Abstract
The anti-CD20 monoclonal antibody rituximab induces remission in 40% to 60% of patients with indolent B-cell lymphoma, but virtually all patients have relapses. We evaluated the efficacy of concurrent administration of another biologic agent, N-(4-hydroxyphenyl) retinamide (4HPR, fenretinide) with rituximab against a variety of human B-cell lymphoma cell lines (Ramos, DHL-4, and FL-18) in vivo. Concurrent 4HPR and rituximab administration prevented tumor progression of lymphoma-bearing mice in a minimal disease model (rituximab + 4HPR, 100% progression free; rituximab alone, 37.5% progression free, P = .01; 4HPR alone, 12.5% progression free, P < .01; controls, 0% progression free, P < .01). Combinations of 4HPR + rituximab exceeded the predicted 50% additive rate of disease control from each agent alone (P = .038). Administering 4HPR and rituximab to mice with established tumors induced complete responses (CRs) in 80% of animals compared with 20% to 40% CRs using either agent alone (P = .07), resulting in significantly improved survival. Tumors harvested from 4HPR + rituximab-treated mice displayed elevated caspase activation compared with untreated controls (P = .02). Adding a broad-spectrum caspase inhibitor in vivo fully abrogated the antitumor effects of 4HPR + rituximab (P = .05). These results establish the efficacy of 4HPR/rituximab combinations, confirm their caspase-mediated mechanism of action, and offer the potential for disease control with minimal toxicity for patients with B-cell malignancies. (Blood. 2004;103:3516-3520)
Introduction
A major recent advance in the therapy for non-Hodgkin lymphoma has been the development of monoclonal antibodies (mAbs) that target tumor-specific antigens and induce tumor cell death. Effector mechanisms evoked by unconjugated antibodies include complement activation, antibody-dependent cellular cytotoxicity (ADCC), and direct induction of apoptosis.1,2 Despite the efficacy and safety demonstrated by antibodies such as rituximab, only a small minority of patients achieve complete responses (CRs), and most patients eventually have relapses.3 Adding chemotherapy to mAb therapy has improved responses and remission durations, but predictable chemotherapy-related toxicities are incurred.4,5 Methods to maximize the efficacy of mAb therapy with minimal toxicity are being investigated.
Fenretinide (4HPR) is a novel synthetic retinoid with anticancer properties that has been shown to induce apoptosis in a variety of malignant cell lines.6-8 In vitro experiments by our group have also suggested that 4HPR alone can induce programmed cell death in human Burkitt lymphoma lines, which may be further augmented by anti-CD20 antibodies.9 In the series of experiments described in this article, we demonstrate the ability of 4HPR and the anti-CD20 antibody rituximab to eradicate palpable B-cell lymphoma xenografts and to prevent the progression of minimal disease in experimental settings in which neither agent alone was effective. We confirm that the in vivo mechanism of action is mediated by caspase-dependent pathways by verifying caspase activation in treated tumors and by demonstrating abrogation of the therapeutic effects with a broad-spectrum caspase inhibitor.
Materials and methods
Cells
The CD20-expressing human Burkitt lymphoma (BL) cell line Ramos was obtained from the American Type Culture Collection (ATCC; Bethesda, MD). The B-lymphoma cell lines SU-DHL4 (DHL-4) and FL-18 were gifts from Dr David Maloney (Fred Hutchinson Cancer Research Center, Seattle, WA). All cell lines were maintained in log-phase growth in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM glutamine, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25μg/mL Fungizone (BioWhittaker, Walkersville, MD).
Antibodies and reagents
The chimeric anti-CD20 mAb C2B8 (rituximab) was purchased from Genentech (South San Francisco, CA). Fenretinide (4HPR) was provided by the Cancer Therapy Evaluation Program of the National Cancer Institute. Q-VD(OMe)-OPh, a broad-spectrum caspase inhibitor, was obtained from Enzyme Systems Products (Livermore, CA). 4HPR and Q-VD(OMe)-Oph were dissolved in 5% ethanol as previously described, resulting in final concentrations of 1.25 mg/mL and 1 mg/mL, respectively.10 Apoptosis assays were conducted using the APO LOGIX Carboxyfluorescein Caspase (FAM-VAD-FMK) Detection Kit (Cell Technology, Minneapolis, MN) for activated caspase detection.
Murine xenograft techniques
Female BALB/c nude mice were obtained from Animal Technologies (Kent, WA) and were housed under protocols approved by the Fred Hutchinson Cancer Research Center Institutional Animal Care and Use Committee. Mice were inoculated subcutaneously with 7 × 106 tumor cells in the right flank. Tumor volumes were calculated twice weekly using 3-dimensional measurements. Toxicity was monitored by evaluation of serial weekly weights and biweekly physical findings, including activity level and skin changes. Fisher exact test or χ2 analysis was used to determine differences between categorical variables. The Kaplan-Meier method was used to estimate the probability of a given event (eg, tumor progression, survival) over time. The log rank test was used to determine differences in the time-to-event end points. Adjustment was not made for multiple comparisons. All animal experiments were approved by the Fred Hutchinson Cancer Research Center Institutional Review Office and the Institutional Animal Care and Use Committee.
Ex vivo apoptosis experiments
Apoptosis was assessed by staining with FAM-VAD-FMK (FAM) and performing flow cytometric analysis (FACScan; Becton Dickinson, Mountain View, CA).11 FAM is a fluoresceinated derivative of zVAD-FMK that enters cells, irreversibly binds to the activated caspases of those undergoing apoptosis, and can be assessed by flow cytometry. FAM-positive cells were scored as apoptotic. The paired t test was used to determine statistical significance of differences in apoptosis between groups.
Results
In vivo administration of 4HPR and rituximab to eradicate lymphoma in a minimal tumor burden model
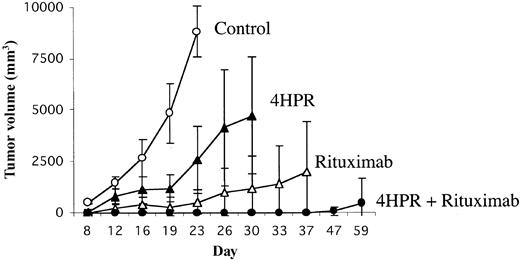
We initially tested the efficacy of 4HPR and rituximab in vivo in a minimal disease model because we thought this would afford an optimal clinical circumstance for demonstrating the efficacy of such biologic agents. Mice were inoculated in the flank with 7 × 106 Ramos Burkitt lymphoma cells because of the predictable tumor engraftment kinetics in this cell line. Seventy-two hours later, mice were randomly assigned to receive daily 4HPR (250 μg/d) intraperitoneally (ip) 5 days per week for 4 weeks (total of 20 doses), rituximab 200 μg once per week ip for 4 weeks (total of 4 doses), the combination of 4HPR + rituximab at the doses and schedules above, or diluent alone. Tumor growth curves for each treatment group are depicted in Figure 1.
Efficacy of 4HPR and rituximab in preventing tumor progression using a minimal disease lymphoma xenograft model. Athymic mice (8 mice per group) were inoculated subcutaneously with 7 × 106 Ramos (Burkitt lymphoma) cells. Seventy-two hours after inoculation, mice were randomly assigned to treatment with 4HPR (250 μg/d, 5 days per week), rituximab (200 μg/wk), 4HPR + rituximab (at the same doses), or solvent only for 4 weeks. Tumor volume in mm3 (y-axis) was measured over time (x-axis). Curves were truncated at the indicated time points when mice in a group required humane killing because of tumor progression. Error bars indicate standard deviation.
Efficacy of 4HPR and rituximab in preventing tumor progression using a minimal disease lymphoma xenograft model. Athymic mice (8 mice per group) were inoculated subcutaneously with 7 × 106 Ramos (Burkitt lymphoma) cells. Seventy-two hours after inoculation, mice were randomly assigned to treatment with 4HPR (250 μg/d, 5 days per week), rituximab (200 μg/wk), 4HPR + rituximab (at the same doses), or solvent only for 4 weeks. Tumor volume in mm3 (y-axis) was measured over time (x-axis). Curves were truncated at the indicated time points when mice in a group required humane killing because of tumor progression. Error bars indicate standard deviation.
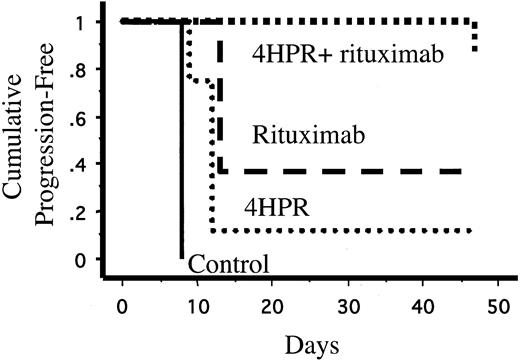
None of the 8 mice treated with 4HPR and rituximab in combination developed palpable tumors during treatment compared with 5 (63%) of 8 mice treated with rituximab alone (P = .01), 7 (88%) of 8 mice treated with 4HPR alone (P = .0007), and 8 of 8 untreated control mice (P = .00008). One mouse treated with 4HPR and rituximab experienced tumor progression 3 weeks after discontinuing therapy. The effects of 4HPR and rituximab appeared supra-additive in vivo. Only 4 of 8 mice would be expected to be free of progressive lymphoma over 4 weeks if the effects were merely additive, but all 8 mice treated with combination 4HPR and rituximab achieved tumor-free status (P = .038). 4HPR and rituximab in combination also significantly lengthened the time to disease progression compared with rituximab alone (log rank, P = .038) and 4HPR alone (log rank, P = .0018) (Figure 2). The Kaplan-Meier plot in Figure 2 demonstrates that single-agent 4HPR and single-agent rituximab delay the growth of lymphoma xenografts, but most mice treated with monotherapy eventually experienced progressive disease. In contrast, 7 of 8 mice treated with combination 4HPR and rituximab remained continuously free of tumor growth for the entire period of observation (47 days).
Kaplan-Meier estimates of time to tumor progression (days) of lymphoma cells (Ramos) in inoculated mice. Athymic mice (8 mice per group) were inoculated subcutaneously with 7 × 106 Ramos (Burkitt lymphoma) cells. Seventy-two hours after inoculation, mice were randomly assigned to treatment with 4HPR (250 μg/d, 5 days per week), rituximab (200μg/wk), 4HPR + rituximab (at the same doses), or solvent only (negative control) for 4 weeks.
Kaplan-Meier estimates of time to tumor progression (days) of lymphoma cells (Ramos) in inoculated mice. Athymic mice (8 mice per group) were inoculated subcutaneously with 7 × 106 Ramos (Burkitt lymphoma) cells. Seventy-two hours after inoculation, mice were randomly assigned to treatment with 4HPR (250 μg/d, 5 days per week), rituximab (200μg/wk), 4HPR + rituximab (at the same doses), or solvent only (negative control) for 4 weeks.
Efficacy of combination 4HPR and rituximab for the treatment of palpable lymphoma xenografts
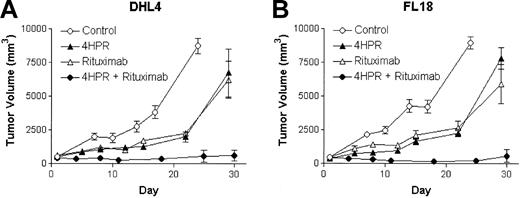
The most rigorous preclinical evaluation of an antineoplastic agent is to determine its ability to induce responses in well-established tumors. To test the hypothesis that the combined use of 4HPR and rituximab could produce superior CR and cure rates of established lymphoma xenografts than either agent alone, we randomly assigned mice with established, palpable DHL-4 and FL-18 lymphoma xenografts (approximately 500 mm3) to treatment with either 4HPR (250 μg/d) ip for 5 days per week for 4 weeks, weekly rituximab (200 μg ip for 4 weeks), combined 4HPR + rituximab at the same doses, or diluent alone. Four of 5 of mice bearing DHL-4 tumors achieved CR when treated with 4HPR + rituximab compared with only 1 of 5 mice achieving CR with 4HPR alone and 0 of 5 mice achieving CR with rituximab alone or with diluent alone. Similarly, in mice bearing FL-18 lymphoma xenografts, CRs were induced in 4 of 5 of those treated with combination 4HPR + rituximab, 2 of 5 treated with rituximab alone, and 0 of 5 remissions with 4HPR alone or in the control group (Figure 3). If these agents' activities were merely additive, one would have expected response rates of 20% to 40% when used in combination compared with the observed 80% rate in each group that achieved CR (P = .07). Furthermore, most remissions achieved with the combination of 4HPR and rituximab were durable; 7 of 8 (88%) mice that achieved CR remained without relapse up to 450 days of observation.
Efficacy of 4HPR and rituximab in tumor killing using a palpable B-cell lymphoma xenograft model. Palpable DHL-4 (A) and FL-18 (B) tumors were established in athymic mice a median of 18 days after inoculation (5 mice per group). Mice were then treated with 4HPR (250 μg/d, 5 days per week), rituximab (200 μg/wk, 4HPR + rituximab (at the noted doses), or no drug (solvent only) for 4 weeks. Tumor volume in mm3 (y-axis) was measured over time (x-axis). Curves were truncated when mice required humane killing because of tumor progression. Error bars indicate standard deviation.
Efficacy of 4HPR and rituximab in tumor killing using a palpable B-cell lymphoma xenograft model. Palpable DHL-4 (A) and FL-18 (B) tumors were established in athymic mice a median of 18 days after inoculation (5 mice per group). Mice were then treated with 4HPR (250 μg/d, 5 days per week), rituximab (200 μg/wk, 4HPR + rituximab (at the noted doses), or no drug (solvent only) for 4 weeks. Tumor volume in mm3 (y-axis) was measured over time (x-axis). Curves were truncated when mice required humane killing because of tumor progression. Error bars indicate standard deviation.
Impact of 4HPR + rituximab combination therapy on survival and toxicity in mice with palpable lymphoma xenografts
The ability of a therapy to prolong survival without imposing significant toxicity is even more important than its ability to induce remission. Hence, it was important that treatment with the combination of 4HPR + rituximab significantly improve long-term survival compared with monotherapy with either agent alone or with survival of control mice (Figure 4). Eighty percent of DHL-4-inoculated mice receiving combination therapy were long-term survivors (more than 9 months) compared with 20% of mice receiving 4HPR alone (P = .06) and 0% of mice receiving rituximab alone (P = .002) or control mice (P = .003). Similarly, 80% of mice with palpable FL18 tumors achieved pronged survival with the combination compared with 40% of mice receiving rituximab alone (P = .35), 0% receiving 4HPR alone mice (P = .04), and 0% for control mice (P = .003). One mouse in the combination group experienced 10% weight loss after achieving CR, but other signs of toxicity, such as skin changes, diarrhea, or huddling behavior, were not seen in this subset (data not shown). In contrast, difficulty ambulating associated with tumor progression was noted in the control cohorts.
Kaplan-Meier estimates of overall survival of mice bearing human lymphoma xenografts. (A) DHL-4. (B) FL-18. Mice were treated for 4 weeks with 4HPR (250 μg/d, 5 days per week), rituximab (200 μg/wk), fenretinide + rituximab (at the noted doses), or control with no drug (solvent only). Survival was measured from the time of initiating therapy.
Kaplan-Meier estimates of overall survival of mice bearing human lymphoma xenografts. (A) DHL-4. (B) FL-18. Mice were treated for 4 weeks with 4HPR (250 μg/d, 5 days per week), rituximab (200 μg/wk), fenretinide + rituximab (at the noted doses), or control with no drug (solvent only). Survival was measured from the time of initiating therapy.
Identification of in vivo caspase-mediated apoptosis
Because apoptosis appeared to be an important mechanism of cell death induced by 4HPR and rituximab in vitro, we hypothesized that programmed cell death was also important in vivo. To test this hypothesis, we randomly assigned mice (5 mice per group) with palpable tumors (Ramos) to treatment with 4HPR (250 μg/d ip 5 days per week), rituximab (200 μg ip once per week), combination therapy (at above doses), or diluent alone. After 8 days of therapy, tumors were harvested, and single-cell suspensions were created, stained with FAM, and assessed by flow cytometry. Positive control cells were incubated ex vivo with 0.2% azide for 4 hours before staining with FAM. FAM-positive cells were quantified and counted as apoptotic, and all assays were performed in triplicate. Untreated tumors had a mean apoptotic fraction of 9.9% compared with a mean apoptotic fraction of 43% with 4HPR-treated mice (P = .02), 49% with rituximab-treated mice(P = .03), 50% with 4HPR + rituximab-treated mice (P = .02), and 57% with azide-treated positive controls (P = .0006).
A second experiment further confirmed that caspases were involved in mediating tumor regression in vivo. Mice with palpable tumors (FL-18) were randomly assigned to treatment with 4HPR + rituximab or diluent alone in the presence or absence of the pan-caspase inhibitor Q-VD(OMe)-Oph at a dose of 200 μg/d ip 5 days a week for 3 weeks. All mice treated with 4HPR + rituximab without the caspase inhibitor attained sustained CR (100% CR by day 18) (Figure 5). In marked contrast, none of the untreated control mice and none of the mice treated with 4HPR + rituximab in the presence of Q-VD(OMe)-Oph achieved CR, and all had to be humanely killed because of progressive tumor growth (P = .05).
Effect of in vivo caspase inhibition on tumor growth in palpable FL-18 xenografts. Palpable FL-18 tumors were established in athymic mice (5 mice per group). Mice were then treated with control (diluent only), 4HPR (250 μg/d, 5 days per week) + rituximab (200 μg/wk + irreversible pan caspase inhibitor Q-VD(OMe)-Oph (200 μg/d ip for 5 days a week), or 4HPR + rituximab (at doses above) without Q-VD(OMe)-Oph. Tumor volume in mm3 (y-axis) was measured over time (x-axis). Error bars indicate standard deviation.
Effect of in vivo caspase inhibition on tumor growth in palpable FL-18 xenografts. Palpable FL-18 tumors were established in athymic mice (5 mice per group). Mice were then treated with control (diluent only), 4HPR (250 μg/d, 5 days per week) + rituximab (200 μg/wk + irreversible pan caspase inhibitor Q-VD(OMe)-Oph (200 μg/d ip for 5 days a week), or 4HPR + rituximab (at doses above) without Q-VD(OMe)-Oph. Tumor volume in mm3 (y-axis) was measured over time (x-axis). Error bars indicate standard deviation.
Discussion
Effective curative therapy without significant toxicity is the ultimate goal of cancer treatment. The development and approval of antitumor monoclonal antibodies, such as rituximab and trastuzumab, were major steps in this direction, although response rates and durations remained moderate at best using antibodies as single agents.3,12,13 Some groups have used monoclonals such as rituximab to “sensitize” cells to killing by a second agent, although traditional cytotoxic chemotherapeutic drugs have been most commonly studied.14,15 Our study focused on a novel vitamin A derivative that induces apoptosis in a wide variety of malignant cell lines and has been shown to be well tolerated for long durations in large clinical trials.6,7,16-20 Previous pilot studies from our group demonstrated that 4HPR is superior to other retinoids in inducing growth arrest and apoptosis in human Burkitt lymphoma cell lines in vitro and that it can augment the apoptotic action of anti-CD20 antibodies.9
In this series of experiments, we demonstrated that the combination of 4HPR and rituximab was significantly superior to either agent used alone in vivo and that the therapeutic efficacy observed appeared to be supra-additive. Most mice that received rituximab and 4HPR and achieved CR did not experience disease progression after therapy was discontinued, even after follow-up for up to 450 days, suggesting that these mice were cured. The ability of this combination to eradicate bcl2-overexpressing tumors such as DHL-4 and FL-18 is consistent with observations by others that 4HPR can induce apoptosis in vitro in bcl2-overexpressing leukemia cell lines.21 We hypothesized that 4HPR and rituximab functioned, at least partially, in vivo through apoptotic pathways on the basis of our in vitro data in lymphoma lines and on results from studies using many other tumor types.6,7,9,22-24 Our xenograft model demonstrated that treatment was associated with caspase activation in vivo because cells derived from tumors treated with rituximab, 4HPR, or both stained as positively for activated caspases as the positive controls. Furthermore, we confirmed that a caspase-dependent mechanism was essential for tumor response because treatment with an irreversible activated pan caspase inhibitor, Q-VD(OMe)-Oph, fully abrogated any apparent antitumor activity of the drug combination.
Determining the exact mechanism of this observed in vivo “synergy,” however, is more complex. Studies in other malignant cell lines suggest that the proapoptotic effect of fenretinide is mediated by retinoic acid receptor-dependent and -independent pathways.7 After exposure to 4HPR, it was hypothesized that malignant cells induce the expression of stress-dependent transcription factors, followed by the generation of reactive-oxygen species, increased mitochondrial permeability, and eventual activation of the caspase cascade leading to apoptosis.6-9,24,25 The ligation of CD20 with monoclonal antibodies has also been demonstrated to induce apoptosis in malignant B cells under optimal culture conditions, especially when cross-linked with secondary antibodies or Fc-receptor-bearing accessory cells.1,26 In contrast to 4HPR, the mechanism of action after CD20 binding is thought to be mediated by src family tyrosine kinase-dependent pathways, resulting in intracellular Ca++ release and eventually leading to activation of the caspase cascade and programmed cell death.1,27-30 Other groups have also suggested that an alternative caspase-independent, CD20-mediated apoptotic pathway exists,31 in contrast to our results demonstrating complete abrogation of the drug effect with in vivo caspase inhibitors. The detailed pathways linking CD20 src kinase activation to the 4HPR-initiated mitochondrial pathway activation have yet to be elucidated.
Our observations in this in vivo lymphoma xenograft model suggest that the combination of fenretinide + rituximab might have a major clinical impact on the 50 000 patients who are diagnosed each year in the United States with B-cell non-Hodgkin lymphomas.32 Most current clinical strategies to improve the efficacy of anti-CD20 antibodies have focused on the addition of traditional chemotherapeutic agents.4,5,33 Although responses may be improved, significant hematopoietic and nonhematopoietic toxicities are inevitably incurred with chemotherapy. Fenretinide affords a potentially less toxic approach to enhance rituximab's efficacy because it has been found to be well tolerated in a large number of chemopreventive and therapeutic trials.16-20,34-36 Studies using fenretinide doses of up to 400 mg/d orally with a 3-day drug holiday each month demonstrated very low rates (less than 10%) of all toxicities.17,34,35,37 Such an approach parallels the 2-day-a-week drug holiday that the experimental animals in our study enjoyed. With a drug holiday regimen, it is likely that such doses of 4HPR could be well tolerated for long periods of time.
Combining 4HPR and anti-CD20 antibody therapy in the clinic is particularly appealing. Unexpected toxicity would be unlikely from this combination based on the known tolerability of 4HPR alone and the moderate toxicity of rituximab that is typically limited to infusional reactions.3,12 More important, any synergistic toxicity should be limited given that CD20 and, thus, the target of this combined effect, is only known to be expressed on normal and malignant B cells.38 Unlike traditional chemotherapy, one could envision use of this drug combination to induce responses and to maintain long-term remissions. Sustained exposure to this combination could be particularly effective in patients with indolent B-cell lymphoma in which the major oncogenic abnormality is thought to be an inability of cells to undergo normal apoptosis.39-41 Fenretinide/rituximab combinations could shift the intracellular milieu of malignant B cells from antiapoptosis and proliferation to proapoptosis and cell death. These hypotheses will ultimately have to be tested in clinical trials. Nevertheless, the in vivo and in vitro data to date demonstrate that 4HPR/rituximab combinations enhance apoptosis induction in malignant human B cells, prevent progression of tumors in the minimal disease state, eradicate established tumors, and prolong survival in mice, suggesting that patients may ultimately benefit from this approach.
Prepublished online as Blood First Edition Paper, December 24, 2003; DOI 10.1182/blood-2003-08-2795.
Supported by National Institutes of Health grants K23 CA85479, NIH K12 CA76930, American Society of Clinical Oncology Young Investigator Award, the Hext Foundation, and the Dr Penny L. Peterson Memorial Endowment Fund.
A.K.G. and J.M.P. contributed equally to the article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal