Abstract
Despite the success of anti-CD20 monoclonal antibody (mAb) in the treatment of lymphoma, there remains considerable uncertainty about their mechanism(s) of action. Here, we show that certain of these reagents (rituximab and 1F5), which redistribute CD20 into membrane rafts, are bound efficiently by C1q, deposit C3b, and result in complement-dependent cytotoxicity (CDC). This activity is important in vivo, because complement depletion using cobra venom factor (CVF) markedly reduced the efficacy of rituximab and 1F5 in 2 lymphoma xenograft models. However, complement depletion had no effect on the potent therapeutic activity of B1, a mAb that does not redistribute CD20 into membrane rafts, bind C1q, or cause efficient CDC. Equivalent immunotherapy also occurred in the presence or absence of natural killer (NK) cells. Perhaps most surprising was the observation that F(ab′)2 fragments of B1 but not 1F5 were able to provide substantial immunotherapy, indicating that non-Fc-dependent mechanisms are involved with B1. In accordance with this, B1 was shown to induce much higher levels of apoptosis than rituximab and 1F5. Thus, although complement is important for the action of rituximab and 1F5, this is not so for B1, which more likely functions through its ability to signal apoptosis. (Blood. 2004;103:2738-2743)
Introduction
Previous studies have suggested that several mechanisms might be involved in providing therapeutic efficacy through anti-CD20 reagents, including antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and the induction of growth arrest or apoptosis. Currently, the bulk of the experimental evidence indicates that rituximab operates through conventional effector mechanisms measured by CDC and ADCC assays, the most conclusive data coming from work in primates showing that an immunoglobulin G4 (IgG4) variant of rituximab, unlike the chimeric IgG1 monoclonal antibody (mAb), was unable to deplete normal B cells.1
The evidence that ADCC and FcγR are important in the therapeutic activity of rituximab comes from several sources, both clinical and experimental. In the former, it has been shown that patients with non-Hodgkin lymphoma (NHL) expressing the high-affinity 158V variant of the FcγRIIIa gene have better response rates after rituximab treatment than those carrying the low-affinity allotype.2 Similar results have now been shown for the depletion of B cells with rituximab in systemic lupus erythematosus (SLE).3 The importance of FcγR in vivo is also supported by elegant work from Clynes et al4 that used FcγR-deficient mice, which indicated that FcγR on macrophages are critical to the ability of mAb to control subcutaneous B-cell lymphoma. Thus, there are clearly data to support a role for FcγR-bearing effectors in mediating the activity of anti-CD20 mAb. Although these experiments seem to indicate the importance of FcγR-expressing cells as cytotoxic effectors, the possible role of these cells in enhancing mAb and receptor cross-linking should not be ignored, as in vitro experiments show that signaling activity is often enhanced by hyper-cross-linking either using anti-Ig antisera, immobilization, or FcγR-bearing ADCC effector cells.5-7
Support for the role of complement comes from the demonstration that complement is consumed during rituximab treatment8 and that on some occasions cells remaining after treatment appear to have increased levels of the complement defense molecule CD59,9,10 consistent with the idea that they had been subject to complement selection. Similarly, it has been shown that the resistance of different lymphoma cells to rituximab in vivo may be related to their sensitivity to CDC in vitro.11 However, it should be noted that certain researchers have reported that expression of complement inhibitors on tumor cells does not predict clinical outcome after rituximab treatment in follicular lymphoma and that no correlation between in vitro complement sensitivity and subsequent therapeutic response was observed.12
In contrast to rituximab, almost no information is available concerning the mode of action of B1 in vivo, which has just been approved for treatment of non-Hodgkin lymphoma by the US Food and Drug Administration as an I131 conjugate, tositumomab (Bexxar). In fact, preclinical data suggest that naked B1 was as effective as the I131-labeled compound.13
Previously, we have demonstrated that CD20 was a particularly good target antigen for evoking high levels of CDC.14 These in vitro studies revealed that much of the potency of anti-CD20 mAb was due to their ability to redistribute CD20 into “lipid-raft” regions of the membrane. However, the previous work did not consider how the ability of an anti-CD20 mAb to translocate CD20 into lipid rafts might relate to its activity in vivo.
Here, we demonstrate that the CDC activity of anti-CD20 mAb relates directly to their binding of the first component of complement, C1q. Furthermore, complement activation is an important component of the therapeutic activity of those mAbs, such as 1F5 and rituximab, that are able to translocate CD20 into lipid rafts and consequently mediate efficient CDC. However, neither complement nor natural killer (NK) cell activity appears to be required for B1-induced immunotherapy in lymphoma xenografts. Instead, B1, unlike 1F5 and rituximab, was able to induce potent apoptosis, which probably accounts for its efficacy in vivo.
Materials and methods
Cell lines
Human cell lines were obtained from the European Collection of Animal and Cell Cultures (ECACC) and were maintained in antibiotic free RPMI-1640 medium with fetal calf serum (FCS; Myoclone, 10%), glutamine (2 mM), and pyruvate (1 mM) (Gibco, Paisley, United Kingdom), at 37°C, 5% CO2. The FCS used was heat inactivated at 56°C for 30 minutes to remove any complement activity.
Antibodies
The mAbs used in this study are detailed in Table 1. B1 and rituximab were kind gifts from Dr T. Illidge (University of Southampton, United Kingdom). The anti-C3b mAb was a kind gift from Prof B. P. Morgan (University of Cardiff, Wales). 1F5 and all other mAbs were produced from hybridoma lines, secreting mAb in tissue culture and purified from culture supernatant using Protein A columns (Amersham Biosciences, Little Chalfont, Bucks, United Kingdom) according to the manufacturer's instructions and the purity of mAb produced was routinely assessed by electrophoresis (Beckman EP system; Beckman, Hialeah, FL). The presence of aggregated IgG was routinely assessed in the mAb preparations by high-performance liquid chromatography (HPLC) and found to be minimal, with aggregates no more prevalent in any given mAb preparation. F(ab′)2 fragments of IgG were produced by standard pepsin (1F5) or bromelain digestions (B1) and were further purified by passing material through an antimouse Fc column.17 To verify absence of IgG contamination, samples of each preparation were taken, boiled for 2 minutes in loading buffer containing 2-mercaptoethanol, and loaded onto 10% polyacrylamide gels containing sodium dodecyl sulfate (SDS), prior to electrophoresis and staining with Coomassie blue. As expected IgG preparations show typical heavy and light chain bands at approximately 50 and 25 kDa, respectively, whereas F(ab′)2 fragments show cleaved heavy (Fd) and light chains only. Note, the Fd and light chain fragments of the reduced B1 F(ab′)2 comigrate.
Specificity, isotype, source, and properties of mAbs used
. | . | . | . | Redistribution to rafts . | . | |
|---|---|---|---|---|---|---|
| mAb . | Specificity . | Isotype . | Source . | No x-link . | + x-link . | |
| OKT3 | CD3 | mlgG2a | ATCC Hybridoma | − | − | |
| IF5 | CD20 | mlgG2a | ECACC Hybridoma | ++ | ++++ | |
| B1 | CD20 | mlgG2a | Coulter, Miami, FL | −/+ | + | |
| Rituximab | CD20 | Chimeric Hu Fc | IDEC, San Francisco, CA | +++ | ++++ | |
| WR17 | CD37 | mlgG2a | Moore et al15 | − | +++ | |
| 1B4 | CD38 | mlgG2a | Funaro et al16 | − | +++ | |
. | . | . | . | Redistribution to rafts . | . | |
|---|---|---|---|---|---|---|
| mAb . | Specificity . | Isotype . | Source . | No x-link . | + x-link . | |
| OKT3 | CD3 | mlgG2a | ATCC Hybridoma | − | − | |
| IF5 | CD20 | mlgG2a | ECACC Hybridoma | ++ | ++++ | |
| B1 | CD20 | mlgG2a | Coulter, Miami, FL | −/+ | + | |
| Rituximab | CD20 | Chimeric Hu Fc | IDEC, San Francisco, CA | +++ | ++++ | |
| WR17 | CD37 | mlgG2a | Moore et al15 | − | +++ | |
| 1B4 | CD38 | mlgG2a | Funaro et al16 | − | +++ | |
In the isotype column, m indicates mouse and Hu indicates human immunoglobulins. Raft redistribution in the presence (+ x-link) or absence (no x-link) of hyper-cross-linking agent was determined by insolubility in 0.5% TX-100 as detailed previously.14 The number of +'s signify the extent of redistribution.
CDC assay
CDC was assessed using a rapid and simple propidium iodide (PI) exclusion flow cytometric assay detailed previously.14 Briefly, cells were first incubated with mAb for 15 minutes at room temperature and then incubated with serum (20% normal human serum [NHS]) for a further 5 minutes at 37°C, before analysis. NHS for CDC assays was prepared from healthy volunteer blood following clotting.
Flow cytometry for C1q and C3b binding
Flow cytometric determination of C1q and C3b binding was performed on Daudi or EHRB cells following incubation with various mAbs for 15 minutes. After incubation, NHS (20% vol/vol) was added for 5 minutes, and the samples were incubated at 37°C before washing in cold phosphate-buffered saline (PBS) 1% bovine serum albumin (BSA) 0.01% Azide. Anti-C1q fluorescein isothiocyanate (FITC)-labeled mAb (Serotec, Oxford, United Kingdom) or anti-C3b FITC (a kind gift from B. P. Morgan) was then added to cells for 15 minutes at 4°C, before further washing and analysis by flow cytometry with a FACScan cytometer (BD Pharmingen, San Diego, CA) equipped with a 488-nm argon ion laser. Data were collected and analyzed using Cellquest (BD Pharmingen). Cell debris was excluded by using the forward scatter (FSC) threshold and at least 7500 events were collected per sample.
Hyper-cross-linking experiments
To move more target antigen into the Triton X-100-insoluble fraction of the cell membrane, additional hyper-cross-linking was provided by adding goat antimouse Ig F(ab′)2 fragments to mAb-coated cells. In these experiments, mAbs were incubated with cells for 15 minutes at room temperature, then washed, and divided into 2 samples. One of these samples was incubated for 10 minutes in RPMI alone, and the other was incubated in RPMI in the presence of 25 μg/mL goat antimouse Ig F(ab′)2 fragments. Under these conditions, almost all of the MB1 and 1B4 mAbs and a proportion of the B1 mAb move into the TX-100-insoluble membrane fraction.14 These separate samples (hyper-cross-linked or not) were then assessed in the C1q binding and CDC assays detailed above.
Immunotherapy
CB-17 severe compromised immune deficiency (SCID) and CB-17 SCID-Beige mice were sourced from Harlan United Kingdom Limited (Blackthorn, Oxon, United Kingdom), bred, and maintained under pathogen-free conditions. Animal immunotherapy was cleared through local ethical committee and was performed under Home Office license. Daudi or EHRB cells (2.5 × 106) were injected intravenously into the tail vein of cohorts of 12- to 16-week-old SCID or SCID-Beige mice, followed 7 days later by the intravenous injection of 100 μg mAb. Animals were culled on presentation of rear-limb paralysis. For complement-depletion groups, animals were injected with 25 μg cobra venom factor (CVF) intravenously on days 5, 9, and 12 after tumor. Each shot of CVF should deplete complement activity for up to 4 days.18 For NK cell depletion, ASGM1 Ab (Wako, Neuss, Germany) was injected intraperitoneally (10 μL in 200 μL PBS) on day 5 and day 9 after tumor inoculation. Depletion was assessed in the tail blood of 2 treated versus 2 control mice on day 7 using FSC/side scatter (SSC) criteria and staining with DX5 and Ly-49c mAb (both from Pharmingen, San Diego, CA) by flow cytometry. Anti-asialoGM1 (ASGM1)-treated mice possessed 3.1% ± 1.5% NK cells compared with 9.5% ± 1.4 % in untreated control mice at this time (n = 4).
Statistical analysis
Statistical analysis of immunotherapy results was performed using the chi-square test of Peto.19
Apoptosis assays
For experiments in which mAbs were placed in free solution, 10 μg/mL was added to cells for 18 to 24 hours. Following treatment, 1 × 105 cells were washed, resuspended in binding buffer (10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.4, 140 mM NaCl, 2.5 mM CaCl2), containing 1 μg/mL FITC-annexin V. PI (10 μg/mL) was also added to the samples to distinguish between early apoptosis and secondary necrosis. Subsequently, cells were assessed by flow cytometry. In experiments with coated mAbs, mAbs were added to 96-well plates (Nunc, Invitrogen, Paisley, United Kingdom) in PBS for 2 hours at 37°C. Unbound material was then removed, and cells were added to the plate for 18 to 24 hours before assessment of apoptosis as detailed in “Immunotherapy.” In some experiments apoptosis was confirmed by using the mitochondrial reporter dye DioC6, exactly as performed previously.20 This assay determines whether cells have undergone the mitochondrial permeability transition. For these experiments, 10 to 20 nM DioC6 was added to 2.5 × 105 cells and then incubated for 30 minutes at 37°C in the dark. The cells were pelleted, washed, and resuspended in PBS before being analyzed by flow cytometry (FL1). Apoptotic cells display less fluorescence than viable cells, allowing calculation of the proportion of each.
Results
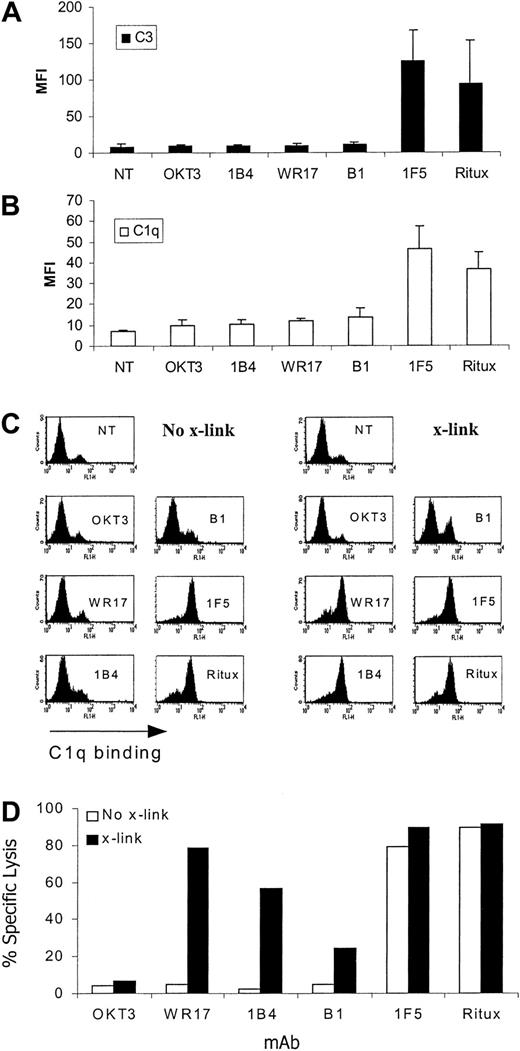
We previously reported that the efficacy with which anti-CD20 mAbs mediate CDC appears to correlate with their readiness to translocate CD20 into Triton X-100-derived lipid rafts.14 However, the explanation for this difference in CDC was not explored. To address how movement into rafts might enhance this activity, we assessed the extent to which different complement proteins could be deposited on target cells after treatment with mAbs that redistribute into membrane rafts and cause good levels of CDC (1F5, rituximab) compared with mAbs that do not (B1, IB4, WR17).14 These experiments were performed in the presence of serum as a source of complement and, with the exception of rituximab, all mAbs are mouse IgG2a isotype, thus excluding Ab subclass as an explanation for their differing CDC activity. Preliminary data showed that, as expected, only mAbs potent at inducing CDC (rituximab, 1F5) caused insertion of the membrane attack complex (MAC) into the target cell membrane as measured by detecting cell-bound C6 (data not shown). Therefore, we assessed an earlier cascade component, C3b, and found that the same profile of reactivity was observed (Figure 1A), with only rituximab and 1F5 causing deposition of high levels of C3b onto the target cells, indicating that the mAb-dependent complement activation and deposition preceded this pivotal step in the complement cascade. These results are in agreement with recent data21 and led us to examine the binding of the first component of the complement cascade, C1. These experiments, measuring cell-bound C1q showed that only mAbs that moved their target antigen into membrane rafts (IF5, rituximab) caused the cell to be coated with C1q (Figure 1B), thus explaining the pattern of C3b and C6 deposition detailed earlier.
C3b, C1q binding, and CDC activity of anti-CD20 mAb. (A-B) EHRB cells were incubated with various mAbs (10 μg/mL) for 15 minutes prior to addition of 20% NHS. Samples were taken after 5 minutes of incubation at 37°C and washed, and C3b and C1q binding was assessed by staining with an anti-C3b (A) or anti-C1q (B) FITC-labeled mAb for 15 minutes at 4°C. Bars represent the mean MFI ± SD for at least 3 experiments. (C-D) To assess whether cross-linking could enhance C1q and CDC activity, mAbs were bound to EHRB cells for 15 minutes at room temperature at concentrations adjusted to provide equivalent levels of Fc at the cell surface. This concentration was determined by titrating primary mAb concentrations to a level that gave equivalent surface binding as measured with the use of a secondary antimouse IgG FITC mAb and flow cytometry. Cells were then washed once, and the sample was divided into 2 samples, one half receiving cross-linking agent 25 μg/mL (x-link) and the other receiving PBS (No-x-link) for 10 minutes at room temperature. NHS (20%) was then added, and the extent of C1q bound (C) and cell lysis (D) apparent after 5 minutes at 37°C was measured. Samples were assayed for lysis by PI staining.
C3b, C1q binding, and CDC activity of anti-CD20 mAb. (A-B) EHRB cells were incubated with various mAbs (10 μg/mL) for 15 minutes prior to addition of 20% NHS. Samples were taken after 5 minutes of incubation at 37°C and washed, and C3b and C1q binding was assessed by staining with an anti-C3b (A) or anti-C1q (B) FITC-labeled mAb for 15 minutes at 4°C. Bars represent the mean MFI ± SD for at least 3 experiments. (C-D) To assess whether cross-linking could enhance C1q and CDC activity, mAbs were bound to EHRB cells for 15 minutes at room temperature at concentrations adjusted to provide equivalent levels of Fc at the cell surface. This concentration was determined by titrating primary mAb concentrations to a level that gave equivalent surface binding as measured with the use of a secondary antimouse IgG FITC mAb and flow cytometry. Cells were then washed once, and the sample was divided into 2 samples, one half receiving cross-linking agent 25 μg/mL (x-link) and the other receiving PBS (No-x-link) for 10 minutes at room temperature. NHS (20%) was then added, and the extent of C1q bound (C) and cell lysis (D) apparent after 5 minutes at 37°C was measured. Samples were assayed for lysis by PI staining.
We previously showed that hyper-cross-linking of certain mAbs can result in their redistribution into a TX-100-insoluble membrane compartment characteristic of membrane rafts.14 Therefore, to address whether the ability to fix C1q (and subsequently evoke lysis) was enhanced in membrane rafts we hyper-cross-linked the previously inert mAbs (B1, WR17, 1B4) and subsequently assessed the extent of C1q binding. These data shown in Figure 1C reveal that hyper-cross-linking caused an elevated level of C1q binding with these mAbs and, subsequently, target cell lysis (Figure 1C). Thus, it appears that the explanation for why rituximab and 1F5 are so efficient in CDC lies in their ability to bind the initial component of the complement cascade, C1. This property presumably results from these 2 mAbs being redistributed into membrane rafts, which may provide a high local concentration of Abs and/or a favorable orientation of Fc regions for efficient C1 binding.
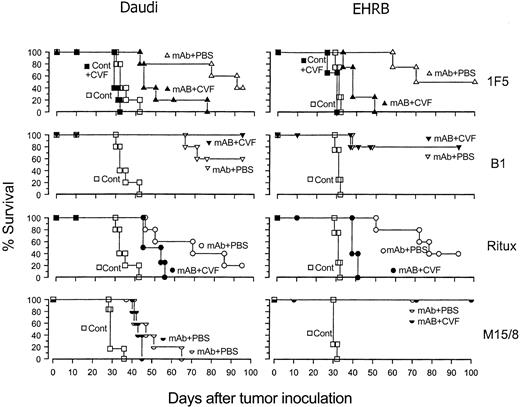
Next, we wanted to determine whether the high levels of in vitro CDC observed for rituximab and 1F5 might indicate a role for complement activation in the therapeutic activity of these anti-CD20 mAbs in vivo. Therefore, we treated lymphoma-bearing SCID mice with different anti-CD20 mAbs with and without complement depletion by using cobra venom factor (CVF). The results (Figure 2) show that in the case of rituximab and 1F5 administering CVF early in the mAb treatment markedly reduced therapeutic activity, with, for example, the median survival times for EHRB-bearing mice being reduced from 76 to 39 days for rituximab and 70 to 39 days for 1F5 with significant differences in the response of these groups (P < .02). Unexpectedly, B1, which induces very little CDC activity, provided a potent therapeutic response, although in accordance with its poor CDC activity, was completely unaffected by depleting complement (P > .5, comparing the B1 and B1 + CVF groups). A similar result was seen with an anti-BCR mAb, M15/8. These results clearly show that, although B1 and M15/8 are highly effective therapeutic mAbs, their activity does not depend on complement activation, presumably because they use other effector mechanisms such as ADCC or the induction of apoptosis. It is important to note that we have previously examined all of these mAbs for their efficacy in ADCC assays and have shown no substantial differences19 (and data not shown). It should also be noted that differences between rituximab and the murine mAb may reflect the fact that rituximab carries a human IgG1 Fc domain. Therefore, it may not interact as effectively with murine effector cells. However, the central principal of what we have shown (different effector mechanisms) is also true when comparing 1F5 and B1 mAbs which are of the same species and isotype.
The effect of the depletion of complement with CVF on the therapeutic activity of rituximab, 1F5, B1, and M15/8 in SCID mice. Groups of 4 to 5 SCID mice were injected with 2.5 × 106 Daudi or EHRB cells intravenously on day 0, and then treated with 100 μg 1F5, rituximab, or B1 (anti-CD20), M15/8 (anti-BCR), or PBS (cont) intravenously on day 7. In the complement-depleted groups (solid symbols), the animals were injected with 25 μg CVF intraperitoneally on days 7, 9, and 12 after tumor inoculation. Mice were culled when rear-leg paralysis was observed.
The effect of the depletion of complement with CVF on the therapeutic activity of rituximab, 1F5, B1, and M15/8 in SCID mice. Groups of 4 to 5 SCID mice were injected with 2.5 × 106 Daudi or EHRB cells intravenously on day 0, and then treated with 100 μg 1F5, rituximab, or B1 (anti-CD20), M15/8 (anti-BCR), or PBS (cont) intravenously on day 7. In the complement-depleted groups (solid symbols), the animals were injected with 25 μg CVF intraperitoneally on days 7, 9, and 12 after tumor inoculation. Mice were culled when rear-leg paralysis was observed.
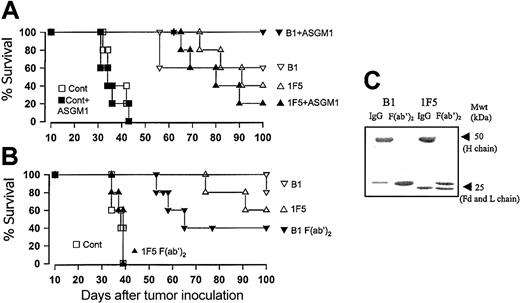
To address whether NK cells might be playing a role in B1-induced tumor cell destruction in vivo, we treated mice with anti-ASGM1, a well-established NK depletion strategy22,23 and assessed the ability of B1 to induce immunotherapy in these mice. As can be seen in Figure 3A, mice depleted of NK cells at the time of tumor inoculation were still protected from challenge with Daudi or EHRB cells following administration of B1 mAb (P > .5 for B1 compared with B1 + anti-ASGM1). Subsequently, we inoculated SCID-Beige mice, which have an inherent defect in NK function, with Daudi cells and found that B1 also protected these mice (data not shown). In an attempt to remove the potential for any conventional effector functions, we produced highly purified F(ab′)2 fragments of the B1 and 1F5 mAbs. The resulting F(ab′)2 fragments were assessed on SDS-polyacrylamide gel electrophoresis (PAGE) gels (Figure 3C) to confirm a lack of contaminating IgG and then injected into tumor-bearing SCID mice on days 7, 8, and 9 after tumor inoculation to allow for the shorter half-life. These experiments revealed that, although the 1F5 mAb F(ab′)2 had no therapeutic activity, as expected, F(ab′)2 fragments from B1 were still highly potent, providing long-term protection in 40% of mice (Figure 3B). The results confirm that in this model 1F5 is completely dependent on natural effectors, particularly complement, for its therapeutic activity, although B1 is not as reliant on these mechanisms.
The effect of NK cells and Fc-dependent mechanisms on the therapeutic activity of B1 mAb in SCID mice. (A) Groups of 4 to 5 SCID mice were injected with 2.5 × 106 Daudi cells intravenously on day 0, and then treated with 100 μg B1, 1F5, or PBS (control) intravenously on day 7. In the NK-depleted groups (solid symbols), the animals were injected with 25 μg anti-ASGM1 (ASGM1) intraperitoneally on days 5 and 9 after tumor inoculation. (B) Groups of 4 to 5 SCID mice were injected with 2.5 × 106 Daudi cells intravenously on day 0 and then treated with 100 μg B1 or 1F5 IgG (open symbols) or F(ab′)2 fragments (solid symbols) or PBS intravenously on day 7. Additional 100-μg injections of F(ab′)2 were given intraperitoneally on day 7, intravenously and intraperitoneally on day 8, and intraperitoneally on day 9, to a total of 500 μg. (C) Samples of the IgG and F(ab′)2 fragments of the IF5 and B1 mAb used in panel B were assessed by SDS-PAGE analysis under reducing conditions. The Fd and light (L) chain fragments of the reduced B1 F(ab′)2 comigrate. Note, the absence of contaminating heavy (H) chains in both F(ab′)2 preparations.
The effect of NK cells and Fc-dependent mechanisms on the therapeutic activity of B1 mAb in SCID mice. (A) Groups of 4 to 5 SCID mice were injected with 2.5 × 106 Daudi cells intravenously on day 0, and then treated with 100 μg B1, 1F5, or PBS (control) intravenously on day 7. In the NK-depleted groups (solid symbols), the animals were injected with 25 μg anti-ASGM1 (ASGM1) intraperitoneally on days 5 and 9 after tumor inoculation. (B) Groups of 4 to 5 SCID mice were injected with 2.5 × 106 Daudi cells intravenously on day 0 and then treated with 100 μg B1 or 1F5 IgG (open symbols) or F(ab′)2 fragments (solid symbols) or PBS intravenously on day 7. Additional 100-μg injections of F(ab′)2 were given intraperitoneally on day 7, intravenously and intraperitoneally on day 8, and intraperitoneally on day 9, to a total of 500 μg. (C) Samples of the IgG and F(ab′)2 fragments of the IF5 and B1 mAb used in panel B were assessed by SDS-PAGE analysis under reducing conditions. The Fd and light (L) chain fragments of the reduced B1 F(ab′)2 comigrate. Note, the absence of contaminating heavy (H) chains in both F(ab′)2 preparations.
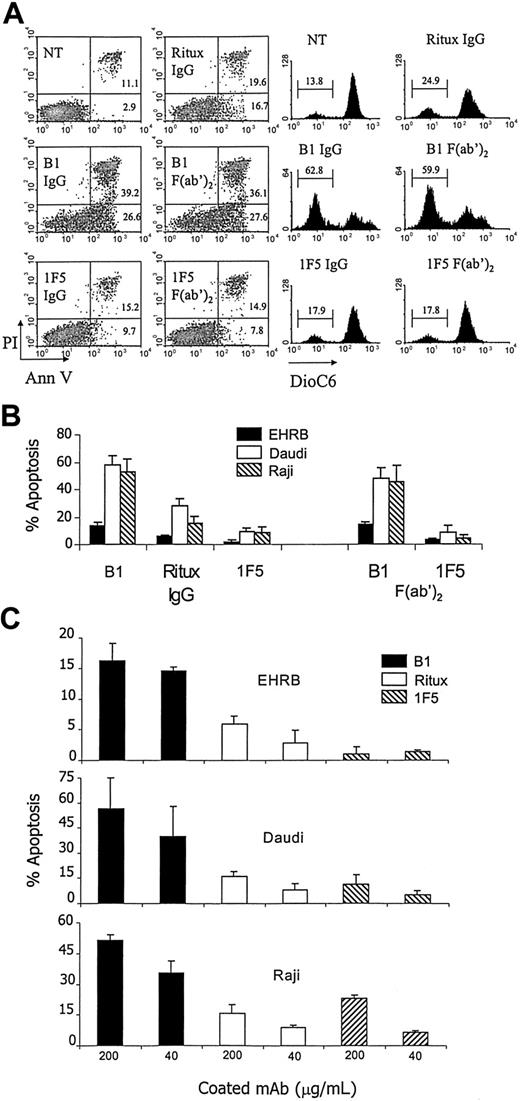
Given the lack of a requirement for conventional effector functions with B1 in our model system, we explored whether apoptosis might explain the potent immunotherapy. Therefore, we assessed the ability of B1 to induce apoptosis compared with 1F5 and rituximab by using a variety of assays, assessing phosphatidylserine translocation, lack of membrane integrity, and mitochondrial dysregulation (Figure 4A). Furthermore, given the efficacy of the B1 F(ab′)2 in vivo, we assessed its ability to evoke apoptosis in these assays, comparing it with the therapeutically inert IF5 F(ab′)2 fragments. As can be seen in Figure 4A, apoptosis induced by B1 IgG in Daudi cells was substantially higher than that induced by 1F5 or rituximab IgG and this activity was retained in the F(ab′)2 fragments. This greater level of apoptosis with B1 was confirmed by all 3 assays (increase in annexin V binding, increase in PI positivity, and decrease of DioC6 fluorescence), although it should be noted that the proportion of cells assessed as apoptotic was generally higher with annexin V than other measures. The same trends were true for EHRB and Raji cells, with B1 IgG and F(ab′)2 fragments inducing the highest levels of apoptosis in all 3 cell lines (Figure 4B). We also assessed whether enhanced cross-linking of the CD20 might enhance the activity of B1. We chose to achieve this by coating the mAbs to the surface of the wells, rather than applying a secondary layer of Ab, facilitating direct comparison with 1F5 and rituximab without introducing experimental variation because of using different cross-linking reagents for the mouse and human mAbs. All 3 mAbs appeared to coat to the culture wells equivalently (data not shown). In the presence of this enhanced cross-linking, B1 induced far greater levels of apoptosis than either rituximab or 1F5 in all 3 cell lines tested, although the level of apoptosis was far lower for EHRB cells than others (Figure 4C).
Apoptosis induced by anti-CD20 mAb. mAbs were either added in free solution (A-B) or coated onto wells for 2 hours at 37°C, and unbound mAbs were removed (C) before Daudi, EHRB, or Raji cells were added to the wells. Numbers in quadrants represent the percentage of cells in each section of the quadrant. Bars represent mean apoptosis value ± SD from at least 3 separate experiments. Apoptosis was assessed 18 to 24 hours later by annexin V/PI staining (left-hand dot plots in A), annexin V staining (B-C), or DioC6 staining (right-hand plots in A).
Apoptosis induced by anti-CD20 mAb. mAbs were either added in free solution (A-B) or coated onto wells for 2 hours at 37°C, and unbound mAbs were removed (C) before Daudi, EHRB, or Raji cells were added to the wells. Numbers in quadrants represent the percentage of cells in each section of the quadrant. Bars represent mean apoptosis value ± SD from at least 3 separate experiments. Apoptosis was assessed 18 to 24 hours later by annexin V/PI staining (left-hand dot plots in A), annexin V staining (B-C), or DioC6 staining (right-hand plots in A).
Discussion
Here, we have shown that anti-CD20 mAbs fall into 2 distinct types of reagents on the basis of their ability to eradicate lymphoma xenografts: type I (rituximab and 1F5) uses complement and type II (B1) does not. Interestingly, both types of mAbs gave excellent prolongation of survival, but depleting complement activity, by administering CVF, considerably diminished the potency of rituximab and 1F5 but had no effect on the activity of B1. These results clearly show for the first time that different CD20 mAbs operate different effector mechanisms in vivo. Furthermore, they are in complete accord with our previous work, showing that rituximab and 1F5 are able to activate complement efficiently as a result of translocating CD20 to lipid rafts in the target cell membrane, something that B1-type mAbs cannot do. We still do not know why some CD20 mAbs move CD20 into rafts and others do not, but it probably relates to the extent to which CD20 is cross-linked in the plasma membrane. We have now also shown that the discrimination between raft-redistributing mAbs (rituximab and 1F5), and non-raft-redistributing mAbs (B1) occurs at the level of C1q binding. As such, rituximab and 1F5 bind C1q effectively, engaging the classical pathway of complement activation and causing effective CDC when bound to target cells, whereas B1 does not.
The notion that complement plays a major role in the function of rituximab in vivo has been proposed previously and has been supported with several lines of circumstantial evidence, showing that complement is activated,8 exerts selective pressure,9 and causes CDC of different target cells in vitro in accordance with their sensitivity to rituximab treatment in vivo.11 However, strong, direct evidence has been lacking until our own work and the recent work of Di Gaetano et al,24 who have demonstrated that eradication of syngeneic murine EL4 tumor cells expressing human CD20 with rituximab or 1F5 is dependent on C1q and independent of NK cells.
However, all of these results are in clear contrast to the FcγRIIIa haplotype data and the work of Clynes et al,4 who suggested ADCC as the central effector mechanism of rituximab. In those experiments, the therapeutic activity of rituximab was shown to be dependent on the expression of the FcR γ chain used by both FcγRI and FcγRIII, and that FcRII, an inhibitor of leukocyte activation, had a negative effect on rituximab immunotherapy, thus providing good evidence that FcR can play an important role in rituximab activity. It is important to note that clear differences exist between the models studied. Clynes et al4 adopted a subcutaneous solid tumor model with Raji cells, whereas we, as well as Di Gaetano et al,24 and have adopted a model involving intravenous administration of tumor cells. Therefore, it is possible to speculate that the mode of action of rituximab may be altered according to the site of the tumor. Bloodborne leukemic or more accessible tumors may be controlled through CDC mechanisms in the presence of high levels of complement proteins in the blood, whereas less vascularized tumors may require penetration with effector cells for destruction. Alternatively, complement and FcγR effector mechanisms may be working cooperatively in effecting the therapeutic activity of rituximab, as in addition to its direct lytic activity, complement activation produces several other important immune modulators. The most obvious of these is the deposition of C3b fragments onto the target cell surface, which will lead to opsonization, activation, and enhanced phagocytic and cytotoxic activity.25,26 Furthermore, several other products of the complement cascade, such as C3a and C5a, are potent chemotactic and inflammatory agents that could readily enhance the cytotoxicity of effector cells.26 To distinguish between the direct lytic activity of complement and these latter immunomodulatory functions, more careful manipulation of the complement proteins than can be achieved with CVF will be required, with the effect of rituximab compared in mice deficient for single complement components such as C1q, C3, C5, and C6.
Interestingly, the other clinically relevant anti-CD20 mAb studied here, B1, shows very weak CDC activity, yet protected mice from EHRB and Daudi tumors very effectively, even in the absence of complement. The B1 mAb was still effective even in the absence of NK cells, arguing that it was not evoking potent ADCC-type mechanisms, although these experiments do not rule out the potential role of macrophages in ADCC. However, in earlier work we showed that B1, 1F5, and rituximab were all similar in their efficacy in ADCC assays27 and so it would be wholly unexpected that this activity could explain the potency of B1. Indeed, when F(ab′)2 fragments of B1 were used, rendering the molecule inert in terms of Fc-dependent effector mechanisms, a substantial proportion of therapeutic activity remained. Although larger doses of F(ab′)2 than IgG were given in these experiments, this was necessary to allow for the far shorter half-life of F(ab)2 compared with IgG in vivo, which, although not directly studied here with B1, we estimate from previous work (Honeychurch and M.S.C., unpublished results, November 1997) to be at least 3- to 4-fold lower than IgG. It might also be argued that contaminating IgG may exist in the F(ab′)2 preparations, accounting for the therapeutic activity. First, it should be noted that all F(ab′)2 preparations used here were passed through an anti-Fc column to eradicate IgG. Previously, we have estimated that contaminating IgG is present at less than 0.01%. Second, the F(ab′)2 preparations were evaluated by SDS-PAGE and HPLC, and by these techniques IgG was undetectable. Furthermore, it would be expected that, even if a very small amount of IgG was present, the F(ab′)2 would out-compete it for binding sites on the tumor, hence blocking its activity. Finally, it is clear that substantial contamination does not occur with the 1F5 F(ab′)2 preparations, as no therapeutic activity was observed. Therefore, together with the data showing that C′ and NK cells were unimportant for B1-induced immunotherapy, it is most likely that another effector mechanism is responsible.
When apoptosis was assessed in vitro, it was clear that B1 IgG and F(ab′)2 were comparable and that B1 was the most potent anti-CD20 mAb of the 3 studied here. This result is in agreement with the data of Cardarelli et al.28 In our recent work we have assessed a large panel of anti-CD20 mAbs and have shown that B1, along with 2 other mAbs (AT80 and 11B8), is unusual in this ability to induce high levels of apoptosis.20 This property appears to relate to the binding properties of these 3 mAbs, which bind at a half-maximal level at saturation compared with other anti-CD20 mAbs, presumably reflecting a difference in the prevalence of the tertiary epitope bound.20
Therefore, the main difference between B1 (type II) and rituximab (and 1F5; type I) is the ability to induce high levels of apoptosis, and we expect that this property provides much of the therapeutic activity observed. Interestingly, in the Ramos subline EHRB, B1 only demonstrated an enhanced apoptotic capacity compared with 1F5 and rituximab when immobilized onto tissue culture plastic. In the EHRB model then, this might provide evidence for the presence of effector cell FcγR hyper-cross-linking of mAbs in vivo as suggested by Shan et al.5 Furthermore, this Fc-dependent function, which is separate from CDC or ADCC activities, may explain why B1 IgG is more potent than B1 F(ab′)2 in vivo. However, what is not clear is why EHRB cells seem less sensitive to B1-induced apoptosis than Daudi cells in vitro, yet achieve a comparable therapeutic effect in vivo.
Several other workers have described the ability of anti-CD20 mAb to regulate apoptosis. Their findings are in good agreement with those reported here, whereby rituximab causes limited apoptosis unless hyper-cross-linked.5-7,29 It is important to note that rituximab has been reported to induce signs of apoptosis (caspase cleavage) in vivo.30 This is perhaps due to additional Ab cross-linking by FcR in vivo or possibly through the reported ability of the complement membrane attack complex to activate caspases and induce apoptosis.31 The ability of B1 to induce apoptosis in vivo remains undocumented.
This work has shown for the first time that mAb directed to the same target antigen, and in the case of 1F5 and B1 with the same Ig isotype, can achieve in vivo clearance of tumor cells by different mechanisms. The major question now is to ask whether a similar distinction can be found in humans and whether a human type II CD20 mAb will outperform rituximab. Interestingly, the failure of such a reagent to activate complement should also have the advantage of reduced toxicity, because most of the unfortunate side effects of rituximab are now known to be as a result of the complement cascade.8
Prepublished online as Blood First Edition Paper, October 9, 2003; DOI 10.1182/blood-2003-06-2031.
Supported by the Biotechnology and Biological Sciences Research Council (BBSRC), Cancer Research UK (CRUK), and Tenovus of Cardiff.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank all members of the Tenovus Cancer Laboratory and the Cancer Sciences Division who provided expert technical support and valuable discussion, in particular Miss Kerry Cox. We also thank Dr T. Illidge (B1, Ritux) and Professor B. P. Morgan (CVF and anti-C3b) for the provision of reagents.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal