A hemorrhagic disorder associated with enhanced thrombin activation and disseminated intravascular coagulation is often observed in acute promyelocytic leukemias (APLs) carrying the t(15;17). In these leukemias high levels of annexin II, a calcium-regulated and phospholipid-binding cell surface protein, correlate with increased propensity to hemorrhage. Treatment with all-trans-retinoic acid (ATRA) resolves the coagulapathy, and is associated with a concomitant down-regulation of annexin II transcript and protein levels. But what of the role of annexin II in the pathogenesis of hemorrhagic disorders associated with leukemia?
Annexin II is thought to have a thromboregulatory role by enhancing the tissue plasminogen activator–dependent formation of plasmin on endothelial cell surfaces. Annexin II overexpression on the surface of APLs may in fact lead to uncontrolled production of plasmin, shifting the hemostatic balance toward overt bleeding.1 A definitive role for annexin II in maintaining fibrin homeostasis and plasmin regulation has been demonstrated in annexin II null mice, providing a dramatic link to coagulopathy.2 FIG1
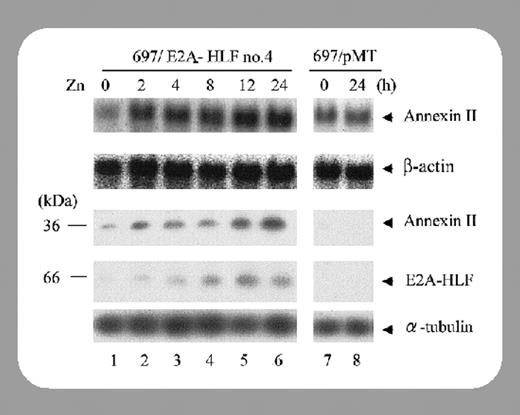
In this issue of Blood, Matsunaga and colleagues (page 3185) report that annexin II is also expressed at high levels in each of 4 t(17;19) acute lymphoid leukemic (ALL) cell lines. The t(17;19) encodes the uncommon but well-studied chimeric oncoprotein E2A-HLF, a basic-region leucine zipper (bZIP) DNA-binding protein that contains the heterologous E2A transactivation domains. Patients with E2A-HLF–induced leukemias are refractory to conventional chemotherapeutic treatment and have a generally poor prognosis that is associated with hypercalcemia and hemorrhagic complications. E2A-HLF has been implicated to transform B-cell progenitors by several potential pathways, including enhanced survival (through induction of SLUG, a transcriptional repressor that shares homology with the Caenorhabditis elegans apoptotic repressor Ces1) and impaired differentiation.3,4 Interestingly, genetic studies have also implicated annexin VIII, another member of the annexin family, as a potential downstream gene, suggesting a possible mechanistic basis for the leukemia-associated bleeding disorder.5
Matsunaga et al now provide experimental evidence that annexin II is a downstream target of E2A-HLF and that in IL-3–dependent cells annexin II expression is regulated by IL-3 and Ras pathways. Moreover, E2A-HLF expression in these cells induced annexin II expression in the absence of IL-3, indicating that E2A-HLF induces annexin II by substituting for cytokines that activate downstream pathways of Ras. They noted that annexin II expression was unlikely to contribute to the cell survival pathways that E2A-HLF trigger since conditional expression of annexin II was unable to stem cytokine deprivation–induced apoptosis. Matsunaga and colleagues asked this important question: what role, if any, does annexin II play in E2A-HLF–induced ALL coagulopathy? They noted that while total annexin II protein levels were increased in 4 E2A-HLF–bearing cell lines tested, the cell surface levels were fairly divergent and in one example did not correlate with patient coagulopathy. More likely, they report, surface annexin II could be correlated with hypercalcemia at onset, the other rare complication in pro-B ALL. One other intriguing aspect is that annexin II interacts with procathepsin B on the surface of tumor cells, and is involved in extracellular proteolysis, facilitating tumor invasion and metastasis. E2A-HLF–positive leukemia is characterized by bone invasion and hypercalcemia, both paraneoplastic syndromes that are rare complications in other types of childhood acute B-lineage leukemia, offering yet another possible clue. Certainly future studies will shed light on the role annexin II expression plays in these rare ALLs.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal