Abstract
We investigated whether the protection from graft-versus-host disease (GVHD) afforded by donor treatment with granulocyte colony-stimulating factor (G-CSF) could be enhanced by dose escalation. Donor treatment with human G-CSF prevented GVHD in the B6 → B6D2F1 murine model in a dose-dependent fashion, and murine G-CSF provided equivalent protection from GVHD at 10-fold lower doses. Donor pretreatment with a single dose of pegylated G-CSF (peg-G-CSF) prevented GVHD to a significantly greater extent than standard G-CSF (survival, 75% versus 11%, P < .001). Donor T cells from peg-G-CSF-treated donors failed to proliferate to alloantigen and inhibited the responses of control T cells in an interleukin 10 (IL-10)-dependent fashion in vitro. T cells from peg-G-CSF-treated IL-10-/- donors induced lethal GVHD; T cells from peg-G-CSF-treated wild-type (wt) donors promoted long-term survival. Whereas T cells from peg-G-CSF wt donors were able to regulate GVHD induced by T cells from control-treated donors, T cells from G-CSF-treated wt donors and peg-G-CSF-treated IL-10-/- donors did not prevent mortality. Thus, peg-G-CSF is markedly superior to standard G-CSF for the prevention of GVHD following allogeneic stem cell transplantation (SCT), due to the generation of IL-10-producing regulatory T cells. These data support prospective clinical trials of peg-G-CSF-mobilized allogeneic blood SCT. (Blood. 2004;103:3573-3581)
Introduction
Graft-versus-host disease (GVHD) remains a major complication following allogeneic hemopoietic stem cell transplantation, with the resultant multiorgan damage and immune deficiency significantly impairing overall transplantation survival. The use of recombinant human granulocyte colony-stimulating factor (G-CSF) mobilized stem cell grafts has led to more rapid immune and hemopoietic reconstitution, reduced transplant-related mortality, and improved leukemia eradication.1 T cells from donors treated with G-CSF have a reduced capacity to induce GVHD on a per cell basis relative to those from control-treated donors.2 In clinical studies, despite an approximate 10-fold increase in the T-cell content of G-CSF-mobilized leukapheresis products compared with unstimulated bone marrow harvests,2,3 there is no increase in the incidence of acute GVHD.1,4 The mechanism by which G-CSF prevents GVHD has been suggested to be the result of T helper 2 (Th2) polarization of donor T cells.2 There is also data suggesting G-CSF may also reduce GVHD through effects on dendritic cells,5 monocytes,6,7 natural killer cells,8 natural killer T cells,9 or direct effects on T cells.10 A recent study suggested that CD4+ T cells exposed to G-CSF in vivo acquire the properties of T regulatory (Tr) cells following T-cell receptor ligation in vitro,11 although regulatory effects in vivo have not been demonstrated.
Interpretation of the large numbers of published studies of G-CSF and GVHD in animal models is hampered by the significant differences in G-CSF doses used (including 0.2 μg/d,2 2 μg/d,12 10 μg/d,13 or 5 μg twice/d14 ), which have provided differing levels of protection. In addition, the majority of the studies have examined the effects of donor pretreatment with human G-CSF in murine models in which there is a species disparate receptor-ligand interaction. We, therefore, investigated whether the protection from GVHD afforded by G-CSF occurred in a dose-dependent fashion and whether efficacy was dependent on the species of origin of G-CSF. Attachment of a polyethylene glycol (peg) molecule to a protein (pegylation) prolongs the plasma half-life of the conjugated agent.15,16 Pegylated filgrastim (hereafter referred to as peg-G-CSF) has a significantly reduced rate of renal clearance and, thus, a longer plasma half-life than standard G-CSF,17 and the ability of peg-G-CSF to mobilize peripheral blood progenitor cells has been demonstrated in both mice and healthy volunteers.18 Although pegylation does not effect the ligand-receptor affinity of G-CSF, we hypothesized that the increased plasma half-life and concomitant increase in the overall duration of receptor occupancy may enhance the ability of G-CSF to reduce GVHD.
We have demonstrated that donor pretreatment with G-CSF is protective in a dose-dependent fashion, and murine G-CSF provides equivalent protection to human G-CSF at a 10-fold lower dose. Peg-G-CSF is shown to be markedly superior to standard G-CSF for the prevention of GVHD because of the generation of interleukin 10 (IL-10)-producing regulatory T cells.
Materials and methods
Mice
Female C57BL/6 (B6, H-2b, Ly-5.2+, CD45.2+), B6 PTPRCA Ly-5a (H-2b, Ly-5.1+, CD45.1+), and B6D2F1 (H-2b/d, Ly-5.2+, CD45.2+)19 mice were purchased from the Animal Research Centre (Perth, Western Australia, Australia). C57BL/6 IL-10-/- mice were supplied by the Australian National University (Canberra, New South Wales, Australia). Mice were housed in sterilized microisolator cages and received acidified autoclaved water (pH 2.5) for the first 2 weeks after transplantation.
Cytokine treatment
Murine G-CSF (Amgen, Thousand Oaks, CA), recombinant human G-CSF (Amgen), and pegylated recombinant human G-CSF (peg-G-CSF) (Amgen) were diluted in 0.9% normal saline before injection. Mice were injected subcutaneously with murine or human G-CSF from days -6to -1, or peg-G-CSF on day -6, at doses as stated, with spleens harvested on day 0.
Stem cell transplantation
Mice received transplants according to a standard protocol as has been described previously.2,12 Briefly, on day -1, B6D2F1 mice received 1100 cGy total body irradiation (137Cs source at 108 cGy/min), split into 2 doses separated by 3 hours to minimize gastrointestinal toxicity. Donor splenocytes were injected intravenously into recipients. T-cell depletion and T-cell purification by nylon wool enrichment and magnetic lineage depletion were performed as previously described.13 Survival was monitored daily, and GVHD clinical scores were measured weekly.
Assessment of GVHD
The degree of systemic GVHD was assessed by a scoring as previously described (maximum index = 10).20-22 Animals with severe clinical GVHD (scores > 6) were killed according to ethics guidelines, and the day of death was deemed to be the following day.
Fluorescence-activated cell sorting (FACS) and engraftment analysis
Fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (mAbs) CD3, CD4, CD8, CD11b, CD11c, CD45.1, CD45.2, class II, B220, Gr-1, and identical phycoerythrin (PE)-conjugated antibodies were purchased from PharMingen (San Diego, CA). For engraftment studies, lethally irradiated B6D2F1 recipients (CD45.2+) received whole spleen from PTPRCA donors (CD45.1+). Complete blood counts were performed on EDTA (ethylenediaminetetraacetic acid) peripheral blood samples by using a Sysmex SE-9000 (Sysmex, Mundelein, IL) analyser. The percentage donor engraftment of peripheral blood nucleated cells and splenocytes was defined as the percentage of CD45.1+ cells divided by the percentage of CD45.2+ + CD45.1+ cells.
Cell cultures
Mixed lymphocyte cultures (MLCs) were undertaken as previously described.13 For in vitro regulation studies, additional nonirradiated purified C57BL/6 T cells or CD4+CD25+ T cells, sorted to more than 85% purity (MoFlo High-Performance Cell Sorter; DakoCytomation, Carpinteria, CA), were added to MLCs (stimulator-effector ratio, 1:4; additional T cells added at doses as indicated).
Cytokine enzyme-lined immunosorbent assays (ELISAs)
The antibodies used in the interferon γ (IFNγ), IL-10, and IL-2 assays were purchased from PharMingen. All assays were performed according to the manufacturer's protocol.
Histology
Formalin-preserved skin, liver, and distal small bowel were embedded in paraffin, and 5-μm-thick sections were stained with hematoxylin and eosin for histologic examination. Slides were coded and examined in a blinded fashion by A.D.C. with the use of a semiquantitative scoring system for abnormalities known to be associated with GVHD as previously described.13,20,21,23 Scores were added to provide a total score of 24 for the skin, 28 for small bowel, and 40 for the liver.
Statistical analysis
Survival curves were plotted by using Kaplan-Meier estimates and compared by log-rank analysis. The Mann Whitney U test was used for the statistical analysis of cytokine data and clinical scores. P < .05 was considered statistically significant.
Results
Donor pretreatment with recombinant human G-CSF prevents GVHD in a dose-dependent fashion
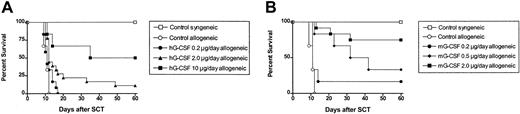
G-CSF prevents GVHD in murine models of stem cell transplantation (SCT), but many studies have demonstrated differing levels of protection following donor pretreatment with different doses of G-CSF. We, therefore, examined the effect of incrementally increasing the dose of G-CSF administered to SC transplant donors in a well-established murine SCT model (C57BL/6 → B6D2F1) that induces GVHD to major and minor histocompatibility antigens. Although this model uses spleen as a stem cell source rather than peripheral blood, its validity has been proven by informative data, indicating beneficial effects of G-CSF on both GVHD and graft versus leukemia (GVL)2,12 that have since been confirmed clinically.1 Allogeneic donor C57BL/6 animals received 6 daily subcutaneous injections of either control diluent, 0.2 μg human G-CSF, 2 μg human G-CSF, or 10 μg human G-CSF, and spleens were harvested on day 7. B6D2F1 recipient mice received 1100 cGy total body irradiation (TBI), and splenocytes (corrected to administer 3 × 106 T cells per inoculum) were transplanted intravenously from respective donors the following day. As shown in Figure 1A, and as previously described,2,13 GVHD induced in this model is severe with all recipients of control splenocytes dying within 2 weeks with characteristic features of GVHD (weight loss, hunching, fur ruffling, etc). In contrast, 100% of non-GVHD controls that received transplants with syngeneic splenocytes survived, confirming that this splenocyte dose contained sufficient stem cells to rescue lethally irradiated recipients. We have previously observed 100% long-term survival in recipients of T-cell-depleted (TCD) splenocytes,13 demonstrating that GVHD in this system is absolutely dependent on the presence of donor T cells. As shown in Table 1, donor engraftment was present at day 8 after transplantation in recipients of T-cell-depleted spleen, as demonstrated by rising blood counts and the exclusive presence of the donor CD45.1 marker. In addition, the percentage and number of donor cells within the spleen were high in TCD recipients (> 98% and 74.8 ± 10.3 × 106 per spleen). In contrast, blood counts in irradiated controls not receiving transplants were very low, without detectable donor cells in the blood or spleen. We have also previously confirmed that the peripheral blood 75 days after SCT in recipients of TCD grafts remains predominantly of donor origin.13 Donor pretreatment with 0.2 μg, 2.0 μg, or 10.0 μg human G-CSF per day for 6 days resulted in dose-dependent protection from GVHD lethality, with allogeneic SC transplant recipient survival at day 60 after transplantation of 0%, 11%, and 50%, respectively (P < .05). Clinical GVHD, assessed by clinical scores in surviving animals, demonstrated that G-CSF did not completely prevent GVHD, but donor pretreatment with G-CSF at 10 μg/d provides greater protection than mobilization with 2 μg/d or 0.2 μg/d (P < .05).
Donor pretreatment with G-CSF reduces the severity of GVHD in a dose-dependent fashion. (A) Survival was determined by Kaplan-Meier analysis. Donor B6 mice were treated for 6 days with human G-CSF (0.2 μg/animal/d, 2 μg/animal/d, or 10 μg/animal/d) or control diluent. Splenocytes were harvested on day 7 and transplanted into lethally irradiated (1100 cGy) B6D2F1 recipient mice; T-cell dose was equilibrated across all groups (3 × 106 T cells/recipient); (control syngeneic recipients, n = 6; control allogeneic, n = 6; G-CSF 0.2 μg/d, n = 12; G-CSF 2.0 μg/d, n = 18; G-CSF 10 μg/d, n = 6). P = .03, 0.2 μg G-CSF versus 2 μg G-CSF; P = .004, 0.2 μg G-CSF versus 10 μg G-CSF. Combined results from 2 identical experiments are shown. (B) Survival was determined by Kaplan-Meier analysis. Donor B6 mice were treated with murine G-CSF (02. μg/animal/d; 0.5 μg/animal/d; or 2 μg/animal/d, for 6 days) or control diluent and transplanted as described earlier. B6D2F1 recipient mice received transplants as described earlier (control syngeneic recipients, n = 6; control allogeneic, n = 6; murine G-CSF 0.2 μg/d, n=6; murine G-CSF 2 μg/d, n=12). Survival P = .003, 0.2 μg murine G-CSF versus 2 μg murine G-CSF. Combined results from 2 identical experiments are shown.
Donor pretreatment with G-CSF reduces the severity of GVHD in a dose-dependent fashion. (A) Survival was determined by Kaplan-Meier analysis. Donor B6 mice were treated for 6 days with human G-CSF (0.2 μg/animal/d, 2 μg/animal/d, or 10 μg/animal/d) or control diluent. Splenocytes were harvested on day 7 and transplanted into lethally irradiated (1100 cGy) B6D2F1 recipient mice; T-cell dose was equilibrated across all groups (3 × 106 T cells/recipient); (control syngeneic recipients, n = 6; control allogeneic, n = 6; G-CSF 0.2 μg/d, n = 12; G-CSF 2.0 μg/d, n = 18; G-CSF 10 μg/d, n = 6). P = .03, 0.2 μg G-CSF versus 2 μg G-CSF; P = .004, 0.2 μg G-CSF versus 10 μg G-CSF. Combined results from 2 identical experiments are shown. (B) Survival was determined by Kaplan-Meier analysis. Donor B6 mice were treated with murine G-CSF (02. μg/animal/d; 0.5 μg/animal/d; or 2 μg/animal/d, for 6 days) or control diluent and transplanted as described earlier. B6D2F1 recipient mice received transplants as described earlier (control syngeneic recipients, n = 6; control allogeneic, n = 6; murine G-CSF 0.2 μg/d, n=6; murine G-CSF 2 μg/d, n=12). Survival P = .003, 0.2 μg murine G-CSF versus 2 μg murine G-CSF. Combined results from 2 identical experiments are shown.
Hemopoietic recovery after transplantation
. | Hematocrit, % . | . | . | Total white cell count, × 109/L (mean absolute neutrophil count, × 109/L) . | . | . | Platelets, × 109/L . | . | . | % donor engraftment . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group . | D + 8 . | D + 10 . | D + 12 . | D + 8 . | D + 10 . | D + 12 . | D + 8 . | D + 10 . | D + 12 . | D + 8 . | ||||||
| Irradiation control | 41.9 ± 1.1 | NA | NA | 0.14 ± 0.02 (<0.1) | NA | NA | 17 ± 2 | NA | NA | <0.1 | ||||||
| Control allogeneic | 34.7 ± 1.5 | 40.3 ± 0.8 | NA | 0.54 ± 0.11* (0.26) | 0.68 ± 0.04 (0.41) | NA | 9 ± 2* | 32 ± 2 | NA | 99.7 ± 0.08 | ||||||
| G-CSF allogeneic | 39.4 ± 0.9 | 42.1 ± 1.4 | 39.2 ± 1.6 | 0.53 ± 0.01* (0.23) | 0.81 ± 0.05 (0.34) | 1.03 ± 0.09 (0.64) | 24 ± 7 | 44 ± 3 | 53 ± 4 | 99.8 ± 0.05 | ||||||
| Peg-G-CSF | ||||||||||||||||
| allogeneic | 41.7 ± 0.8 | ND | 39.6 ± 1.1 | 3.05 ± 0.27* (2.2) | ND | 2.13 ± 0.05 (0.93) | 110 ± 13* | ND | 246 ± 46 | >99.9 | ||||||
| Control syngeneic | 40.6 ± 0.9 | 43.8 ± 0.6 | 39.4 ± 1.7 | 0.32 ± 0.05* (0.24) | 0.71 ± 0.08 (0.48) | 0.95 ± 0.11 (0.68) | 33 ± 6* | 38 ± 5 | 53 ± 9 | NA | ||||||
| Control allogeneic | ||||||||||||||||
| TCD | 41.5 ± 0.6 | ND | ND | 0.33 ± 0.03* (0.15) | ND | ND | 33 ± 5* | ND | ND | >99.9 | ||||||
. | Hematocrit, % . | . | . | Total white cell count, × 109/L (mean absolute neutrophil count, × 109/L) . | . | . | Platelets, × 109/L . | . | . | % donor engraftment . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group . | D + 8 . | D + 10 . | D + 12 . | D + 8 . | D + 10 . | D + 12 . | D + 8 . | D + 10 . | D + 12 . | D + 8 . | ||||||
| Irradiation control | 41.9 ± 1.1 | NA | NA | 0.14 ± 0.02 (<0.1) | NA | NA | 17 ± 2 | NA | NA | <0.1 | ||||||
| Control allogeneic | 34.7 ± 1.5 | 40.3 ± 0.8 | NA | 0.54 ± 0.11* (0.26) | 0.68 ± 0.04 (0.41) | NA | 9 ± 2* | 32 ± 2 | NA | 99.7 ± 0.08 | ||||||
| G-CSF allogeneic | 39.4 ± 0.9 | 42.1 ± 1.4 | 39.2 ± 1.6 | 0.53 ± 0.01* (0.23) | 0.81 ± 0.05 (0.34) | 1.03 ± 0.09 (0.64) | 24 ± 7 | 44 ± 3 | 53 ± 4 | 99.8 ± 0.05 | ||||||
| Peg-G-CSF | ||||||||||||||||
| allogeneic | 41.7 ± 0.8 | ND | 39.6 ± 1.1 | 3.05 ± 0.27* (2.2) | ND | 2.13 ± 0.05 (0.93) | 110 ± 13* | ND | 246 ± 46 | >99.9 | ||||||
| Control syngeneic | 40.6 ± 0.9 | 43.8 ± 0.6 | 39.4 ± 1.7 | 0.32 ± 0.05* (0.24) | 0.71 ± 0.08 (0.48) | 0.95 ± 0.11 (0.68) | 33 ± 6* | 38 ± 5 | 53 ± 9 | NA | ||||||
| Control allogeneic | ||||||||||||||||
| TCD | 41.5 ± 0.6 | ND | ND | 0.33 ± 0.03* (0.15) | ND | ND | 33 ± 5* | ND | ND | >99.9 | ||||||
B6D2F1 irradiation control animals (n = 4) received 1100 cGy total body irradiation, as described in “Materials and methods,” but did not receive a transplant inoculum. Control allogeneic animals (n = 8), G-CSF allogeneic animals (n = 8), peg-G-CSF animals (n = 8), and control syngeneic animals (n = 4) received splenocytes from CD45.1+ B6 donors pretreated with control diluent or cytokine as indicated and as described in “Materials and methods.” Control allogeneic T-cell—depleted (TCD) animals (n = 4) received TCD spleen from control pretreated donors. Blood was collected on days 8, 10, and 12 after transplantation, as shown and analyzed as described in “Materials and methods.” Data are presented as mean ± SEM. *P < .05 versus irradiation control. All values are mean ± SEM. NA indicates not assessable; ND, not determined.
Donor pretreatment with murine G-CSF provides equivalent protection from GVHD as human G-CSF at a 10-fold lower dose
Studies to date have examined the effects of pretreatment of mice with human G-CSF. We sought to determine the relative efficacy of murine G-CSF to prevent GVHD compared with human G-CSF. Allogeneic donor C57BL/6 animals received 6 daily injections of either control diluent, 0.2 μg murine G-CSF, 0.5 μg murine G-CSF, or 2 μg murine G-CSF. As shown in Figure 1B, donor pretreatment with 0.2 μg, 0.5 μg, or 2 μg murine G-CSF again provided dose-dependent protection from GVHD lethality, with survival at day 60 of 17%, 33%, or 75%, respectively (P < .05). Survival at day 60 for recipients of splenocytes pretreated with 0.2 μg murine G-CSF was equivalent to recipients of splenocytes pretreated with a 10-fold higher dose of human G-CSF (0.2 μg murine G-CSF day 60 survival 17% versus 2.0 μg human G-CSF day 60 survival 11%, P = .63).
Donor pretreatment with peg-G-CSF is markedly superior to standard G-CSF in preventing graft-versus-host disease
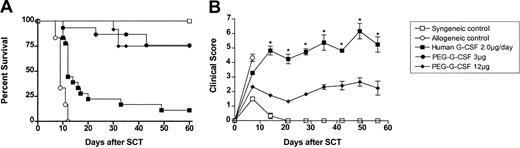
We next examined whether the increase in plasma half-life attributable to pegylation of G-CSF leads to increased protection from GVHD. Allogeneic donor C57BL/6 animals received either control diluent, standard G-CSF (2 μg/d for 6 days), or a single dose of peg-G-CSF (3 or 12 μg at day -6). Lethally irradiated B6D2F1 recipient mice received transplants as described earlier, and grafts were normalized to contain equal numbers of T cells. As shown in Figure 2, donor pretreatment with 3 μg or 12 μg peg-G-CSF resulted in 75% recipient survival at day 60. Donor pretreatment with peg-G-CSF leads to significantly better survival than the same dose of “standard” human G-CSF given over 6 days (11%, P < .0001). Furthermore, a single 3-μg dose of peg-G-CSF at day -6 was superior to standard G-CSF at all doses tested (up to 10 μg/d for 6 days). As shown in Figure 2B, GVHD clinical scores (weight loss, hunching, fur ruffling, etc) in recipients of peg-G-CSF-pretreated spleen were consistent with mild GVHD only (mean scores < 3), whereas recipients of G-CSF-treated splenocytes had severe GVHD (scores > 5, P < .05 at all time points). However, the recipients of allogeneic peg-G-CSF splenocytes that did die after SCT had classical features of GVHD (mean clinical score, 7.0 immediately before death), and so protection was not complete. At day 14 after transplantation, GVHD clinical scores were significantly higher in recipients of spleen from donors pretreated with 3 μg peg-G-CSF when compared with 12 μg peg-G-CSF (mean score, 3.32 ± 0.22 versus 2.29 ± 0.32, P = .02). Following this, however, clinical scores were similar (data not shown).
Donor pretreatment with peg-G-CSF is more effective than standard G-CSF in preventing GVHD. (A) Survival was determined by Kaplan-Meier analysis. Donor B6 mice received either control diluent, standard human G-CSF 2 μg/animal/d for 6 days, 3 μg peg-G-CSF, or 12 μg peg-G-CSF as a single injection on day -6. Lethally irradiated B6D2F1 recipient mice received transplants as in Figure 1 (control syngeneic recipients, n = 6; control allogeneic, n = 6; peg-G-CSF 3 μg, n = 12; peg-G-CSF 12 μg, n = 12; human standard G-CSF 2 μg/d, n = 18). P = .82, 3 μg peg-G-CSF versus 12 μg peg-G-CSF; P = .0001, 2 μg G-CSF (for 6 days) versus 3 μg, and 12 μg peg-G-CSF (single dose).
Donor pretreatment with peg-G-CSF is more effective than standard G-CSF in preventing GVHD. (A) Survival was determined by Kaplan-Meier analysis. Donor B6 mice received either control diluent, standard human G-CSF 2 μg/animal/d for 6 days, 3 μg peg-G-CSF, or 12 μg peg-G-CSF as a single injection on day -6. Lethally irradiated B6D2F1 recipient mice received transplants as in Figure 1 (control syngeneic recipients, n = 6; control allogeneic, n = 6; peg-G-CSF 3 μg, n = 12; peg-G-CSF 12 μg, n = 12; human standard G-CSF 2 μg/d, n = 18). P = .82, 3 μg peg-G-CSF versus 12 μg peg-G-CSF; P = .0001, 2 μg G-CSF (for 6 days) versus 3 μg, and 12 μg peg-G-CSF (single dose).
To understand the mode of death in allogeneic SC transplant recipients, hemopoietic recovery was assessed in conjunction with histopathology. As shown in Table 1, lethally irradiated animals not receiving transplants did not achieve hemopoietic recovery and were severely leukopenic and thrombocytopenic by day 8 after transplantation. In contrast, recipients of peg-G-CSF-pretreated splenocytes experienced almost complete hematologic reconstitution by this time. Early donor white blood count recovery had also occurred in recipients of control allogeneic, G-CSF allogeneic, control syngeneic, and control T-cell-depleted allogeneic splenocytes. Although recipients of control and G-CSF allogeneic splenocytes begin to die shortly after this time point in association with rising blood counts, control syngeneic and control T-cell-depleted allogeneic recipients remained well, despite equivalent or lower blood counts (Table 1; Figure 2B). This finding confirms that control allogeneic and G-CSF allogeneic recipients were not dying as a consequence of engraftment failure. To confirm that mortality was indeed due to GVHD in recipients of allogeneic splenocytes, we evaluated histologic evidence of GVHD prior to and at the time of death. Skin, small bowel, and liver tissue samples were taken from recipients at day 8 after transplantation, the day of maximum mortality (day 10 after transplantation in control allogeneic and day 12 in G-CSF allogeneic and relevant control groups), and at day 60 in surviving animals (control syngeneic and peg-G-CSF allogeneic recipients). As shown in Table 2, there was evidence of GVHD in all target organs in recipients of allogeneic splenocytes at day 8 after transplantation, regardless of donor pretreatment with G-CSF. However, as shown in Figure 2B, GVHD clinical scores (weight loss, hunching, fur ruffling, etc) are similar at day 7 after transplantation but diverge after this time (coinciding with the period of rapid death). Histologic analysis of tissue taken from recipients of control and G-CSF allogeneic splenocytes at the time of maximal mortality (day 10 and 12, respectively; Table 2) demonstrated severe GVHD of the gastrointestinal tract (GI) (mean score, 24.0 ± .3 and 18.9 ± 4.6), whereas the GVHD in recipients of peg-G-CSF-pretreated allogeneic splenocytes demonstrated resolving GVHD of the GI tract at this time (mean score, 4.7 ± 0.3). Furthermore, recipients of G-CSF-treated allogeneic splenocytes that died by day 12 had mean GI tract scores of 23.2 ± 1.2 (n = 5), whereas GI tract scores of surviving animals were 9.5 ± 1.9 (n = 6, P < .01). In contrast, the skin and liver histopathology scores were not increased in dying animals relative to animals surviving (data not shown), confirming that GVHD of the GI tract was the cause of mortality in these animals. Interestingly, by day 60 the GVHD of the skin and GI tract in recipients of peg-G-CSF grafts had completely resolved, whereas that in the liver remained stable. Thus, recipients of peg-G-CSF-pretreated splenocytes develop early GVHD which is maximal at engraftment (day 8) and, subsequently, resolves in the skin and gut, suggesting a process of active regulation. In contrast, recipients of control or G-CSF-pretreated splenocytes develop progressive and ultimately fatal gastrointestinal GVHD at engraftment. This finding is consistent with published data in this transplantation model in which mortality is known to be a consequence of severe gastrointestinal GVHD.20,21,24
Effect of donor pretreatment with peg-G-CSF on GVHD histopathology
. | Day 8 after treatment . | . | . | . | Day 10 to 12 after treatment . | . | . | Day 60 after treatment . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Control syngeneic . | Control allogeneic . | G-CSF allogeneic . | Peg-G-CSF allogeneic . | Control allogeneic . | G-CSF allogeneic . | Peg-G-CSF allogeneic . | Control syngeneic . | Peg-G-CSF allogeneic . | ||||||
| Cutaneous GVHD | 1.0 ± 0.2 | 10.1 ± 1.1 | 10.5 ± 1.2 | 13.0 ± 1.3 | 11.4 ± 0.7 | 12.3 ± 0.7 | 15.1 ± 0.6 | 0.5 ± 0.5 | 0.3 ± 0.1 | ||||||
| GI tract GVHD | 2.3 ± 0.3 | 14.7 ± 1.9 | 14.1 ± 2.9 | 13.8 ± 0.4 | 24.0 ± 1.3 | 18.5 ± 4.6 | 4.7 ± 0.3 | 0.3 ± 0.3 | 1.4 ± 0.6 | ||||||
| Hepatic GVHD | 0.6 ± 0.2 | 5.0 ± 0.8 | 3.3 ± 0.7 | 11.2 ± 1.3 | 6.3 ± 0.5 | 19.2 ± 0.9 | 12.7 ± 0.9 | 1.8 ± 0.3 | 8.6 ± 1.6 | ||||||
. | Day 8 after treatment . | . | . | . | Day 10 to 12 after treatment . | . | . | Day 60 after treatment . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Control syngeneic . | Control allogeneic . | G-CSF allogeneic . | Peg-G-CSF allogeneic . | Control allogeneic . | G-CSF allogeneic . | Peg-G-CSF allogeneic . | Control syngeneic . | Peg-G-CSF allogeneic . | ||||||
| Cutaneous GVHD | 1.0 ± 0.2 | 10.1 ± 1.1 | 10.5 ± 1.2 | 13.0 ± 1.3 | 11.4 ± 0.7 | 12.3 ± 0.7 | 15.1 ± 0.6 | 0.5 ± 0.5 | 0.3 ± 0.1 | ||||||
| GI tract GVHD | 2.3 ± 0.3 | 14.7 ± 1.9 | 14.1 ± 2.9 | 13.8 ± 0.4 | 24.0 ± 1.3 | 18.5 ± 4.6 | 4.7 ± 0.3 | 0.3 ± 0.3 | 1.4 ± 0.6 | ||||||
| Hepatic GVHD | 0.6 ± 0.2 | 5.0 ± 0.8 | 3.3 ± 0.7 | 11.2 ± 1.3 | 6.3 ± 0.5 | 19.2 ± 0.9 | 12.7 ± 0.9 | 1.8 ± 0.3 | 8.6 ± 1.6 | ||||||
Recipients received transplants as described in “Materials and methods.” Tissue was collected from control allogeneic animals (n = 11), G-CSF allogeneic animals (n = 6), peg-G- CSF allogeneic animals (n = 6), and control syngeneic animals (n = 4) at day 8 after treatment. Tissue was also collected from recipients on the day of maximal GVHD mortality (control pretreated donors [n = 6] at day 10 and from G-CSF pretreated at day 12 [n = 5]). Tissue was also collected from recipients of peg-G-CSF splenocytes at day 12 after transplantation as a comparison. The cutaneous, GI tract, and hepatic histopathology scores in syngeneic recipients at day 12 were 0.1 ± 0.1, 1.6 ± 0.2, and 2.4 ± 0.6, respectively. Finally, tissue was collected from syngeneic (n = 3) and peg-G-CSF allogeneic recipients (n = 5) at the termination of the experiment on day 60. Histopathology was scored as described in “Materials and methods.” Data presented as mean ± SEM. The scores at day 10 to 12 in control and G-CSF allogeneic recipients that are significantly higher than those in peg-G-CSF allogeneic recipients are shown in bold (P < .05). Histopathology scores in all allogeneic recipients were significantly higher than syngeneic recipients at days 8 and 10 to 12 (P < .05). At day 60, only the hepatic score in peg-G-CSF allogeneic recipients was significantly higher than that in syngeneic recipients (P < .05).
Cellular expansion following donor pretreatment with standard and pegylated G-CSF
G-CSF has been shown to alter antigen-presenting cell (APC) phenotype in stem cell grafts, and this may contribute to the attenuation of GVHD. We, therefore, examined both overall spleen expansion and cellular composition following G-CSF or peg-G-CSF pretreatment. Donor pretreatment with 2 μg per day standard G-CSF for 6 days led to an average 53% increase in spleen size (P < .0001 versus control). Pretreatment with a single dose of 12 μg peg-G-CSF led to an average 65% increase in spleen size (P < .0001 versus control). The difference in spleen size between 2 μg G-CSF for 6 days and 12 μg peg-G-CSF as a single dose was not statistically significant (P = .11). Interestingly, despite the marked reduction in GVHD afforded by a single 3-μg dose peg-G-CSF, the spleens in these donors were approximately 25% smaller than donors receiving 2 μg/d standard G-CSF for 6 days.
Pretreatment with 12 μg peg-G-CSF did not alter the total T-cell number or subset proportions, and in particular the numbers of CD11c+ DC and CD4+CD25+ regulatory T cells were not altered (Figure 3). The granulocyte lineage was expanded 2-fold in peg-G-CSF-treated spleens and bone marrow, and to a lesser degree in G-CSF-treated spleens (data not shown). In addition, a population of CD11bpos/Gr-1dim precursor cells was disproportionately increased relative to other APC subsets in peg-G-CSF-treated donors (G-CSF versus peg-G-CSF P = .001).
Splenocyte expansion following donor pretreatment with standard orpegylated G-CSF. Donor B6 mice (n = 4 per group) received either control diluent, 2 μg human G-CSF/d for 6 days or single injection of 12 μg peg-G-CSF day -6, and splenocytes were harvested on day 7. (A) Relative proportions of each cell lineage. (B) Absolute numbers of each cell lineage. *P < .05 control versus peg-G-CSF, +P < .05 peg-G-CSF versus control and G-CSF. Data are presented as mean ± SD.
Splenocyte expansion following donor pretreatment with standard orpegylated G-CSF. Donor B6 mice (n = 4 per group) received either control diluent, 2 μg human G-CSF/d for 6 days or single injection of 12 μg peg-G-CSF day -6, and splenocytes were harvested on day 7. (A) Relative proportions of each cell lineage. (B) Absolute numbers of each cell lineage. *P < .05 control versus peg-G-CSF, +P < .05 peg-G-CSF versus control and G-CSF. Data are presented as mean ± SD.
Donor treatment with peg-G-CSF impairs T-cell function and induces regulatory T-cell activity
GVHD induced in these models is dependent on T-cell function,12,25 and we, therefore, examined the effect of G-CSF and peg-G-CSF on T-cell function in vitro. C57BL/6 T cells were stimulated with alloantigen, and T-cell proliferation and cytokine production were determined. Pretreatment of donors with both G-CSF and peg-G-CSF inhibited T-cell proliferation to alloantigen but did not prevent IL-2 production (Figure 4A). Interferon-γ secretion to alloantigen was reduced 10-fold following donor treatment with peg-G-CSF. The response of donor T cells from peg-G-CSF animals to mitogen (CD3 and CD28) was also reduced 10-fold both before and after transplantation relative to T cells from control-treated donors (data not shown). Because the impairment of T-cell proliferation was not associated with reductions in IL-2 production, we next sought to determine whether T cells from cytokine-pretreated donors exhibited regulatory function and were able to inhibit the proliferation of T cells from control-treated donors. T cells from noncytokine exposed C57BL/6 donors were stimulated with alloantigen, with or without the addition of T cells from wild-type or IL-10-/- donors pretreated with a single dose (12 μg) of peg-G-CSF. As shown in Figure 4B, T cells from peg-G-CSF-pretreated donors but not T cells from control-pretreated donors regulated proliferation of control T cells to alloantigen (P < .05). T cells from peg-G-CSF-treated IL-10-/- donors regulated proliferation to a lesser degree (Figure 4B), suggesting that IL-10 production is required, at least in part, to provide regulatory function. As shown in Figure 4C, CD4+CD25+ T cells from control-pretreated donors suppress allogeneic T-cell proliferation to a similar degree as those from peg-G-CSF-pretreated donors. There does not, therefore, appear to be a change in the “potency” of the CD4+CD25+ T cells because of donor pretreatment with peg-G-CSF. Because IL-10 appeared to be playing a role in the regulation of T-cell function by peg-G-CSF-treated T cells in vitro, we next studied the ability of grafts from these animals to produce IL-10 in response to inflammatory stimuli. Surprisingly, spleen from peg-G-CSF-treated donors produced 8-fold more IL-10 in response to LPS and CpG relative to control-treated spleen (Figure 4D), whereas the G-CSF spleen produced IL-10 at an intermediate level.
Donor treatment with peg-G-CSF impairs T-cell function and induces regulatory T-cell activity. (A) C57BL/6 T cells from donors pretreated with control diluent, G-CSF 2 μg/d for 6 days, or peg-G-CSF 12 μg as a single dose at day -6 were stimulated with irradiated B6D2F1 peritoneal macrophages. Proliferation was measured at 72 hours by way of standard 3[H]thymidine incorporation assay as described in “Materials and methods.” P < .05 control versus G-CSF and P < .05 control versus peg-G-CSF. IFN-γ and IL-2 production were determined in culture supernatants by ELISA. (B) Control C57BL/6 T cells were stimulated with irradiated B6D2F1 macrophages as described earlier. Additional nonirradiated T cells from wild-type C57BL/6 donors pretreated with control diluent, peg-G-CSF 12 μg day -6, or IL-10-/- donors pretreated with peg-G-CSF 12 μg day -6 were added at the doses shown. Proliferation was measured at 72 hours by way of standard 3[H]thymidine incorporation assay. *P < .05 control versus wild-type peg-G-CSF. (C) Control C57BL/6 T cells were stimulated with irradiated B6D2F1 macrophages as described earlier. Purified CD4+CD25+ T cells (nonirradiated) from wild-type C57BL/6 donors pretreated with control diluent or peg-G-CSF 12 μg day -6 were added at the doses shown. Proliferation was measured at 72 hours by way of standard 3[H]thymidine incorporation assay. (D) Whole spleen from control-, G-CSF-, or peg-G-CSF-pretreated donors as described earlier was stimulated with lipopolysaccharide (LPS) and cytosine-phosphate-guanosine (CpG), and IL-10 was measured in supernatants at 48 hours by ELISA. P = .0002 control versus G-CSF; P = .001 control versus peg-G-CSF. Data (A-D) are presented as mean ± SD of triplicate wells and represent one of 2 identical experiments.
Donor treatment with peg-G-CSF impairs T-cell function and induces regulatory T-cell activity. (A) C57BL/6 T cells from donors pretreated with control diluent, G-CSF 2 μg/d for 6 days, or peg-G-CSF 12 μg as a single dose at day -6 were stimulated with irradiated B6D2F1 peritoneal macrophages. Proliferation was measured at 72 hours by way of standard 3[H]thymidine incorporation assay as described in “Materials and methods.” P < .05 control versus G-CSF and P < .05 control versus peg-G-CSF. IFN-γ and IL-2 production were determined in culture supernatants by ELISA. (B) Control C57BL/6 T cells were stimulated with irradiated B6D2F1 macrophages as described earlier. Additional nonirradiated T cells from wild-type C57BL/6 donors pretreated with control diluent, peg-G-CSF 12 μg day -6, or IL-10-/- donors pretreated with peg-G-CSF 12 μg day -6 were added at the doses shown. Proliferation was measured at 72 hours by way of standard 3[H]thymidine incorporation assay. *P < .05 control versus wild-type peg-G-CSF. (C) Control C57BL/6 T cells were stimulated with irradiated B6D2F1 macrophages as described earlier. Purified CD4+CD25+ T cells (nonirradiated) from wild-type C57BL/6 donors pretreated with control diluent or peg-G-CSF 12 μg day -6 were added at the doses shown. Proliferation was measured at 72 hours by way of standard 3[H]thymidine incorporation assay. (D) Whole spleen from control-, G-CSF-, or peg-G-CSF-pretreated donors as described earlier was stimulated with lipopolysaccharide (LPS) and cytosine-phosphate-guanosine (CpG), and IL-10 was measured in supernatants at 48 hours by ELISA. P = .0002 control versus G-CSF; P = .001 control versus peg-G-CSF. Data (A-D) are presented as mean ± SD of triplicate wells and represent one of 2 identical experiments.
Protection from GVHD is dependent on production of IL-10 from the donor T cell
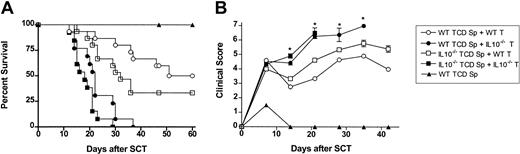
Splenocytes pretreated with peg-G-CSF produced large amounts of IL-10 in response to inflammatory stimuli, and T cells from peg-G-CSF-pretreated donors regulated proliferation of alloantigen stimulated T cells in vitro in an IL-10-dependent fashion. We, therefore, next examined whether the protection from GVHD afforded by peg-G-CSF was dependent on IL-10 production by the donor T cell, the non-T-cell compartment, or both. C57BL/6 IL-10-/- or wild-type donors were all pretreated with 12 μg peg-G-CSF on day -6. Survival at day 60 was 100% in recipients of wild-type or IL-10-/- TCD spleen alone, confirming that adequate numbers of stem cells were transferred to allow hemopoietic reconstitution (data for IL-10-/- TCD spleen not shown). Wild-type or IL-10-/- TCD splenocytes plus purified T cells from either wild-type or IL-10-/- donors were infused into lethally irradiated B6D2F1 recipients as indicated (Figure 5A-B). Recipients of allogeneic wild-type T cells had delayed mortality (Figure 5A) and moderate GVHD as assessed by clinical scores (Figure 5B), regardless of whether the non-T-cell component was from wild-type or IL-10-/- donors. In contrast, recipients of allogeneic IL10-/- T cells all died from GVHD by day 37 regardless of whether the non-T-cell component was from wild-type or IL-10-/- donors. Recipients of control-treated T-cell-replete grafts from wild-type or IL-10-/- donors all died by day 14 at an equivalent rate (data not shown). There was a nonstatistical improvement in survival in recipients of wild-type TCD splenocytes plus wild-type T cells when compared with recipients of IL-10-/- TCD splenocytes plus wild-type T cells. Thus, the production of IL-10 by cells outside the T-cell compartment may provide some protection from GVHD under certain conditions.
The protection from GVHD afforded by peg-G-CSF is dependent on IL-10 production from the donor T cell. All donors were pretreated with a single dose of 12 μg peg-G-CSF at day -6. T-cell-depleted (TCD) splenocytes from wild-type or IL-10-/- donors plus purified CD3+ T cells from wild-type or IL-10-/- B6 donors were combined as indicated and injected into lethally irradiated B6D2F1 recipients (wild-type TCD spleen only, n = 6; wild-type T cells plus wild-type or IL-10-/- spleen, n = 15; IL-10-/- T cells plus wild-type or IL-10-/- spleen, n = 13). (A) Survival was determined by Kaplan-Meier analysis. P < .001, wild-type TCD spleen + wild-type T cells versus wild-type TCD spleen + IL10-/- T cells; P < .0001, IL10-/- TCD spleen + wild-type T cells versus IL10-/- spleen + IL10-/- T cells; (B) GVHD clinical scores were determined as a measure of GVHD severity in surviving animals. *P < .05, wild-type TCD spleen + wild-type T cells versus wild-type TCD spleen + IL10-/- T cells. Data are presented as mean ± standard deviation.
The protection from GVHD afforded by peg-G-CSF is dependent on IL-10 production from the donor T cell. All donors were pretreated with a single dose of 12 μg peg-G-CSF at day -6. T-cell-depleted (TCD) splenocytes from wild-type or IL-10-/- donors plus purified CD3+ T cells from wild-type or IL-10-/- B6 donors were combined as indicated and injected into lethally irradiated B6D2F1 recipients (wild-type TCD spleen only, n = 6; wild-type T cells plus wild-type or IL-10-/- spleen, n = 15; IL-10-/- T cells plus wild-type or IL-10-/- spleen, n = 13). (A) Survival was determined by Kaplan-Meier analysis. P < .001, wild-type TCD spleen + wild-type T cells versus wild-type TCD spleen + IL10-/- T cells; P < .0001, IL10-/- TCD spleen + wild-type T cells versus IL10-/- spleen + IL10-/- T cells; (B) GVHD clinical scores were determined as a measure of GVHD severity in surviving animals. *P < .05, wild-type TCD spleen + wild-type T cells versus wild-type TCD spleen + IL10-/- T cells. Data are presented as mean ± standard deviation.
IL-10-producing protective donor T cell has regulatory function
Because the protection from GVHD afforded by peg-G-CSF administration was dependent on IL-10 production by the donor T cell, we next studied whether these T cells were able to suppress or regulate GVHD by T cells that would otherwise induce lethal GVHD. T cells from wild-type donors pretreated with control diluent, G-CSF, or peg-G-CSF or T cells from IL-10-/- donors pretreated with peg-G-CSF were added to wild-type T-cell-replete grafts from untreated donors. As shown in Figure 6, the addition of T cells from control-treated donors to control grafts had no effect on GVHD mortality with all animals dying by day 12. In contrast, the addition of T cells from peg-G-CSF-treated donors to control grafts resulted in 45% survival at day 50 (P < .001). This ability to regulate GVHD was significantly greater in T cells from peg-G-CSF-treated donors than in T cells from standard G-CSF-treated donors because the latter provided only a modest 10-day delay in mortality. The regulation of GVHD by T cells from peg-G-CSF-treated donors was largely, although not completely, dependent on IL-10 production by the donor T cell, because T cells from peg-G-CSF-pretreated IL-10-/- donors delayed, but did not prevent, GVHD mortality.
The protective IL-10 producing donor T cell has regulatory function. Lethally irradiated B6D2F1 recipients received splenocytes from wild-type B6 donors plus additional purified T cells from control, G-CSF, or peg-G-CSF pretreated donors as shown (syngeneic control, n = 3; allogeneic control, n = 5; allogeneic control + wild-type control T cells, n = 9, ○; allogeneic control + wild-type G-CSF T cells, n = 10; allogeneic control + wild-type peg-G-CSF T cells, n = 14; allogeneic control + IL-10-/- peg-G-CSF T cells, n = 13). Survival was determined by Kaplan-Meier analysis. P < .001, allogeneic control + wild-type peg-G-CSF T cells versus all other groups. Data were combined from 2 identical experiments.
The protective IL-10 producing donor T cell has regulatory function. Lethally irradiated B6D2F1 recipients received splenocytes from wild-type B6 donors plus additional purified T cells from control, G-CSF, or peg-G-CSF pretreated donors as shown (syngeneic control, n = 3; allogeneic control, n = 5; allogeneic control + wild-type control T cells, n = 9, ○; allogeneic control + wild-type G-CSF T cells, n = 10; allogeneic control + wild-type peg-G-CSF T cells, n = 14; allogeneic control + IL-10-/- peg-G-CSF T cells, n = 13). Survival was determined by Kaplan-Meier analysis. P < .001, allogeneic control + wild-type peg-G-CSF T cells versus all other groups. Data were combined from 2 identical experiments.
Discussion
We have shown that donor pretreatment with recombinant human G-CSF protects recipients from GVHD in a dose-dependent fashion and that murine G-CSF is approximately 10-fold more potent than human G-CSF in this murine model. In addition, donor pretreatment with a single dose of peg-G-CSF significantly reduces GVHD when compared with the same dose of standard G-CSF given over 6 days. The protection from GVHD is dependent on the production of IL-10 by donor T cells, and T cells from cytokine-pretreated donors have transferable regulatory activity both in vitro and in vivo.
The concept of protection from GVHD by donor pretreatment with G-CSF being a dose-dependent phenomenon has not been previously described. In the absence of altered receptor affinity or plasma half-life, this may reflect increased numerical receptor occupancy for a short time following administration of higher doses of G-CSF. Murine G-CSF was 10-fold more potent at preventing GVHD mortality than human G-CSF in our murine model. Human G-CSF is a 174-amino acid polypeptide, and murine G-CSF consists of 178 amino acids, with 72.6% homology at the amino acid sequence level.26 Renal clearance would not be expected to differ between the 2 forms of G-CSF, suggesting their plasma profiles, following administration of comparable doses, are likely to be very similar. Species cross-reactivity of human and murine G-CSF has previously been demonstrated,27 although the affinity of cytokine for its receptor is not influenced by species-specific receptor-ligand interaction.28 The protection provided by species-specific G-CSF at a 10-fold lower dose may thus reflect altered ligand-receptor signalling between murine G-CSF and the murine G-CSF receptor than between human G-CSF and murine G-CSF receptors. Pegylation of G-CSF significantly increases the plasma half-life of G-CSF, without altering receptor affinity.16 Thus, increased receptor occupancy over a prolonged period leads to further increases in therapeutic efficacy, with significantly improved survival of animals receiving splenocytes from donors pretreated with a single dose of peg-G-CSF, compared with recipients receiving splenocytes from donors pretreated with the same dose of standard G-CSF over 6 days. There are a number of differences between this study and that of Pan et al12 that may explain the differences in survival following donor treatment with similar doses of G-CSF. Our institution's animal ethics committee requires that animals with severe GVHD (scores > 6) be killed which was not the case in the study of Pan et al12 which predates this scoring system. No histopathology was shown in surviving animals in the Pan et al12 study to demonstrate relative severity of GVHD in these animals, although persistent weight loss was evident. More importantly, the 2 studies were performed in different animal facilities, using mice sourced from suppliers in different continents, and using different irradiation sources. The ability of different animal facilities (and associated microflora) to affect GVHD lethality has been widely described, despite apparently identical strain combinations and transplantation procedures.29,30
Histologic examination of skin, small bowel, and liver demonstrated similar levels of GVHD at day 8 after transplantation in recipients of control allogeneic, G-CSF-pretreated allogeneic, or peg-G-CSF-pretreated allogeneic splenocytes despite subsequent differences in survival. However, recipients of control or G-CSF-pretreated splenocytes develop progressive gastrointestinal GVHD that is frequently fatal between days 9 to 12. Clinically, acute GVHD most frequently manifests at the time of blood count recovery;31 thus, one may expect that the rapid hemopoietic recovery in recipients of peg-G-CSF allogeneic splenocytes would be associated with the early development of GVHD. Conversely, the comparatively slower hemopoietic recovery in recipients of control and G-CSF allogeneic splenocytes may be expected to be associated with a later onset of GVHD. Thus, although histologic GVHD scores were similar in all groups at day 8, GVHD in recipients of peg-G-CSF allogeneic splenocytes had peaked in severity, whereas GVHD in recipients of control or G-CSF allogeneic splenocytes was similar but rapidly progressed as ongoing hemopoietic recovery occurred thereafter. The fact that cutaneous and GI tract GVHD had resolved by day 60 after transplantation in recipients of peg-G-CSF-treated splenocytes is intriguing and consistent with the finding of potent regulatory activity in these grafts. The pathologic processes responsible for the development of GVHD are recognized to differ in the GI tract,24,32-34 skin,35-37 and liver.33 The Fas-FasL pathway appears important in hepatic GVHD,38 and the persistence of hepatic GVHD at low levels (mean GVHD score, 8.6 ± 1.6 of potential maximum of 40) suggests that attenuation of GVHD in the liver is less sensitive to the regulatory process induced by peg-G-CSF. However, the severity of hepatic GVHD in peg-G-CSF recipients was less severe than in G-CSF recipients at day 12, suggesting that peg-G-CSF does attenuate hepatic GVHD to some extent. The relevance of the mild hepatic GVHD observed in the peg-G-CSF recipients will require assessment in the clinical setting in which GVHD prophylaxis is operative and may serve to minimize GVHD isolated to the liver.
Although increasing the duration of receptor occupancy or receptor-ligand affinity increases the ability of T cells from donors pretreated with G-CSF or peg-G-CSF to prevent GVHD, the precise cellular mechanisms responsible remain to be defined. Donor treatment with G-CSF is known to reduce GVHD in murine models,2,12,13 and in clinical practice rates of acute GVHD are not increased despite the 10-fold increase in transferred T cells associated with peripheral blood SCT.1 The ability of G-CSF to alter T-cell function is not a new finding, but previously it has been linked to Th2 differentiation.2 Administration of other cytokines that induce Th2 differentiation also protect from GVHD, supporting this finding.21 Other studies, however, have documented that G-CSF inhibits both Th1 and Th2 cytokine production.3,13 Rutella et al11 reported the presence of T cells with regulatory function in vitro in humans following G-CSF treatment. CD4+ T-cell cytokine synthesis was compared before and after G-CSF treatment and demonstrated an increase in IL-10 production, no change in TGF-β1 production, and reduced IL-2 and IL-4 synthesis. The in vitro CD4+ T-cell proliferation in responses to alloantigen and recall antigens was reduced following G-CSF pretreatment. Thus, G-CSF administration results in T-cell function that varies between Th2 and Th3/Tr1 differentiation which may be the result of the different doses studied. To date, however, there has been no demonstration of regulatory function in vivo. A single in vivo study demonstrated that transfer of G-CSF-mobilized donor leukocytes following allogeneic heart transplantation in a rat model led to increased IL-10 (and reduced IFN-γ and IL-2) within grafts and significantly prolonged graft survival.39 The mechanism of this effect was not established, however, and tolerance may reflect the induction of a mixed chimeric state, as previously described,40-42 rather than the presence of regulatory function. G-CSF may alter T-cell function either directly10 or indirectly by way of expansion of APCs or other cells of the innate immune system. We have previously demonstrated that neither CD11chi dendritic cells (DCs) nor CD11cdim/B220hi DCs from cytokine-treated donors confer protection from GVHD.13 The treatment of transplant recipients (but not donors) with G-CSF does not prevent GVHD in murine models,43 suggesting that the modulation of GVHD by G-CSF is due to indirect effects of undefined cellular subsets rather than the direct effect of G-CSF on T cells.
The nature of the protective IL-10-secreting T cell observed in our studies is unclear at the present time. CD4+CD25+ regulatory T cells have been shown to regulate both autoimmune disease,44,45 the rejection of solid organ transplants,46 and GVHD.47 Cohen et al48 examined the regulatory effects of CD4+CD25+ T cells (which represent 5%-10% of the normal T-cell compartment49 ) in the B6 to B6D2F1 murine SCT model. This study reported that removal of the CD4+CD25+ T-cell compartment from a transplant inoculum resulted in earlier GVHD mortality. Addition of CD4+CD25+ T cells reduced, although did not prevent, GVHD mortality because of the limited half-life of transferred cells. Treatment with peg-G-CSF does not lead to expansion of CD4+CD25+ T cells, nor does it appear to increase the potency of these cells. Thus, the protective IL-10-producing T cell does not appear to be a classical CD4+CD25+ regulatory T cell. Regulatory function has been described in a wide variety of other cell types, including CD8+ T cells,50,51 TCR+CD4-CD8- T cells,52,53 and natural killer T (NKT) cells.54,55 In addition, T cells from peg-G-CSF-treated IL-10-/- donors have residual (albeit significantly reduced) regulatory function, suggesting the presence of additional effector molecules such as transforming growth factor β (TGFβ). Ongoing work is attempting to elucidate the exact nature of the IL-10-producing T cells in question.
These studies confirm that peg-G-CSF is markedly superior to standard G-CSF for the prevention of GVHD in the C57BL/6 to B6D2F1 murine model of allogeneic hemopoietic stem cell transplantation. This effect is predominantly due to the enhanced generation of IL-10-producing regulatory donor T cells. Further studies examining other donor-recipient strain combinations are required to confirm the general applicability of the findings, and prospective clinical trials examining the ability of peg-G-CSF-mobilized allogeneic peripheral blood stem cell grafts to promote transplant tolerance in both stem cell and solid organ settings are indicated. Furthermore, the induction of IL-10-producing regulatory T cells following peg-G-CSF administration suggests applicability to a wider variety of diseases characterized by autoimmunity and failure of regulatory tolerance to self-antigens.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-08-2864.
Supported in part by grants from the NHMRC and Queensland Cancer Fund. G.R.H. is a Wellcome Trust Senior Overseas Research Fellow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Figure 4. Donor treatment with peg-G-CSF impairs T-cell function and induces regulatory T-cell activity. (A) C57BL/6 T cells from donors pretreated with control diluent, G-CSF 2 μg/d for 6 days, or peg-G-CSF 12 μg as a single dose at day -6 were stimulated with irradiated B6D2F1 peritoneal macrophages. Proliferation was measured at 72 hours by way of standard 3[H]thymidine incorporation assay as described in “Materials and methods.” P < .05 control versus G-CSF and P < .05 control versus peg-G-CSF. IFN-γ and IL-2 production were determined in culture supernatants by ELISA. (B) Control C57BL/6 T cells were stimulated with irradiated B6D2F1 macrophages as described earlier. Additional nonirradiated T cells from wild-type C57BL/6 donors pretreated with control diluent, peg-G-CSF 12 μg day -6, or IL-10-/- donors pretreated with peg-G-CSF 12 μg day -6 were added at the doses shown. Proliferation was measured at 72 hours by way of standard 3[H]thymidine incorporation assay. *P < .05 control versus wild-type peg-G-CSF. (C) Control C57BL/6 T cells were stimulated with irradiated B6D2F1 macrophages as described earlier. Purified CD4+CD25+ T cells (nonirradiated) from wild-type C57BL/6 donors pretreated with control diluent or peg-G-CSF 12 μg day -6 were added at the doses shown. Proliferation was measured at 72 hours by way of standard 3[H]thymidine incorporation assay. (D) Whole spleen from control-, G-CSF-, or peg-G-CSF-pretreated donors as described earlier was stimulated with lipopolysaccharide (LPS) and cytosine-phosphate-guanosine (CpG), and IL-10 was measured in supernatants at 48 hours by ELISA. P = .0002 control versus G-CSF; P = .001 control versus peg-G-CSF. Data (A-D) are presented as mean ± SD of triplicate wells and represent one of 2 identical experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/9/10.1182_blood-2003-08-2864/6/m_zh80090460480004.jpeg?Expires=1765924129&Signature=Ha8MhMB-5xo8mQYS97~wXT23-Y9sWKkM843k~PHwxzVr0kUICRMqiZ9YP32vjuvrRhmOegudvYfoCsfI0kxj0FUIE6vsDEfJe1981ceWJyJJagSaeQd75OoxqkE3IGevgqkCqR2Zqp8R9bipwySigbL330KzE9jORtIUx0yft4c0VajSfOl5Pn13MFIYwquVVtYNtH3HBVrmqYvfhOAJn-HBJxOQZ7gQnoHFCA3hGC~sQ~vtkst3BVid1eDagxaxymRNAq1MJVa1xcctmxKnx~t2UiMtHjJE5wok5yE21hEzt-1FtbvUfAJSA26EsHNkf-6hvucY7vvOSrqeP826PQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal