Abstract
The nuclear factor of activated T cells (NFAT) proteins are a family of transcription factors whose activation is controlled by calcineurin, a Ca2+-dependent phosphatase. Once dephosphorylated, these proteins move to the nucleus where they interact with cofactors to form transcription factor complexes. Inhibition of NFAT proteins by immunosuppressants, such as cyclosporin A (CsA) and FK506, is used clinically to prevent transplant rejection. Although these drugs have revolutionized organ transplantation, their use is associated with severe side effects in other organs in which NFAT proteins are important. One of the signal transducers that controls NFAT activity is Vav1, which is exclusively expressed in the hematopoietic system. Vav1 contains numerous modular domains that enable its function as a guanine exchange factor (GEF) toward RhoGTPases as well as participate in protein-protein interactions. This review focuses on the mechanisms by which Vav1 regulates NFAT through GEF-dependent and -independent cascades, emphasizing the newly assigned role of Vav1 in the regulation of Ca2+ release. Because of its restriction to hematopoietic cell lineages and its importance in the regulation of NFAT, targeting Vav1 and, in particular, its association with other proteins may offer a highly selective means of modifying T-cell behavior, thus allowing the development of more specific immunosuppressive therapies.
Introduction
Signaling events tightly control the function of nuclear factor of activated T cells (NFAT) in T cells.1 Although NFAT was originally identified in T cells as a regulator of cytokine gene expression,1-6 additional members of the NFAT family were found to play varied roles in nonhematopoietic cells.1-6 One of the signal transducers critical for NFAT activation in T cells is Vav1.7 This review presents recent data demonstrating the link between Vav1 and NFAT. Furthermore, I raise here the possibility of targeting Vav1 to block NFAT specifically in T cells and thereby prevent immune reaction during organ transplantation. The benefits of such a molecular-targeted approach to immunosuppression for organ transplantation will be discussed.
NFAT
Interest in NFAT increased when it became apparent that the clinically important immunosuppressants cyclosporin A (CsA) and FK506, used to facilitate organ transplantation, act by inhibiting NFAT responses.1,8 Consequently, initial studies focused on the function of NFAT in the immune system, particularly in T cells. T cells play a primary role in the ability of the immune system to respond potently to foreign assaults. The stimulation of T cells by foreign antigens leads to the induction of complex signal transduction pathways that result in the production of various cytokines and the expression of immunoregulatory molecules on the cell surface. One of the major mediators in these events is NFAT. NFAT induction is associated with the secretion of numerous cytokines such as interleukin-2 (IL-2),9 IL-3,10 IL-4,11 IL-5,12 granulocytemacrophage colony-stimulating factor (GM-CSF),10 interferon-γ (IFN-γ),13 and tumor necrosis factor α (TNFα).14 NFAT is also involved in the expression of a number of immunoregulatory molecules on the cell surface of T cells such as CD40L15 and cytotoxic T lymphocyte antigen 4 (CTLA-4).16 Thus, NFAT is a cardinal participant in T-cell biology. NFAT proteins also play varied roles in other cells of the immune system such as B cells17 and mast cells.18
In recent years, it has become apparent that NFAT is also involved in regulating multiple other processes in many different cell lineages. Thus, NFAT plays a role in the cardiovasculature,19,20 where it contributes to the pathogenesis of cardiac hypertrophy21 and regulates heart valve formation and vascular development.22 Additionally, NFAT is involved in skeletal muscle hypertrophy, adipocyte differentiation, and memory and plasticity.23,24
The ability of NFAT to perform these many diverse tasks relies mainly on the fact that there are several members of the NFAT family. At least 4 structurally related NFAT cytoplasmic subunit members have been identified. These proteins are designated NFATc1 (also known as NFAT2/c), NFATc2 (NFAT1/p), NFATc3 (NFAT4/x), and NFATc4 (NFAT3).3,25 A fifth putative member of the family (NFAT5) is a constitutively nuclear phosphoprotein that has only limited sequence homology to other members of the NFAT family.26 A hallmark of this family is a shared NFAT homology region in the N-terminal portion of the protein, which mediates regulatory functions of the molecule. This region contains nuclear localization and nuclear export sequences, and phosphorylation sites for a number of serine/threonine kinases, many of which are localized to a serine-rich region and 3 SP (serine-proline) repeats.3,25 NFAT proteins also share a conserved domain located toward the C terminus that binds DNA and also participates in cooperative protein-protein interactions with transcription factors.3,25 This region shows moderate sequence homology to the DNA-binding domains of Rel-family proteins.3
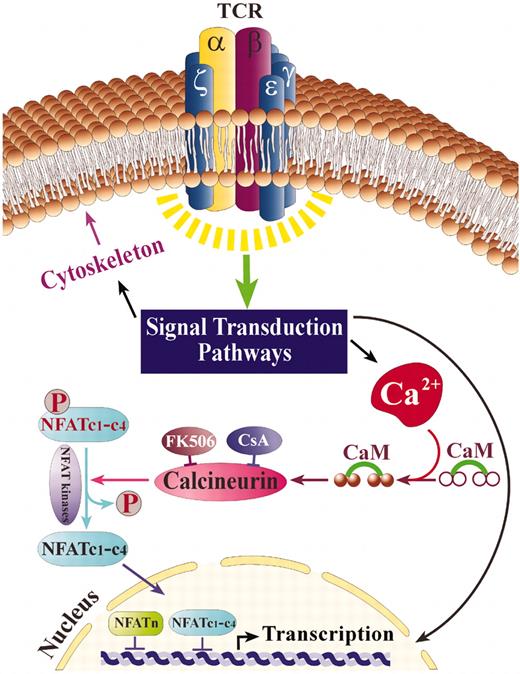
NFAT activation is regulated primarily through control of its subcellular localization (Figure 1). In unstimulated cells, NFAT is a hyperphosphorylated cytosolic protein. An elevation in intracellular Ca2+, induced by a variety of mechanisms, increases the activity of the Ca2+/calmodulin-dependent phosphatase, calcineurin.27 Activated calcineurin dephosphorylates multiple serine residues within the regulatory region of the NFAT molecule, inducing a conformational change in NFAT that exposes its nuclear localization signals and allows import of NFAT into the nucleus.28,29 NFAT is translocated as a complex with calcineurin through nuclear pores by a mechanism that depends on Ran and specific nuclear importins.30 Calcineurin also plays a role in promoting nuclear retention of NFAT by masking nuclear export signals recognized by the exportin protein, chromosomal region maintenance 1 (Crm-1), and by maintaining NFAT in a dephosphorylated state.30 In the nucleus, NFAT interacts with cofactors to form transcription factor complexes. These cofactors may include activator protein-1 (AP-1), a heterodimer composed of members of the Jun and Fos families of oncogenes,31 myocyte enhancer factor-2 (MEF2),32 and members of the homeopoietic lineage-specific transcription factor GATA family.33 Their association with the specific NFATc is subject to regulation by activated kinases, including c-Jun N-terminal kinase 1 (JNK1), JNK3, and the extracellular signal-regulated kinases ERK1 and ERK234 and may also be tissue dependent. At the next level, vigorous nuclear kinases such as protein kinase A (PKA) and glycogen synthase kinase-3 (GSK3) act to rephosphorylate NFAT and hasten its export from the nucleus, thus opposing the activity of calcineurin (Figure 1).35 Phosphorylation by cytosolic kinases may also promote cytosolic retention by disrupting NFAT-calcineurin interactions36 or masking nuclear localization signals.37 Some of these regulatory mechanisms rely on NFAT target sites that are conserved in all NFAT isoforms and are likely to apply universally, whereas other kinases target some NFAT isoforms, but not others.37,38 In sum, the activation of NFAT is the result of complex signaling cascades that affect both the Ca2+/calcineurin pathway and additional tyrosine kinase receptor effectors.
NFAT activation following TCR stimulation. TCR stimulation leads to calcium flux and additional signaling events that result in the activation of calcineurin, a Ca+2-calmodulin–dependent phosphatase that dephosphorylates NFAT proteins, enabling their movement to the nucleus (NFATc1-c4). Once in the nucleus, NFATc1 to NFATc4 cooperate with various coactivators (NFATn) to promote gene transcription. NFAT kinases, such as GSK-3, rephosphorylate NFATc1 to c4, thus leading to their relocalization to the cytoplasm.
NFAT activation following TCR stimulation. TCR stimulation leads to calcium flux and additional signaling events that result in the activation of calcineurin, a Ca+2-calmodulin–dependent phosphatase that dephosphorylates NFAT proteins, enabling their movement to the nucleus (NFATc1-c4). Once in the nucleus, NFATc1 to NFATc4 cooperate with various coactivators (NFATn) to promote gene transcription. NFAT kinases, such as GSK-3, rephosphorylate NFATc1 to c4, thus leading to their relocalization to the cytoplasm.
Immunosuppressant drugs block NFAT activity
NFAT and the pathways leading to its activation are considered to be targets for potential therapeutic intervention especially in the efforts to search for and design better immunosuppressants. The commonly used drugs in current use that block NFAT are CsA and FK506.39 These act by binding to their respective immunophilins, cyclophilin (CsA) and FKBP12 (FK506).39,40 The immunophilin-drug complex binds to calcineurin and inhibits its phosphatase activity, thus preventing the dephosphorylation and translocation of NFAT to the nucleus.40 Although CsA and FK506 are effective immunosuppressive drugs, side effects such as nephrotoxicity, neurotoxicity, diabetogenicity, and gastrointestinal toxicity have considerably reduced their impact.41 These side effects are likely caused by the pleiotropic metabolic effects these agents exert through binding to immunophilins and inhibiting calcineurin in cells outside the immune system.42 Indeed, NFATc1 to NFATc4 are all regulated by Ca2+/calcineurin and therefore are susceptible to CsA and FK-506 treatment3 (Figure 1). NFAT5 differs in its regulation by osmotic stress and integrin activation.3 The identification of NFAT as a molecular target in T-cell activation suggests that a more directed, molecular-based approach to the development of immunosuppressive agents would have the potential for improved efficacy and reduced side effects. Manipulating NFAT pathways to treat disease will be facilitated by drugs that directly inhibit NFAT activation in cells of the immune system. One such potential activator to target is Vav1, a protein that is expressed only in cells of the immune system and is critical for NFAT induction.
Vav1
Although the vav proto-oncogene (Vav1) was initially identified as an oncogene,43 results from the past several years have placed the molecule as an important signal transducer protein, serving a pivotal role in hematopoietic cell activation as a guanine exchange factor (GEF) for Rho GTPases, as well as having a role as a scaffolding protein.44
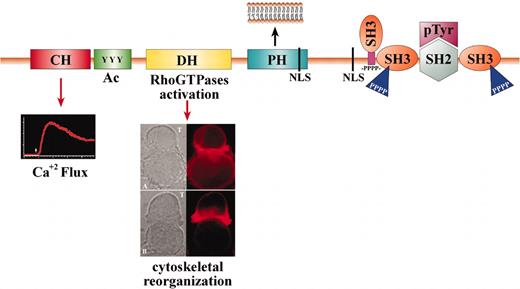
The Vav proteins contain numerous domains that are involved in multiple functions44 (Figure 2). These domains include: a Calponin-homology (CH), which is considered in other proteins to associate with F-actin; an Acidic (Ac) motif that contains 3 regulatory tyrosines in mammalian Vav proteins; a DBL-homology (DH) domain that promotes the exchange of guanosine diphosphate (GDP) for guanosine triphosphate (GTP) on Rho GTPases; a Pleckstrin homology (PH) domain that enables its movement to the inner face of the plasma membrane; and a proline-rich region that mediates the binding of Vav proteins to SRC-homology 3 (SH3)– containing proteins. Additional regions are as follows: 2 SRC-homology 3 (SH3) domains that interact with proteins that contain proline-rich sequences and an SRC-homology 2 (SH2) domain that interacts with proteins that contain phosphorylated tyrosines. There are also 2 putative nuclear localization signals (NLS) that are important for transport to the nucleus.43 In fact, Vav1 and the other ubiquitously expressed members of the Vav family of proteins (Vav2 and Vav3) are the only known Rho GEFs that have SH2 domains, suggesting that their GEF activity is regulated by tyrosine phosphorylation.44
Vav1 functions as a GEF and scaffold protein. The domain structure and function of Vav1 is depicted. The Vav proteins encode the following domains: Calponin-homology (CH) domain—This domain is involved in Ca+2 mobilization (inset). Acidic (Ac) motif—A motif that contains 3 regulatory tyrosines in mammalian Vav and 1 in DroVav. DBL-homology (DH) domain—A conserved region that promotes the exchange of GDP for GTP on Rac/Rho GTPases, thus affecting cytoskeleton reorganization. The inset depicts the reorganization of actin during the creation of the immunologic synapse. Jurkat T cells were incubated for 15 minutes at 37°C with antigen-presenting cells (APCs) (Raji) prepulsed with medium alone (upper panel of inset) or with staphylococcus enterotoxin E (SEE) superantigen (lower panel of inset). F-actin was detected using Alexa546-coupled phalloidin (red). The cells were then analyzed by confocal microscopy. The position of the T cell and the APC (B cell) is indicated in the differential interference contrast (DIC) image by arrows. Pleckstrin homology (PH) domain—A domain that binds phosphatidylinositol 3-kinase (PI3K)–generated lipid products that enable its movement to the inner face of the plasma membrane (inset). Proline-rich region (-Pro-)—A short amino acid sequence rich in prolines that enables the binding of Vav proteins to SH3-containing proteins. SRC-homology 3 (SH3) domain—This region interacts with proteins that contain proline-rich sequences. SRC-homology 2 (SH2) domain—This domain interacts with proteins that contain phosphorylated tyrosines. Nuclear localization signal (NLS)—A motif involved in translocation to the nucleus.
Vav1 functions as a GEF and scaffold protein. The domain structure and function of Vav1 is depicted. The Vav proteins encode the following domains: Calponin-homology (CH) domain—This domain is involved in Ca+2 mobilization (inset). Acidic (Ac) motif—A motif that contains 3 regulatory tyrosines in mammalian Vav and 1 in DroVav. DBL-homology (DH) domain—A conserved region that promotes the exchange of GDP for GTP on Rac/Rho GTPases, thus affecting cytoskeleton reorganization. The inset depicts the reorganization of actin during the creation of the immunologic synapse. Jurkat T cells were incubated for 15 minutes at 37°C with antigen-presenting cells (APCs) (Raji) prepulsed with medium alone (upper panel of inset) or with staphylococcus enterotoxin E (SEE) superantigen (lower panel of inset). F-actin was detected using Alexa546-coupled phalloidin (red). The cells were then analyzed by confocal microscopy. The position of the T cell and the APC (B cell) is indicated in the differential interference contrast (DIC) image by arrows. Pleckstrin homology (PH) domain—A domain that binds phosphatidylinositol 3-kinase (PI3K)–generated lipid products that enable its movement to the inner face of the plasma membrane (inset). Proline-rich region (-Pro-)—A short amino acid sequence rich in prolines that enables the binding of Vav proteins to SH3-containing proteins. SRC-homology 3 (SH3) domain—This region interacts with proteins that contain proline-rich sequences. SRC-homology 2 (SH2) domain—This domain interacts with proteins that contain phosphorylated tyrosines. Nuclear localization signal (NLS)—A motif involved in translocation to the nucleus.
The first indication for the involvement of Vav1 in NFAT stimulation was the demonstration that overexpression of Vav1 in Jurkat T cells leads to activation of NFAT.7 A more robust enhancement of NFAT- and IL-2–dependent transcription was obtained following T-cell receptor (TCR) stimulation.7 The activity of Vav1 as an inducer of NFAT stimulation was dependent on tyrosine phosphorylation and required an intact TCR signaling pathway.7 In vivo, the effect of Vav1 on the enhancement of the NFAT/AP-1 complex ranges from moderate45 to profound,46,47 depending on the experimental system used. Holsinger et al45 noted that lymphocytes from Vav1-/- mice were deficient in IL-2 production, yet the induction of the transcription factor, NFAT, was normal. In contrast, others reported the complete loss of both NFAT and IL-2 activation in T cells from Vav1-/--deficient mice.46,47 Additionally, a Vav1-deficient Jurkat T-cell line (J.Vav1) exhibits dramatic defects in TCR-dependent IL-2 promoter activation.48 Despite the diverse in vivo range of responses, it is unambiguous that Vav1 plays a critical role in signaling cascades that are responsible for IL-2 production.
The activation of NFAT/AP-1 complex requires several converging pathways. Vav1, a protein with multiple domains, could participate in more than 1 pathway and in more than one fashion, that is, either as a GEF protein that could lead to the induction of NFAT and AP-1 cascades through activation of Rho/Rac GTPases and/or through GEF-independent mechanisms. The answer to this question is as complex as the structure and function of Vav1. I will therefore review the data that indicate that the GEF activity of Vav1 is critical for its ability to induce the NFAT/AP-1 complex in T cells and also discuss the possible role of various GEF-independent Vav1 interactions in this process.
NFAT induction in T cells: the role of Vav1 asaGEF
Vav1 is the only known GEF protein whose activity depends on its phosphorylation on tyrosines.44 The tyrosine kinase Lck was shown to activate the guanine nucleotide exchange activity of Vav1 in vitro.49 Furthermore, the ability of Vav1 to induce NFAT was found to be dependent on TCR stimulation, which leads to enhanced phosphorylation of Vav1 as well as other proteins.7,50 Additionally, the protein tyrosine kinase (PTK) inhibitor, herbimycin A, which markedly impairs TCR signaling was shown to block NFAT stimulation in Jurkat T cells that overexpress Vav1, suggesting that PTKs are required for Vav1 function.7 The idea that the GEF activity of Vav1 depends on its tyrosine phosphorylation is consistent with the fact that the induction of NFAT/AP-1 is regulated and elevated following TCR stimulation.51 Although there is still no direct evidence that a specific phosphorylation event is required for Vav1 function in vivo, a critical question is whether the GEF activity of Vav1 is necessary for NFAT/AP-1 stimulation.
The GEF activity of Vav1 toward Rac appears cardinal for Vav1-induced NFAT stimulation, and activation of Rac by Vav1 stimulates c-Jun N-terminal kinase (JNK), followed by enhanced transcriptional and DNA-binding activities of AP-1 and increased phosphorylation of c-Jun.51 Several lines of evidence support this belief. First, overexpression of Vav1 enhances the phosphorylation of c-Jun in T cells, correlating with the transcriptional activation of AP-1 and NFAT/IL-2, which is a composite transcriptional element that binds both an NFAT family member and a Fos · Jun heterodimer.51 Second, J.Vav1 cells display a severe defect in TCR-mediated JNK activation, concomitant with loss of NFAT/IL-2–dependent transcriptional response.48 Third, a Vav1 GEF defective mutant (L213A) suppresses the TCR-induced c-Jun phosphorylation51 and fails to rescue the JNK and AP-1 activation defects in J.Vav1 cells,48 thus indicating that these responses require the Rho/Rac GEF activity of Vav1. Despite these clear in vitro correlations between Vav1's activity as a GEF and NFAT transcription, the physiologic role of a Vav1/Rac pathway in JNK–AP-1 activation and the function of activated JNK in the induction of NFAT are not entirely understood. Nonetheless, the results to date strongly suggest that intact GEF activity is required for the effects of Vav1 on transcriptional activation.
This dependence on Vav1's GEF activity suggests that the mechanism of Vav1-mediated NFAT stimulation might involve the ability of Rac, which is activated by Vav1 GEF activity, to alter cytoskeletal organization.52,53 The catalytic function of Vav1 as a GEF toward Rho family GTPases is the key for transferring the signal from the activated TCR to Rac and thereby to the cytoskeleton52,53 (Figure 2). Vav1 was shown to be important for cytoskeletal reorganization processes such as capping of T cells,45,46 lipid raft formation,54 and the organization of the immunologic synapse (IS)53 (Figure 2). Furthermore, the fact that inhibitors of actin polymerization result in defects in NFAT responses similar to those demonstrated in Vav1-/- T cells46 suggests that cytoskeletal reorganization is required for NFAT activity in T cells. A dominant-negative Vav1 mutant defective in its catalytic DH domain (L213A) inhibits antigen-induced lipid raft aggregation at the IS.54 A dominant-negative Rac1 mutant caused similar effects, suggesting that raft polarization mediated by Vav1 proceeds through the enzymatic activation of Rac. These effects seem to be independent of Pak1, a well-characterized target of Rac1, since dominant-negative Pak1 did not inhibit raft polarization.54 This apparent lack of requirement for Pak1 might stem from the specific experimental system used or alternatively suggest that another Rac1 effector links Vav1/Rac to lipid raft clustering.
Rac might control cytoskeletal alterations by activating phosphatidylinositol-4 phosphate 5-kinase (PIP5K), which phosphorylates phosphatidylinositol 4 phosphate (PIP), producing phosphatidylinositol 4,5-biphosphate (PIP2).55 PIP2 is a substrate of phospholipase C-γ (PLCγ), which mediates the release of Ca2+, a mandatory event for NFAT stimulation. In this manner, the activity of Vav1 as a GEF for Rac could eventually lead to NFAT stimulation.
NFAT induction in T cells: the role of GEF-independent Vav1 activities
Rho/Rac-independent pathways are also involved in Vav1-induced NFAT stimulation.7 Overexpression of oncogenic Vav1 (oncVav1), which lacks 66 residues of its amino-terminus but retains the ability to function as a GEF and as a regulator of the development of cell-mediated killing,56,57 failed to induce NFAT in Jurkat T cells.7 Wild-type Vav1 does induce NFAT under the same experimental conditions.7 Moreover, Kuhne et al demonstrated that a GEF mutant in which residues 338 to 344 were substituted was able to enhance NFAT activity although it was unable to activate Rac.58 Finally, dominant-active Rac1, RhoA, or Cdc42 were unable to stimulate NFAT or Ca2+ fluxes in Vav1-/- T cells, implying that activation of Rho family GTPases by GEF activity is not sufficient for Vav1-mediated NFAT activation.
The cascade leading from Vav1 activation to NFAT stimulation was recently found to include Ca2+ mobilization, which is mandatory for the enhancement of NFAT activity. Wild-type Vav1, but not oncVav1 mutants, can induce Ca2+ flux in T cells57,59,60 (Figure 2). Since the oncVav1 mutants retain GEF activity, this implies that the induction of Ca2+ is mediated by GEF-independent Vav1 activities. T cells from Vav1-/- mice have moderate to profound defects in TCR-dependent Ca2+ mobilization, further supporting the link between Vav1 and Ca2+ release.45-47,61 This greatly reduced TCR-induced calcium flux of Vav1-deficient T cells can be restored with calcium ionophores. Additional defects were noted in these Vav1-deficient cells, including a failure to sustain the [Ca2+]I elevation at later times after receptor stimulation, when the Ca2+ elevation is largely attributable to the opening of inactivation of the Ca2+ release-activated Ca2+ current (ICRAC) channels in the plasma membrane.48
Vav1 induces Ca2+ release: potential signaling cascades
Several recent studies indicate that Vav1 controls Ca2+ flux by participating in the regulation of PLCγ activation. Tyrosine phosphorylation of PLCγ1 and PLCγ2 as well as calcium mobilization were markedly inhibited in Vav1-deficient bone marrow–derived mast cells.60 Reconstitution of these cells with Vav1 restored normal tyrosine phosphorylation of PLCγ1 and PLCγ2 as well as calcium responses. Similar results were obtained in thymocytes from mice with transgenic TCR on a β2 microglobulin/Vav1–deficient background in which phosphorylation and activation of PLCγ were defective.61 Furthermore, TCR-inducible phosphorylation of Itk and Tec is defective in the absence of Vav1.61 These data suggest that Vav1 participates in a multiprotein complex that leads to the activation of PLCγ.
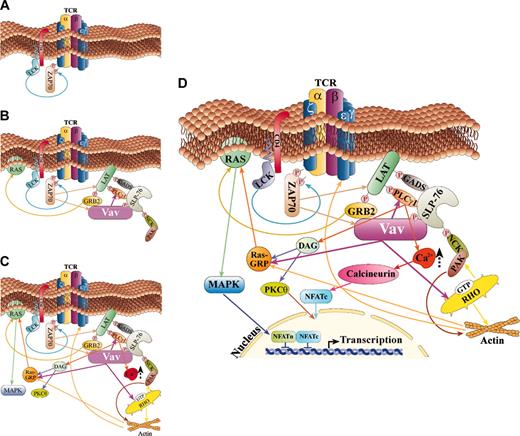
The current model for how this multiprotein complex is formed and how it regulates Ca2+ flux is based on the multiple protein-protein interactions induced following TCR stimulation62 (Figure 3). Once the TCRs recognize an antigen (Ag), a series of events lead to the phosphorylation of the tyrosine kinase Syk family member, zeta-associated protein 70 (ZAP-70)63 (Figure 3A). Several proteins are then tyrosine phosphorylated by ZAP-70. One of these proteins is presumably Vav1, which associates with ZAP-70 through its SH2 region64 (Figure 3B). A point mutation in tyrosine 315 of ZAP-70 (Y315F), a putative Vav1 SH2 domain binding site, eliminated the interaction between these proteins and abolished NFAT stimulation.65 Surprisingly, this mutation also resulted in marked reduction in the tyrosine phosphorylation of ZAP-70, Vav1, SH2 domain–containing leukocyte protein of 76 kDa (SLP-76), and Shc.65 These data demonstrate that the Vav1 binding site in ZAP-70 plays a critical role in antigen receptor–mediated signal transduction.65 PLCγ1 and linker for activation of T cells (LAT), an adapter protein with multiple tyrosine residues, are also phosphorylated by ZAP-70 following TCR stimulation (Figure 3B). LAT is then able to recruit several SH2 domain–containing proteins including PLCγ1, the p85 subunit of phosphatidylinositol 3-kinase (PI3-K), and the growth factor receptor-bound protein-2 (Grb2) and its family member Gads.66,67 Gads constitutively associate through their SH3 region with the proline-rich region of SLP-76,68 a protein that also associates with the SH3 region of PLCγ69 and becomes tyrosine phosphorylated by ZAP-70 (Figure 3B). The N terminus of SLP-76 contains 3 tyrosines critical for SLP-76 function and is proposed to directly associate with the SH2 domain of Vav1,70 the adapter Nck,71 and Itk,72 a Tec family kinase (Figure 3B). A third functional region of SLP-76 is its C-terminal SH2 domain, which associates with adhesion- and degranulation-promoting adapter protein (ADAP)73 and the serine-threonine kinase HPK-1,74 following TCR stimulation. Coexpression of Vav1 with SLP-76 induces a synergistic response, leading to enhanced activation of the NFAT transcriptional activator.70 However, Fang and Koretzky have reported that the synergistic response between Vav1 and SLP-76 occurs also with mutants of SLP-76 that cannot bind to Vav1.75 Although this issue is still unresolved, it is evident that both proteins are critical for NFAT induction. The inducible association of PLCγ1 with SLP-76/Gads is defective in Vav1-/- thymocytes,61 indicating that Vav1 is important for the formation and/or stabilization of this complex. The association between SLP-76 and Itk, a PLCγ activator, might also play a role in PLCγ activation, although its mechanism is unclear. Apparently, the complex web of proteins, LAT–PLCγ–Gads–SLP-76–Vav1, is recruited to the glycolipid-enriched membrane (GEM) microdomains through LAT,62 resulting in maximum activation of PLCγ (Figure 3C).
Vav1 is involved in NFAT activation in more that one pathway in T cells. A scheme of the cardinal regulatory pathways in which Vav1 is involved as an organizer of the cytoskeleton and a regulator of Ca2+ flux, thus leading to NFAT activation, is depicted. (A) This scheme depicts the initial steps in TCR activation and tyrosine kinase stimulation. (B) Tyrosine phosphorylation of Vav1 and other signaling proteins leads to association between these proteins and their activation. (C) Following TCR stimulation and activation of additional proteins, Vav1 activates Rac and is involved in Ca2 release as well as Ras activation. (D) A summary scheme of the various pathways in which Vav1 is involved in T cells that culminate in NFAT activation. A detailed explanation for each pathway is included in “Vav1 induces Ca2+ release: potential signaling cascades.”
Vav1 is involved in NFAT activation in more that one pathway in T cells. A scheme of the cardinal regulatory pathways in which Vav1 is involved as an organizer of the cytoskeleton and a regulator of Ca2+ flux, thus leading to NFAT activation, is depicted. (A) This scheme depicts the initial steps in TCR activation and tyrosine kinase stimulation. (B) Tyrosine phosphorylation of Vav1 and other signaling proteins leads to association between these proteins and their activation. (C) Following TCR stimulation and activation of additional proteins, Vav1 activates Rac and is involved in Ca2 release as well as Ras activation. (D) A summary scheme of the various pathways in which Vav1 is involved in T cells that culminate in NFAT activation. A detailed explanation for each pathway is included in “Vav1 induces Ca2+ release: potential signaling cascades.”
Activated PLCγ cleaves the PIP2 to generate 2 products: inositol 1,4,5-triphosphate (IP3) and diacylglycerol. IP3 leads to the opening of IP3-gated Ca2+ release channels in the endoplasmic reticulum (ER) membrane, while diacylglycerol leads to the induction of protein kinase C (PKC), followed by activation of the Ras pathway (Figure 3C).
Additionally, the link between Vav1/SLP-76 and SLP-76/Nck might also serve a role in the potentiation of NFAT.76 In this complex, Vav1 and Nck bind via their SH2 domains to different phosphotyrosine residues of SLP-76. At the same time, Rac1 binds to the DH domain of Vav1 and Pak to the SH3 domains of Nck (Figure 3C). The final outcome of this multimeric complex is the close proximity between activated Rac1 and its effector Pak77 that results in NFAT stimulation. Indeed, Yablonski et al78 showed that Pak1 is activated after stimulation of the TCR by a pathway involving the Src-family tyrosine kinase Lck and Vav1. Pak1 also undergoes TCR-inducible association with the Nck adapter, which is tyrosine phosphorylated following stimulation. Dominant-negative alleles of Pak1 or Nck specifically block TCR-induced NFAT activation.78 Interestingly, Pak1 is required for TCR- or Vav1-mediated signaling to the nucleus by a pathway that is independent of JNK but requires Erk2. Thus, Pak1 is essential for activation of the transcription factor NFAT following TCR stimulation78 (Figure 3C).
PI3-K may also be part of the cascade from Vav1 to Ca2+ release. PI3-K catalyzes the phosphorylation of inositol phospholipids at the 3 position of the inositol ring to generate lipids called PI(3,4)P2 or PI(3,4,5)P3. Several studies implicate PI3-K as an upstream regulator of Vav1, since its lipid products play a role in binding to the PH region of Vav1 that brings it to the cytoplasmic face of the plasma membrane, where it may enhance Ca2+ release.79 In contrast, the fact that several PI3-K downstream effectors such as protein kinase B, and the Tec-family kinases, Itk and Tec, are defective in Vav1-/- thymocytes suggests that Vav1 is an upstream regulator of PI3-K activation.61 Thus, it possible that Vav1 influences PI3-K activity, which in turn leads to activation of the Tec kinases required for PLCγ enhancement. This potential pathway remains to be elucidated.
Taken together, Vav1 participates in the generation of multiprotein complexes that lead to Ca2+ release and to cytoskeleton reorganization, events critical for NFAT stimulation (Figure 3D). Many facets of the model suggested in Figure 3D remain to be definitively shown to be part of this particular pathway. In addition, alternate pathways for Vav1's stimulation of Ca2+ release may exist, as is suggested by the fact that this model cannot explain several of the results obtained with oncVav1.
Vav1 and Ly-GDI: a novel mechanism underlying Vav1-induced activity
OncVav1, which lacks the Vav1 amino-terminus, does not induce Ca2+ release and is defective in NFAT transcription.7,57,59,61 However, all the domains needed for its participation in the multiprotein complex detailed in Figure 3D are intact. Thus, the amino-terminus of Vav1 might be involved in the regulation of Ca2+ release. The amino-terminus region (residues 3-124) resembles a calponin homology region, yet it is unlikely to directly associate with F-actin since 2 such regions in tandem are needed for association with actin.80 As this region is devoid of any catalytic activity and is rich in alpha-helical content, it may participate in protein-protein interactions. Intriguingly, we have recently demonstrated that the amino-terminus region of Vav1 interacts in vitro and in vivo with another potential regulator of Rho GTPases, the hematopoietic-specific guanine dissociation inhibitor (GDI) protein, Ly-GDI.59,81 Ly-GDI seems to inhibit NFAT stimulation, which correlates with its capacity to block calcium mobilization.81 This activity most probably stems from its ability to inhibit members of the Rho GTPases. However, rather than counteracting the effects of Vav1, Ly-GDI further enhances the induction of NFAT following TCR stimulation in T cells overexpressing Vav1.81 Furthermore, when coexpressed with Vav1, Ly-GDI also enhances Vav1's effects on PLCγ phosphorylation and possibly calcium mobilization. However, it does not alter the regulation of these phenomena when overexpressed with oncVav1, with which it does not associate.59,81 These results strongly support the hypothesis that Vav1 and Ly-GDI must physically associate to exert their cooperative functions.
It is conceivable that Vav1 removes Ly-GDI from its target GTPase, thus enabling Vav1 to function more efficiently as a GEF. However, this suggestion does not entirely explain the observed synergism of Vav1 and Ly-GDI on NFAT activity, unless the target RhoGTPases for Vav1 and Ly-GDI are different. It is possible that the association of Vav1 and Ly-GDI also influences other signaling pathways. The observed increase in PLCγ tyrosine phosphorylation in cells transfected with Vav1 and Ly-GDI compared with cells transfected with Vav1 alone may be one example of this. Additional signaling cascades might also be influenced by Ly-GDI as suggested by the fact that Ly-GDI associates with the SH2 domain of the adapter protein Shc.81 Shc was shown to be involved in activation of c-Rel and mitogen-activated protein kinase (MAPK) and it is required for TCR-induced IL-2 production.82 Vav1 also binds multiple signaling proteins (Figure 3). The fact that Ly-GDI and Vav1 associate with adapter proteins that form multicomplexes as well as with signaling proteins and of course with each other suggests that they might influence various signaling pathways in T cells, including stimulation of PI3-K and/or the Ras/MAPK pathway. Our data suggest that the interaction of Vav1 and Ly-GDI create a fine-tuning mechanism for the regulation of intracellular signaling pathways leading to NFAT stimulation.
Additional proteins were found to associate with the amino-terminus of Vav1. One such protein is ENX-1, which represents the human homolog of the Drosophila enhancer of zeste gene, a member of the Polycomb group of genes, which are transcriptional regulators of homeobox gene expression.83 No further information is available regarding the importance of the interaction between these proteins to the role of Vav1 in T cells. Suppressor of cytokine signaling-1 (Socs1) was also reported to bind to the N-terminal regulatory region of Vav1.84 However, oncVav1, which lacks 66 N-terminal residues, associates with Socs1 at the same efficiency as wild-type Vav1, making it less likely that this association plays a role in NFAT/AP-1 complex formation.85 Thus, only the association between the amino-terminus of Vav1 and Ly-GDI is known to allow Vav1 to enhance NFAT. Nonetheless, the possibility remains that additional proteins might associate with this region and also contribute to its regulatory role.
Vav1 and Ras, 2 important proteins for NFAT stimulation
Numerous studies suggest that Ras is involved in Vav1-induced NFAT stimulation, but the exact link between these proteins is still unclear. Indeed, both a dominant-negative mutant of Ras and a dominant-negative mutant of Raf, which functions downstream of Ras, inhibit the activation of NFAT in Jurkat cells overexpressing Vav1.7 More directly, Vav1 promotes the activation of ERK,51 a known Ras-dependent kinase in the TCR signaling pathway, and receptor-mediated ERK activation is deficient in Vav1-/- T cells.48 Additionally, the MAPK kinase (MEK) inhibitor PD90859 inhibited Vav1-induced activation of ERK and Vav1- or anti-CD3–induced activation of NFAT.51 A dominant-negative Ras mutant inhibited all of these Vav1-mediated functions, indicating that Ras functions as an important downstream target of Vav1 in signaling pathways leading to NFAT and ERK activation.51 In contrast, Ras and ERK activation in TCR-stimulated J.Vav1 cells was found to be normal.48 Yet, the J.Vav1 cells displayed significant defects in TCR-dependent CD69 expression, a response that is tightly linked to activation of the Ras signaling cascade in T cells.48 A possible link between Vav1 and Ras might stem from the constitutive association of Vav1 with Grb2, which functions in the Ras pathway,86,87 though this possibility has to be further substantiated. A recent study demonstrated a novel link between Vav1 and the Ras pathway through the stimulation of RasGRP1, an exchange factor for the Ras subfamily GTPases.88 This interaction is critical for Ras activation in lymphoid cells. The activation of RasGRP1 requires both the generation of diacylglycerol via PLCγ and the induction of actin polymerization, 2 responses induced by Vav1 and Rac188 (Figure 3D).
In summary, Vav1 appears to be involved in Ras signaling in T cells in a GEF-dependent and -independent manner, but many details of these pathways are lacking. Furthermore, it is not entirely clear whether the Vav1/Ras link reflects parallel signaling pathways or converging pathways.
Is Vav1 a component of transcriptionally active complexes?
Thus far the common notion was that Vav1 could lead to NFAT stimulation through its activities as a GEF and a scaffold protein. However, a recent study suggests that Vav1 can be part of transcriptionally active complexes, including NFAT.89 Vav1 is a cytoplasmic protein that moves to the plasma membrane upon stimulation of hematopoietic cells.90 Several reports indicated that Vav1 might also be found in the nucleus under diverse stimulation means of various cells.89,91,92 Thus, Vav1 translocates into the nucleus of a T-cell line following prolactin-stimulated proliferation.91 It also translocates to the nucleus during interferon (IFN) stimulation.92 In this study it was demonstrated that disruption of Vav1 expression with antisense oligonucleotides reverses the antiproliferative effects of IFNα in megakaryocytic cell lines, suggesting that Vav1 is part of a type I IFN–activated pathway that mediates growth inhibitory responses.92 Furthermore, Vav1 translocates to the nucleus upon prolonged stimulation of the Fc receptor I (FcRI), and its movement to the nucleus is dependent on 1 of its 2 NLS sequences.89 The Vav1 nuclear targeting is under the control of the carboxy-terminal SH3 (C-SH3) domain, which serves to sequester its presence in the cytoplasm by an unknown mechanism. It is possible that the association of the C-SH3 with cytoskeletal proteins such as zyxin influences its localization.93 Alternatively, the C-SH3 domain may mask the NLS within the PH domain by an intramolecular interaction. Unlike wild-type Vav1, a Vav1 mutant that lacks the C-SH3 region was unable to induce NFAT activity.89 According to this study, Vav1 facilitates the movement of NFAT to the nucleus.89 Vav1 also participates in a transcriptionally active complex that attaches to the NFAT binding site of the IL-2 promoter. Once Vav1 gets into the nucleus, it acts as a nuclear partner of the transcription factor NFAT and the nuclear factor (NF) B-like factor.89 Indeed, supershift analysis of the activated complex, using 2 different antibodies, established the presence of Vav1.89 It is conceivable that the activity of Vav1 in the nucleus is exerted by its role as an adapter protein. The C-SH3 region was shown, for instance, to associate with Ku-70.94 Ku-70 plays a role as part of the DNA-dependent protein kinase complex. Furthermore, it interacts with homeobox transcription factors.95 A concomitant increase of Vav1's association with Ku-70 was also observed in the nucleus of cells treated with type I IFN.92
Thus, a novel putative role has been assigned to Vav1 in the nucleus, where it may be a component of an active transcriptional complex. However, the questions of how widely such a mechanism is used and following which types of activation await further studies.
Involvement of other members of the Vav family, Vav2 and Vav3, in NFAT stimulation
Like Vav1, Vav2 and Vav3 are also expressed in the hematopoietic system and may potentially participate in NFAT regulation.44 Although Vav2 is tyrosine phosphorylated following antigen receptor engagement in both B and T cells, it potentiates NFAT-dependent transcription only in B cells.96,97 The enhancement of NFAT-dependent transcription by Vav2 can be inhibited by dominant-negative GTPases. Vav2 augments the calcium signal in B cells, and a truncated form of Vav2 can neither activate NFAT nor augment calcium signaling.96,97 In vivo experiments showed that deletion of both Vav1 and Vav2 in mice resulted in a marked reduction in numbers of mature B but not T lymphocytes.98,99 These B cells were unresponsive to B-cell antigen receptor (BCR)–driven proliferation and calcium release in vitro and to thymus-independent antigen response in vivo.98,99 Although not much is known regarding the activity of Vav3, it is clear that, like Vav2, it does not activate NFAT in T cells.97 Thus, despite the similarity in protein structure between the Vav proteins, only Vav1 potentiates NFAT stimulation when overexpressed in T cells.96,97 The reason for the difference in NFAT activation by the various Vav proteins is not known, but may stem from differences in their activity as GEFs toward different RhoGTPases. Several in vitro and in vivo studies have shown that Vav1 is primarily a GEF for Rac1, Rac2, and RhoG.44 The ability of Vav1 to mediate the activation of Cdc42 in vitro is controversial. Although it has been reported that the activation of Cdc42 in Vav1-/- T cells is defective, it is not clear whether this indicates that Vav1 has a direct effect on this GTPase or whether this defect is a by-product of the inactivity of Rac.44 On the other hand, Vav2 and Vav3 activate RhoA, RhoB, and RhoG.44 Since the activation of different GTPases could trigger distinct signaling cascades and thereby different biologic processes, this could explain the observed differences in the activities of the Vav proteins. Alternatively, different proteins may associate with the amino-terminus of each of the Vav proteins. It is noteworthy that the amino-terminus of Vav2 does not associate with Ly-GDI (M. Groysman and S.K., unpublished data, May 2002), so that this difference between Vav1 and Vav2 might contribute to their divergent activities in Ca2+ release and NFAT stimulation in T cells. It remains to be determined which proteins associate with the amino-terminus of Vav2 and Vav3 and how these interactions affect their activity. Additional in vivo studies will be required to fully establish the contribution of all Vav forms to the biology of T lymphocytes and other cell lineages.
Conclusions: targeting Vav1 in hematopoietic cells
Since Vav1, but not Vav2 or Vav3, seems to be highly critical for the function of NFAT in T cells, eliminating Vav1 in T cells might be an effective means to specifically block NFAT activity. One powerful tool for silencing gene expression in mammalian cells is the use of short interfering RNA (siRNA), and it will be of interest to use this technique to eliminate Vav1 and also its various partners to validate their roles in NFAT stimulation. Results of these silencing experiments should speed the identification of potential targets for small molecules that impede or mimic their function, thereby regulating NFAT activity. This approach in turn may allow development of more effective and more specific agents for T-cell inhibition or stimulation.
Prepublished online as Blood First Edition Paper, October 30, 2003; DOI 10.1182/blood-2003-08-2834.
Supported by grants from the Israel Academy of Sciences and the Israel Cancer Association.
I am grateful to Dr M. K. Brenner for critical reading of the manuscript, Dr Susan Lewis for editing assistance, and members of my laboratory for their suggestions. I am indebted to Mr Y. Markson for preparing the figures and to Dr A. Alcover for contribution of the IS figure.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal