| APOA4 | T347S | 675 |
| Q360H | 5110 | |
| APOB | T711 | 1367117 |
| R3500Q | 5742904 | |
| APOC3 | -641C>A | 2542052 |
| -482C>T | 2854117 | |
| -455T>C | 2854116 | |
| 1100C>T | 4520 | |
| 3175C>G | 5128 | |
| 3206T>G | 4225 | |
| APOE | C112R | 429358 |
| R158C | 7412 | |
| CETP | -629C>A | 1800775 |
| I405V | 5882 | |
| D442G | 2303790 | |
| LDLR | Ncol+/- | 5742911 |
| LIPC | -480C>T | 1800588 |
| LPA | 93C>T | 1652503 |
| 121G>A | 1800769 | |
| LPL | -93T>G | 1800590 |
| D9N | 1801177 | |
| N291S | 268 | |
| S447X | 328 | |
| PON1 | M55L | 3202100 |
| Q192R | 662 | |
| PON2 | S311C | 7493 |
| PPARG | P12A | 1801282 |
| Cellular adhesion | ||
| ICAM1 | K56M | 5491 |
| G241R | 1799969 | |
| SELE | S128R | 5361 |
| L554F | 5355 | |
| SELP | S330N | 6131 |
| V640L | 6133 | |
| VCAM1 | -1594T>C | 1041163 |
| Inflammation | ||
| C3 | R102G | 2230199 |
| C5 | I802V | 17611 |
| CCR2 | V62I | 1799864 |
| CCR3 | P39L | 5742906 |
| CCR5 | wt/Δ580-611 | 333 |
| -2454G>A | 1799987 | |
| CD14 | -260C>T | 2569190 |
| CSF2 | I117T | 25882 |
| CTLA4 | -318C>T | 5742909 |
| T17A | 231775 | |
| FCERB1 | E237G | 569108 |
| GC | E416D | 7041 |
| T420K | 4588 | |
| IL1A | -889T>C | 1800587 |
| IL1B | -1418C>T | 16944 |
| F105F | 1143634 | |
| IL4 | -590C>T | 2243250 |
| IL4R | I75V | 1805010 |
| S503P | 1805015 | |
| Q576R | 1801275 | |
| IL5RA | -80G>A | 2290608 |
| IL6 | -572G>C | 1800796 |
| -174G>C | 1800795 | |
| IL9 | T113M | 2069885 |
| IL10 | -571C>A | 1800872 |
| IL13 | 4045C>T | 1295686 |
| Inflammation | ||
| LTA | T26N | 1041981 |
| 252A>G | 909253 | |
| LTC4S | -444A>C | 730012 |
| MMP3 | -1171A5>A6 | 3025058 |
| SCYA11 | A23T | 3744508 |
| -1328G>A | 4795895 | |
| SDF1 | +800 G>A | 1801157 |
| TCF7 | P19T | 5742913 |
| TGFB1 | -509C>T | 1800469 |
| TNF | -376G>A | 1800750 |
| -308G>A | 1800629 | |
| -244G>A | 673 | |
| -238G>A | 361525 | |
| UGB | +38G>A | 3741240 |
| VDR | M1T | 2228570 |
| Bsml+/- | 1544410 | |
| Homocysteine metabolism | ||
| CBS | I278ins/T | 5742905 |
| MTHFR | 677 C>T | 1801133 |
| Thrombosis | ||
| F2 | 20210G>A | 1799963 |
| F5 | R506Q | 6025 |
| F7 | -323 ins/del | 5742910 |
| R353Q | 6046 | |
| FGB | -455G>A | 1800790 |
| ITGA2 | 873G>A | 1062535 |
| ITGB3 | L33P | 5918 |
| PAI1 | -675G5>G4 | 1799768 |
| 11053G>T | 7242 | |
| Blood pressure regulation | ||
| ADD1 | G460W | 4961 |
| ADRB2 | R16G | 1042713 |
| Q27E | 1042714 | |
| T164I | 1800888 | |
| AGT | M235T | 699 |
| AGTR1 | 1166A>C | 5186 |
| ACE | intron 16 ins/del | 1799752 |
| GNB3 | 825C>T | 5443 |
| NOS2A | 231C>T | 1137933 |
| NOS3 | -922A>G | 1800779 |
| -690C>T | 3918226 | |
| E298D | 1799983 | |
| NPPA | 664G>A | 5063 |
| 2238T>C | 5065 | |
| SCNN1A | T493R | 5742912 |
| A663T | 2228756 |
Locus . | Marker . | rs no. . |
|---|---|---|
| Lipid metabolism | ||
| ADRB3 | W64R | 4994 |
| APOA4 | T347S | 675 |
| Q360H | 5110 | |
| APOB | T711 | 1367117 |
| R3500Q | 5742904 | |
| APOC3 | -641C>A | 2542052 |
| -482C>T | 2854117 | |
| -455T>C | 2854116 | |
| 1100C>T | 4520 | |
| 3175C>G | 5128 | |
| 3206T>G | 4225 | |
| APOE | C112R | 429358 |
| R158C | 7412 | |
| CETP | -629C>A | 1800775 |
| I405V | 5882 | |
| D442G | 2303790 | |
| LDLR | Ncol+/- | 5742911 |
| LIPC | -480C>T | 1800588 |
| LPA | 93C>T | 1652503 |
| 121G>A | 1800769 | |
| LPL | -93T>G | 1800590 |
| D9N | 1801177 | |
| N291S | 268 | |
| S447X | 328 | |
| PON1 | M55L | 3202100 |
| Q192R | 662 | |
| PON2 | S311C | 7493 |
| PPARG | P12A | 1801282 |
| Cellular adhesion | ||
| ICAM1 | K56M | 5491 |
| G241R | 1799969 | |
| SELE | S128R | 5361 |
| L554F | 5355 | |
| SELP | S330N | 6131 |
| V640L | 6133 | |
| VCAM1 | -1594T>C | 1041163 |
| Inflammation | ||
| C3 | R102G | 2230199 |
| C5 | I802V | 17611 |
| CCR2 | V62I | 1799864 |
| CCR3 | P39L | 5742906 |
| CCR5 | wt/Δ580-611 | 333 |
| -2454G>A | 1799987 | |
| CD14 | -260C>T | 2569190 |
| CSF2 | I117T | 25882 |
| CTLA4 | -318C>T | 5742909 |
| T17A | 231775 | |
| FCERB1 | E237G | 569108 |
| GC | E416D | 7041 |
| T420K | 4588 | |
| IL1A | -889T>C | 1800587 |
| IL1B | -1418C>T | 16944 |
| F105F | 1143634 | |
| IL4 | -590C>T | 2243250 |
| IL4R | I75V | 1805010 |
| S503P | 1805015 | |
| Q576R | 1801275 | |
| IL5RA | -80G>A | 2290608 |
| IL6 | -572G>C | 1800796 |
| -174G>C | 1800795 | |
| IL9 | T113M | 2069885 |
| IL10 | -571C>A | 1800872 |
| IL13 | 4045C>T | 1295686 |
| Inflammation | ||
| LTA | T26N | 1041981 |
| 252A>G | 909253 | |
| LTC4S | -444A>C | 730012 |
| MMP3 | -1171A5>A6 | 3025058 |
| SCYA11 | A23T | 3744508 |
| -1328G>A | 4795895 | |
| SDF1 | +800 G>A | 1801157 |
| TCF7 | P19T | 5742913 |
| TGFB1 | -509C>T | 1800469 |
| TNF | -376G>A | 1800750 |
| -308G>A | 1800629 | |
| -244G>A | 673 | |
| -238G>A | 361525 | |
| UGB | +38G>A | 3741240 |
| VDR | M1T | 2228570 |
| Bsml+/- | 1544410 | |
| Homocysteine metabolism | ||
| CBS | I278ins/T | 5742905 |
| MTHFR | 677 C>T | 1801133 |
| Thrombosis | ||
| F2 | 20210G>A | 1799963 |
| F5 | R506Q | 6025 |
| F7 | -323 ins/del | 5742910 |
| R353Q | 6046 | |
| FGB | -455G>A | 1800790 |
| ITGA2 | 873G>A | 1062535 |
| ITGB3 | L33P | 5918 |
| PAI1 | -675G5>G4 | 1799768 |
| 11053G>T | 7242 | |
| Blood pressure regulation | ||
| ADD1 | G460W | 4961 |
| ADRB2 | R16G | 1042713 |
| Q27E | 1042714 | |
| T164I | 1800888 | |
| AGT | M235T | 699 |
| AGTR1 | 1166A>C | 5186 |
| ACE | intron 16 ins/del | 1799752 |
| GNB3 | 825C>T | 5443 |
| NOS2A | 231C>T | 1137933 |
| NOS3 | -922A>G | 1800779 |
| -690C>T | 3918226 | |
| E298D | 1799983 | |
| NPPA | 664G>A | 5063 |
| 2238T>C | 5065 | |
| SCNN1A | T493R | 5742912 |
| A663T | 2228756 |
All markers were screened using linear array technology from Roche Molecular Systems. See Appendix 2 for abbreviations of markers listed. All markers are referenced by reference SNP ID (rs) number in the National Center for Biotechnology Information (NCBI) SNP database.
Genotyping was performed using multilocus polymerase chain reaction (PCR)–based assays, essentially as previously described.28 Briefly, every sample was amplified using 3 “cocktails” of biotinylated primer pairs, each targeting between 24 and 50 genomic fragments. Amplified fragments within each PCR product pool were then detected colorimetrically with sequence-specific oligonucleotide probes immobilized in a linear array on nylon membranes.29 Probe specificities were previously confirmed by sequencing, use of DNAs genotyped independently by other methods such as restriction length polymorphism analysis, or by confirming observed frequencies against published values. Interpretation of the alleles represented by positive probe signals was performed independently by 2 investigators blinded to case-control status. Ambiguous interpretations (< 1%) were resolved with repeat genotyping.
Statistical analysis
Given that all patients were homozygous for HbSS, we assumed that linkage equilibrium was present among all loci in the overall sample. Although we expect the overall study sample to meet Hardy-Weinberg proportions, the 3 stroke subgroups examined may not meet these expectations if disease associations are present. Thus, any genotypic ratio distortion resulting from a strong disease association between a particular gene locus and stroke subgroup will be necessarily balanced by the other 2 stroke subgroups within the overall study population. In order to eliminate testing with low statistical power, the minimum allele frequency required for testing a particular gene locus was set at 10%. For those markers known to be in linkage disequilibrium, we chose a single marker for the analysis based on allelic frequency distributions (ie, those that were closest to an allelic frequency of 0.5). For each of the diallelic markers examined at a particular gene locus, the 2 genotypes with the less common allele were compared with the homozygous genotype of the more common allele. Univariate analyses were performed using contingency table testing to compute the χ2. To reduce type 2 statistical error, the maximum associated P value for reporting individual marker effects was set at .10. Marker effects with associated P values less than .10 on univariate analysis were entered into a logistic regression model (STATA 6.0). As we had previously found independent HLA associations with stroke risk in the same study population,15 we included those significant HLA-A, DPB1, and HLA homozygosity effects in the present multivariate model.
Interaction between specific markers was examined using log-linear modeling. This approach tests for rejection of a model containing each of 3 possible 2-way interactions between the 2-locus genotypes and stroke subgroup (LV, SV, and MRI(-)). Significantly deviant results imply the presence of 3-way interaction between the 2-loci and stroke subgroup. Within each subgroup, those markers identified by univariate analyses that share common physiologic pathways were examined. Tests of heterogeneity (2 × 2) for each of the 2-locus genotype categories within each stroke subgroup were also performed. Interactive effects due to specific genotypes were estimated with odds ratios.
Results
The clinical and laboratory features of the CSSCD newborn cohort have been previously described.24,25 Of the 415 patients enrolled in the newborn cohort of the CSSCD, 266 were HbSS genotype and have been previously characterized.27 Of these, 230 were eligible and included in the present study. The study population was composed of 115 females and 115 males with a mean age of 8.4 ± 1.7 years (median, 7.9 years; range, 5.0-14.6 years) at the time of baseline MRI.
Brain MRI results
Of the 230 SCA patients included in this study, 159 (69%) had normal brain MRI scans with a mean age of 12.4 ± 2.3 years at the time of the last follow-up MRI. These patients remained free of clinical stroke during the entire study period. At the time of a first positive MRI, 71 patients (31%) demonstrated infarctive lesions on MRI and had a mean age of 9.3 years (median, 9.0 years). Of these 71 patients, 36 (51%) had MRI evidence of LV involvement (with or without SV involvement), and 35 (49%) had exclusively SV involvement. A clinical history of cerebrovascular accidents (CVAs) was documented in 26 (37%) of these 71, with the majority (92%) due to large vessel (LV) and only 2 cases (8%) due to exclusively small vessel (SV) disease. There were 2 patients who demonstrated cerebral hemorrhage along with infarction on MRI.
Genotypic associations
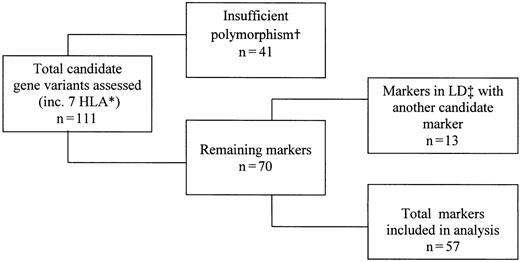
Among 104 variant sites examined, 57 were sufficiently informative for statistical testing (Figure 1).
Process for inclusion of candidate gene variants in statistical analyses.*From previously published data.15 †Insufficient polymorphism was defined as an allele frequency less than 10%. ‡LD denotes linkage disequilibrium.
Process for inclusion of candidate gene variants in statistical analyses.*From previously published data.15 †Insufficient polymorphism was defined as an allele frequency less than 10%. ‡LD denotes linkage disequilibrium.
After classifying the MRI(+) patients by stroke subtype (LV vs SV), several markers were identified by univariate analysis (Table 2). These variants met criteria for inclusion into the logistic regression model. Among the 7 HLA effects previously identified in the same study population, 3 met criteria for inclusion in the model.
Logistic regression analysis of gene variant effects by stroke subtype
. | . | Large-vessel stroke . | . | Small-vessel stroke . | . | ||
|---|---|---|---|---|---|---|---|
| Genetic locus . | Allele frequency* . | OR ± SE . | Z statistic, P . | OR ± SE . | Z statistic, P . | ||
| IL4R 503P | 0.39 | 2.50 ± 0.83 | .006 | 1.30 ± 0.46 | .45 | ||
| HLA-A | 0.06 | 7.71 ± 1.96 | .013 | 1.98 ± 1.01 | .18 | ||
| ADRB2 27E | 0.16 | 0.53 ± 0.16 | .033 | 0.94 ± 0.21 | .78 | ||
| TNF (-308)A | 0.13 | 0.52 ± 0.17 | .048 | 0.97 ± 0.22 | .90 | ||
| VCAM1 (-1594)C | 0.22 | 1.08 ± 0.24 | .72 | 1.98 ± 0.43 | .002 | ||
| HLA-DPB1 | 0.16 | 1.77 ± 0.70 | .16 | 3.50 ± 1.41 | .002 | ||
| HLA homozygosity | 0.16 | 1.20 ± 0.25 | .39 | 1.58 ± 0.32 | .023 | ||
| LDLR Ncol- | 0.20 | 1.01 ± 0.05 | .96 | 0.53 ± 0.139 | .002 | ||
. | . | Large-vessel stroke . | . | Small-vessel stroke . | . | ||
|---|---|---|---|---|---|---|---|
| Genetic locus . | Allele frequency* . | OR ± SE . | Z statistic, P . | OR ± SE . | Z statistic, P . | ||
| IL4R 503P | 0.39 | 2.50 ± 0.83 | .006 | 1.30 ± 0.46 | .45 | ||
| HLA-A | 0.06 | 7.71 ± 1.96 | .013 | 1.98 ± 1.01 | .18 | ||
| ADRB2 27E | 0.16 | 0.53 ± 0.16 | .033 | 0.94 ± 0.21 | .78 | ||
| TNF (-308)A | 0.13 | 0.52 ± 0.17 | .048 | 0.97 ± 0.22 | .90 | ||
| VCAM1 (-1594)C | 0.22 | 1.08 ± 0.24 | .72 | 1.98 ± 0.43 | .002 | ||
| HLA-DPB1 | 0.16 | 1.77 ± 0.70 | .16 | 3.50 ± 1.41 | .002 | ||
| HLA homozygosity | 0.16 | 1.20 ± 0.25 | .39 | 1.58 ± 0.32 | .023 | ||
| LDLR Ncol- | 0.20 | 1.01 ± 0.05 | .96 | 0.53 ± 0.139 | .002 | ||
The MRI (-) group was used as the comparison group in the logistic regression model.
Likelihood ratio χ2 = 73.1; probability greater than χ2 = 0.000; pseudo R2 = 0.19.
OR indicates odds ratio; SE, standard error.
Variant allele frequencies are shown for the total study population (N = 230). For the HLA loci, carrier frequencies are given, as previously published15
The multivariate model accounted for 19% of the overall variance (P < .0001). The IL4R 503P, ADRB2 27E, TNF (-308)A, VCAM1 (-1594)C, and LDLR NcoI variants revealed distinctive associations when analyzed by stroke subgroup (Table 2).
In the LV stroke subgroup, both the IL4R 503P variant and HLA-A variation predisposed to stroke, whereas ADRB2 27E and TNF(-308)A were associated with protection from stroke. The apoC3 3206G and NOS2A 231T variants showed a trend toward protection from LV stroke, but did not reach significance. In the SV stroke subgroup, the VCAM (-1594)C variant, HLA-DPB1, and HLA homozygosity effects predisposed to stroke, while the LDLR (exon18)NcoI variant appeared protective.
We further analyzed the data for interactive effects between these markers, focusing upon those involved in similar physiologic pathways (Table 1). Log-linear modeling for 2-locus interactions between NOS2A and ADRB2 in the LV stroke group and between HLA homozygosity and HLA-DPB1 in the SV stroke group failed to uncover significant interactive effects. However, the model revealed evidence for gene interaction at the 2 loci, IL4R and TNF, in the LV stroke group. In this unselected study population, the frequencies of the predisposing IL4R 503P and TNF G(-308) alleles were 0.39 and 0.87, respectively. A large excess of individuals with the combined IL4R 503P variant and wild-type TNF (-308)G allele in the LV stroke group (81%) compared with the MRI (-) control group (43%) was present (Table 3).
TNF and IL4R genotypic proportions (%) by stroke subgroup
Two-locus genotypes IL4R S503P - TNF G(-308)A . | Large-vessel stroke . | Small-vessel stroke . | MRI(-) . | Total . |
|---|---|---|---|---|
| (SS) - (GG) | 11.1 | 22.9 | 31.4 | 27.0 |
| (SS) - (GA or AA) | 5.6 | 8.6 | 6.2 | 6.5 |
| (SP or PP) - (GG) | 80.6 | 48.6 | 42.8 | 49.6 |
| (SP or PP) - (GA or AA) | 2.8 | 20.0 | 19.5 | 17.0 |
| No. in group | 36 | 35 | 159 | 230 |
Two-locus genotypes IL4R S503P - TNF G(-308)A . | Large-vessel stroke . | Small-vessel stroke . | MRI(-) . | Total . |
|---|---|---|---|---|
| (SS) - (GG) | 11.1 | 22.9 | 31.4 | 27.0 |
| (SS) - (GA or AA) | 5.6 | 8.6 | 6.2 | 6.5 |
| (SP or PP) - (GG) | 80.6 | 48.6 | 42.8 | 49.6 |
| (SP or PP) - (GA or AA) | 2.8 | 20.0 | 19.5 | 17.0 |
| No. in group | 36 | 35 | 159 | 230 |
A 3-way interaction test in 2 (IL4R) × 2 (TNF) × 3 (stroke subgroups) log-linear model: log likelihood ratio statistic = 6.719; degrees of freedom = 2; P = .035.
Alleles are abbreviated in parentheses. The less common polymorphisms are indicated in italics (eg, SP or PP - GG indicates patients carrying 1 or 2 copies of the less common IL4R 503 P allele and are homozygous for the TNF(-308) G allele).
IL4R indicates interleukin-4 receptor; TNF, tumor necrosis factor.
The test of heterogeneity revealed genotypic proportions in the LV stroke group that differed from the other 2 stroke subgroups (P = .01). This effect was significantly greater than expected from the individual effects of these 2 genotypes. SCA patients having this combined genotype (IL4R 503 SP or PP/TNF -308 GG) had a 5.5-fold increased risk of LV stroke, compared with the MRI-negative control group (Table 4).
Risk of combined IL4R/TNF genotype with LV stroke
. | No. with IL4R (SP or PP)/TNF (GG) genotype . | . | . | |
|---|---|---|---|---|
. | + . | - . | Total no. . | |
| No. LV | 29 | 7 | 36 | |
| No. MRI (-) | 68 | 91 | 159 | |
| Total no. | 97 | 98 | 195 | |
. | No. with IL4R (SP or PP)/TNF (GG) genotype . | . | . | |
|---|---|---|---|---|
. | + . | - . | Total no. . | |
| No. LV | 29 | 7 | 36 | |
| No. MRI (-) | 68 | 91 | 159 | |
| Total no. | 97 | 98 | 195 | |
OR = 5.54 (95% Cl, 2.33-13.11); χ2 = 16.77; P = .0000.
Discussion
Although the natural history of stroke in SCA has been well characterized, the pathogenesis of stroke remains poorly defined. The development of cerebrovascular lesions in SCA begins with endothelial injury, perhaps influenced by abnormal adherence of sickle cells or leukocytes to endothelial cells,30 followed by intimal damage and smooth muscle hypertrophy.31
Our previous pilot study was limited to a small sample size and a genotypically and phenotypically distinct group of patients,23 so we could not justify inclusion of our pilot data in the present study. Our present findings on a larger, representative cohort of children participating in the CSSCD show unique associations with specific stroke subtypes and provide evidence that several candidate genes involved in pathways of thrombosis, inflammation, and cellular adhesion may confer stroke risk in children with SCA.
We found that the observed SV stroke risk was partly attributable to the VCAM (-1594)C variant. Adhesion molecules, such as VCAM-1, regulate the attachment and migration of leukocytes and play a dominant role in the development of vascular disease in the general population.32 Furthermore, endothelial VCAM1 has been shown to be up-regulated in response to sickle erythrocytes and appears to be involved in the pathophysiology of microvascular occlusion in sickle cell disease.33-35 Interestingly, the VCAM1 (-1594)C variant was associated exclusively with risk for small-vessel stroke in our study population. Taylor et al recently documented a protective association between the VCAM1 1238C variant and stroke, but this study was limited by a relatively small sample of SCA patients with clinically overt stroke.16 Although we examined only the VCAM1 (-1594)C variant in our group, it is plausible that other SNPs in linkage disequilibrium at the VCAM1 locus may contribute as a “haplotype” to the development of stroke.
The IL4R 503P allelic variant was specifically associated with an increased stroke risk in the LV stroke subgroup. Polymorphisms in the IL4R gene have been studied extensively in immune-mediated diseases (including asthma and insulindependent diabetes mellitus), as the IL4 receptor is critical in T-cell development.36,37 In addition, both IL4R and specific HLA genes have been reported to influence the risk of diabetes.38 Our previous findings of HLA associations, along with our present findings revealing the IL4R variant contribution, suggest possible immune-mediated pathways by which stroke may occur (interleukin-dependent modulation of HLA expression on activated T cells). However, mechanistic studies are clearly required to determine the etiologic factors involved in the development of stroke in SCA. As TNF genes are known to be in linkage disequilibrium with the HLA locus, the TNF(-308) association with LV stroke may reflect linkage disequilibrium (LD) with HLA “risk” alleles previously identified in this study population. However, haplotype analyses on a larger population are needed to confirm whether these effects are due to linkage disequilibrium between TNF and HLA alleles.
Because specific cytokines, including IL4 and TNF, may have synergistic functions in proinflammatory pathways leading to the development of stroke, we investigated whether specific gene interactions could be contributing to stroke in our population. We detected a significant gene-gene interaction between the IL4R 503P variant and the common TNF G(-308) promoter allele. Individuals with the risk genotype for IL4R in combination with the common genotype for TNF had a 5.5-fold increased risk for the development of LV stroke compared with all other possible genotypes in the MRI-negative group (Table 4). Given a prevalence of 50% for the combined genotype in our study population of SCA patients (Table 3), the proportion of LV strokes attributable to this combined genotype may be as high as 69%.39
In our study, subclassification of MRI-positive patients by large- and small-vessel disease localized the observed candidate gene associations to a specific stroke subtype, suggesting that distinct pathogenetic mechanisms may underlie these 2 types of stroke. Determining which genes are responsible for severe phenotypic expression (eg, stroke due to LV disease) versus those that confer milder effects (eg, lacunar infarction) may lead to a better understanding of the distinctive pathophysiologies of stroke.
In conclusion, our results provide evidence for the involvement of multiple candidate genes predisposing to stroke in children with SCA. Our study is the first to investigate genetic associations with stroke in SCA by simultaneously screening many candidate gene variants in a large, representative cohort of patients. Both single gene effects and gene-gene interactions appear to influence the risk of specific vascular subtypes of stroke. Additional investigations in other larger SCA patient cohorts are indicated to corroborate our findings and determine the predictive value of a subset of alleles with the greatest combined impact on stroke risk in SCA. Adults with SCA also have an increased risk for stroke, distinct from that in children with SCA. Further studies investigating genetic modifiers of stroke in adults are also needed. The development of a genetic “risk profile” for primary stroke in SCA might thus be ultimately translated into clinical benefit through early identification and prevention of stroke in patients at greatest risk.
Appendix 1: senior investigators in the Cooperative Study of Sickle Cell Disease (CSSCD)
Clinical centers: R. Johnson, Alta Bates Hospital (Berkeley, CA); L. McMahon, Boston City Hospital (Boston, MA); F. Gill and K. Ohene-Frempong, Children's Hospital (Philadelphia, PA); G. Bray, J. Kelleher, and S. Leikin, Children's National Medical Center (Washington, DC); E. Vichinsky and B. Lubin, Children's Hospital (Oakland, CA); A. Bank and S. Piomelli, Columbia Presbyterian Hospital (New York, NY); W. Rosse, J. Falletta, and T. Kinney, Duke University (Durham, NC); L. Lessin, George Washington University (Washington, DC); J. Smith and Y. Khakoo, Harlem Hospital (New York, NY); R. Scott, O. Castro, and C. Reindorf, Howard University (Washington, DC); H. Dosik, S. Diamond, and R. Bellevue, Interfaith Medical Center (Brooklyn, NY); W. Wang and J. Wilimas, LeBonheur Children's Hospital (Memphis, TN); P. Milner, Medical College of Georgia (Augusta, GA); A. Brown, S. Miller, R. Rieder, and P. Gillette, State University of New York Health Science Center at Brooklyn (Brooklyn, NY); W. Lande, S. Embury, and W. Mentzer, San Francisco General Hospital (San Francisco, CA); D. Wethers and R. Grover, St. Luke's–Roosevelt Medical Center (New York, NY); M. Koshy and N. Talishy, University of Illinois (Chicago, IL); C. Pegelow, P. Klug, and J. Temple, University of Miami (Miami, FL); M. Steinberg, University of Mississippi (Jackson, MS); A. Kraus, University of Tennessee (Memphis, TN); H. Zarkowsky, Washington University (St Louis, MO); C. Dampier, Wyler Children's Hospital (Chicago, IL); H. Pearson and A. K. Ritchey, Yale University (New Haven, CT); Statistical coordinating centers: P. Levy, D. Gallagher, A. Koranda, Z. Flournoy-Gill, and E. Jones, University of Illinois School of Public Health (Chicago, IL; 1979-1989); S. McKinlay, O. Platt, D. Gallagher, D. Brambilla, and L. Sleeper, New England Research Institutes (Watertown, MA; 1989-1997); M. Espeland, Bowman-Gray School of Medicine (Winston-Salem, NC); Program administration: M. Gaston, C. Reid, and J. Verter, National Heart, Lung, and Blood Institute (Bethesda, MD).
Appendix 2: abbreviations for candidate gene polymorphisms assessed inTable 1
ADD1 indicates adducin alpha; ADRB, beta adrenergic receptor; AGT, angiotensinogen; AGTR1, angiotensin receptor 1; APO, apolipoprotein; CBS, cystathionine beta-synthase; CCR, chemokine receptor; CETP, cholesteryl ester transfer protein; CD14, monocyte differentiation antigen; CSF2, colony-stimulating factor 2; CTLA4, cytotoxic T-lymphocyte antigen 4; DCP1, dipeptidyl carboxypeptidase 1/angiotensin converting enzyme; F2, coagulation factor II; F5, factor V; F7, factor VII; FCER1B, immunoglobulin E receptor 1 beta; FGB, fibrinogen beta polypeptide; GC, human vitamin D-binding protein gene; GNB3, guanine nucleotide binding protein beta 3; ICAM1, intracellular adhesion molecule 1; IL, interleukin; IL1A, interleukin 1 alpha; IL1B, interleukin 1 beta; IL4R, interleukin 4 receptor; IL5RA, interleukin 5 receptor alpha; ITGA2, platelet glycoprotein 1a; ITGB3, platelet glycoprotein IIIa; LDLR, low density lipoprotein receptor; LIPC, hepatic lipase; LPA, apolipoprotein(a); LPL, lipoprotein lipase; LTA, tumor necrosis factor beta; LTC4S, leukotriene C4 synthase; MMP3, matrix metalloproteinase 3; MTHFR-5, 10, methylenetetrahydrofolate reductase; NOS2A, nitric oxide synthase 2A; NOS3, nitric oxide synthase 3 endothelial; NPPA, atrial natriuretic peptide; PAI1, plasminogen activator inhibitor type 1; PON, paraoxonase; PPARG, peroxisome proliferator activated receptor gamma; SCNN1A, sodium channel epithelial alpha subunit; SCYA11, eotaxin; SDF1, stromal-derived factor 1; SELE, E-selectin; SELP, P-selectin; TCF7, T-cell transcription factor 7; TGFB1, transforming growth factor-beta 1; TNF, tumor necrosis factor; UGB, uteroglobin; VCAM, vascular cell adhesion molecule; and VDR, vitamin D receptor.
Prepublished online as Blood First Edition Paper, November 13, 2003; DOI 10.1182/blood-2003-09-3015.
Supported in part by the Doris Duke Charitable Foundation, Clinical Scientist Development Award, and by National Institutes of Health grants NS40292, HL64556-01, and M01RR01271
Several of the authors (S.C., R.A., L.S.) are employed by a company (Roche Molecular Systems) whose research assays were used in the present work.
A complete list of the Cooperative Study of Sickle Cell Disease (CSSCD) Investigators appears in Appendix 1.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Michael Grow, Rebecca Reynolds, Arkadiy Silbergleit, and Karen Walker (Roche Molecular Systems) for their efforts in developing the genotyping reagents used for this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal