Recently, Houghtaling et al1 reported that the Fanconi anemia (FA) group D2 knock-out (KO) mouse model presented novel phenotypes not observed in other FA mouse models reported (Fanca, Fancc, and Fancg); these included microphthalmia, perinatal lethality, and epithelial cancers. This prompted the authors to conclude that the Fancd2-/- mice had important differences and to propose 2 models explaining the divergence of phenotypes between Fancd2 and the so-called “nuclear complex genes.” The first model showed that the Fancd2 protein is monoubiquitinated in other Fanc KO mouse models, a difference from human cells, and thus may possess residual activity. The other explanation may be that Fancd2 has other functions unrelated to the FA pathway, an explanation already used for the FANCC protein, which is mainly cytoplasmic in comparison with other FA proteins. In other words, based on the Fancd2-/- phenotype, Houghtaling et al indicate that Fancd2 may play a more critical role in the FA pathway than other FA proteins.
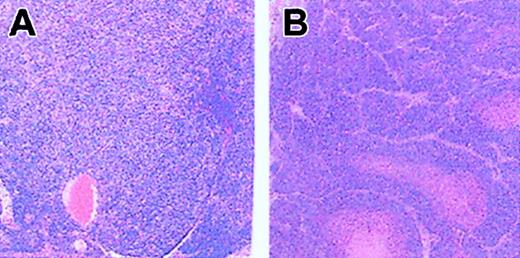
The first phenotype considered new in Fancd2-/- mice is microphthalmia. I and others2 have observed microphthalmia in Fancc and Fanca knock-out mice, respectively. For instance, microphthalmia was observed in 2 Fancc-/- mouse strains, C57Bl/6 and C57Bl/6Ly-Pep3b, at 63.5% (57 of 90) and 63.3% (54 of 85), respectively, compared with 78% (33 of 41) of Fancd2-/- mice. Like those of Houghtaling et al, my nonmutant C57Bl/6 mice showed a low incidence of microphthalmia (2% or 4 of 199). Since microphthalmia seems to be more frequent in a C57Bl/6 background and in view of the fact that the first FA mouse model reported3 has been done using mixed background (129sv/C57Bl/6j), this may explain why microphthalmia had not been previously observed. The second so-called novel phenotype is perinatal lethality. Houghtaling et al reported that only 16.5% (50 of 303) of Fancd2-/- mice were observed versus the expected 25% at the time of genotyping, suggestive of perinatal lethality. I observed a ratio of 19.6% (121 of 616) and 16.4% (132 of 804) in Fancc-/- mouse strains C57Bl/6 and C57Bl/6Ly-Pep3b, respectively, also indicating perinatal lethality. The third phenotype observed in Fancd2-/- mice that was suggested to be specific to this complementation group is the increased incidence of tumors. Again the authors believe that this occurrence is due to the complete loss of Fancd2 function, a function that is dependent on its monoubiquitination. However, Wong et al recently reported tumor formation (lymphoma) in 33% (2 of 6) of Fanca-/- mice from the 129S6:CD-1 background.2 We also observed tumors in older Fancc-/- mice (> 13 months of age; M.C., C. McKerlie, M. Buchwald, unpublished observation, May 2001). Unfortunately, we did only histologic analysis of 2 Fancc-/- C57Bl/6 mice, and in these 2 cases, tumors were observed; the first tumor was mammary adenocarcinoma (same as Fancd2-/- mice) and the second, histiocytic sarcoma (Figure 1). Since only 2 mice had been kept for a longer period of time, these results have not been reported before. Nevertheless, I believe that increased incidence of tumor formation in FA mouse models is not solely observable in the Fancd2 subgroup and is probably a phenotype directly linked to the FA pathway, that is, resulting from lack of a functional FA gene, any FA gene. Thus, Houghtaling et al's statement that Fancd2-/- mice have important differences to other FA mouse models is not accurate.
Hematoxylin and eosin–stained, paraffin-embedded tissue sections from old C57Bl/6 Fancc-/- mice. (A) Small intestine lymph node showing histiocytic sarcoma with sheets of neoplastic cells characterized by eosinophilic cytoplasm and pleomorphic nuclear morphology. (B) Mammary adenocarcinoma with well-circumscribed and encapsulated mass composed of neoplastic cells with oval or polyhedral nucleus with either amphophilic nuclei and stippling or coarse pattern to their chromatin. Anisokaryosis is 2-fold and mitotic index is 5. Original magnification × 100.
Hematoxylin and eosin–stained, paraffin-embedded tissue sections from old C57Bl/6 Fancc-/- mice. (A) Small intestine lymph node showing histiocytic sarcoma with sheets of neoplastic cells characterized by eosinophilic cytoplasm and pleomorphic nuclear morphology. (B) Mammary adenocarcinoma with well-circumscribed and encapsulated mass composed of neoplastic cells with oval or polyhedral nucleus with either amphophilic nuclei and stippling or coarse pattern to their chromatin. Anisokaryosis is 2-fold and mitotic index is 5. Original magnification × 100.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal