Abstract
Neutropenia is a common laboratory finding in systemic lupus erythematosus (SLE). However, the molecular mechanism of SLE neutropenia has not been fully explained. In this study, we examined whether TNF-related apoptosis-inducing ligand (TRAIL) is involved in the pathogenesis of SLE neutropenia using samples from SLE patients. Serum TRAIL levels in SLE patients with neutropenia were significantly higher than those of SLE patients without neutropenia and healthy volunteers. Serum TRAIL levels showed a significant negative correlation with neutrophil counts in SLE patients. The expression of TRAIL receptor 3 was significantly lower in SLE patients with neutropenia than in patients without neutropenia or in healthy volunteers. Treatment with glucocorticoids negated the decrease of TRAIL receptor 3 expression on neutrophils of SLE patients. TRAIL may accelerate neutrophil apoptosis of neutrophils from SLE patients, and autologous T cells of SLE patients, which express TRAIL on surface, may kill autologous neutrophils. Interferon gamma and glucocorticoid modulated the expression of TRAIL on T cells of SLE patients and also modulated the expression of cellular Fas-associating protein with death domain–like interleukin-1β–converting enzyme (FLICE)–inhibitory protein (cFLIP), an inhibitor of death receptor signaling, in neutrophils. Thus, our results provide a novel insight into the molecular pathogenesis of SLE neutropenia.
Introduction
Neutropenia in systemic lupus erythematosus (SLE) was first described more than 70 years ago1 and is found in about 50%-60% of patients with SLE.2 Clinically, increased susceptibility to infections is a major cause of morbidity and mortality in patients with SLE.3,4 In this regard, not only treatment with adrenal glucocorticoids and/or immunosuppressive drugs but also decreased numbers of polymorphonuclear neutrophils (PMNs) is obviously responsible for the increased incidence of infections.5-9 However, the detailed molecular mechanism of neutropenia in SLE has not been fully elucidated.
Traditionally, PMNs have been considered to be the first line cell component of the body defense mechanism against bacterial pathogens and were regarded as terminally differentiated cells incapable of protein synthesis and committed to death within 72 hours.10-12 Recently, it was indicated that neutrophils were not only capable of receiving signals from different proinflammatory cytokines and chemokines, but also could synthesize many important proinflammatory cytokines and chemokines to modulate immune responses, such as interferon-gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukin-8 (IL-8).13,14 And these proinflammatory mediators, relevant to the inflamed site in vivo, also act to modulate the constitutive death of neutrophils.15,16 Regarding neutrophil apoptosis, Fas17 and TNF-α18 were reported to be able to shorten neutrophil lifespan at early time points. Recently, it was reported that TNF-related apoptosis-inducing ligand (TRAIL) could accelerate neutrophil apoptosis.19 Also, TRAIL was reported to be involved in the monocyte apoptosis induced by T cells in SLE.20 However, the role of TRAIL in neutropenia of SLE is still unclear.
In this study, we have investigated the TRAIL receptors on neutrophils and TRAIL-induced neutrophil apoptosis using samples from SLE patients. A difference in the expression pattern of TRAIL receptors between SLE patients and healthy volunteers has been discovered. We also have found that autologous T cells of SLE patients, which expressed TRAIL on the surface, could kill autologous PMNs. IFN-γ and glucocorticoids could modulate the expressions of TRAIL on T cells of SLE patients and also modulate the expressions of cellular Fas-associating protein with death domain–like interleukin-1β–converting enzyme (FLICE)–inhibitory protein (cFLIP), an inhibitor of death receptor signaling, in PMNs.19,20 It is suggested that TRAIL is involved in the pathogenesis of neutropenia in SLE.
Patients, materials, and methods
This study was reviewed and approved by the Kagoshima University Faculty of Medicine Committee on Human Research.
Reagents
Recombinant human TRAIL, neutralizing antibody against human TRAIL, and biotinylated goat anti-human TRAIL-R1, R2, R3, and R4 antibodies were acquired from R&D Systems, Minneapolis, MN. Rabbit polyclonal antibody against human cFLIP and mouse monoclonal antibody against human actin were acquired from Santa Cruz Biotechnology, Santa Cruz, CA. Sheep anti-rabbit IgG, anti-mouse IgG coupled with horseradish peroxidase, and α[32-P] dCTP were acquired from Amersham Pharmacia Biotech, Piscataway, NJ. Phosphate-buffered saline (PBS), RPMI 1640, and TRIzol Reagent were acquired from Invitrogen, Gaithersburg, MD. Fetal calf serum (FCS) was acquired from HyClone, Logan, UT. Corticosterone (glucocorticoid), paraformaldehyde, formamide, human serum, Hist-paque, and propidium iodide (PI) were acquired from Sigma, St Louis, MO. Human recombinant IFN-γ was acquired from PeproTech, Rocky Hill, NJ. Protease inhibitor cocktail tablets, complete mini, were acquired from Roche, Indianapolis, IN.
Patients
Twenty-eight SLE patients (male to female, 13:15; mean age, 39.5 ± 19.2) and 8 healthy volunteers (male to female, 3:5; mean age, 43.2 ± 22.3) participated in this study. All the cases and volunteers gave written consent to participate in the study. All patients with SLE fulfilled the 1997 criteria from the American College of Rheumatology.21 We diagnosed the patients whose absolute blood neutrophil counts (ANC) were below 1.5 × 103/μL22,23 as having neutropenia. None of the patients received immunomodulatory agents, which can affect neutrophil survival, because the blood samples were obtained before the patients underwent medication. We measured anti-nuclear antibody, rheumatoid factor, SSB/La antibody, and SSA/Ro antibody in serum before the patients underwent medication.
Measurement of TRAIL, G-CSF, and Fc γ receptor III in serum
TRAIL and granulocyte colony-stimulating factor (G-SCF) concentrations in serum were measured in duplicate for each sample using a commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems) that recognizes recombinant and natural human TRAIL or G-CSF. This assay is sensitive from 10 pg/mL and does not cross-react with other homologous cytokines. Optical density at 450 nm was measured on a Titertek Multiskan MC plate reader (Flow Laboratories, Helsinki, Finland), and TRAIL or G-CSF concentrations were determined by linear regression from the standard curve using GraphPad software (Flow Laboratory, Rockville, MD) for analysis. We also measured serum-soluble Fc gamma receptor III (sFc γ IIIR) levels using anti–human CD16 monoclonal antibody (Exalpha Biologicals, Watertown, MA) as previously described.24 Briefly, ELISA plates (Nunc Immunoplate Maxisorp, Roskilde, Denmark) were coated with 5 μg/mL CD16 monoclonal antibody. Unbound sites were blocked with PBS containing 2% (vol/vol) milk (PBS/milk). Patient serum samples were diluted in high performance ELISA (HPE) buffer (CLB, Amsterdam, The Netherlands). After washing the plates with PBS containing 0.02% Tween-20 (vol/vol), the wells were incubated with duplicated serum samples at room temperature. The plates were then incubated for 1 hour with appropriate concentrations of a biotin-labeled polyclonal anti–pan-Fc γ RIII antibody (Exalpha Biologicals), diluted in an HPE buffer. After incubation for 30 minutes with horseradish peroxidase–labeled streptavidin and dilution in PBS/milk, 100 μL substrate buffer was added. The colorimetric reaction was stopped by the addition of 2 M H2SO4, and the absorbance at 450 nm was measured by a Titertek multiscan ELISA reader (Flow Laboratory). The pooled serum of 80 healthy individuals was used to obtain a calibration curve. The concentration of sFc γ RIII in this pool was set at 100 arbitrary units.
Preparation of PMNs and T cells
Human PMNs were obtained from heparinized venous blood of 16 SLE patients and 8 healthy volunteers. Heparinized venous blood was mixed with one-fourth volume of 2% dextran solution (Sigma) to precipitate red blood cells. After 30 minutes' incubation at room temperature, the leukocyte-rich plasma was laid onto Hist-paque and centrifuged at 800 × g for 20 minutes at room temperature. PMNs were separated from erythrocytes by lysis in 0.2% NaCl, washed in PBS 3 times at 4° C, and resuspended in RPMI 1640 containing 10% FCS and streptomycin/penicillin. Contamination level of mononuclear leukocytes was lower than 0.5% by morphological examination after staining with Diff-Quick (Wako, Tokyo, Japan).
Peripheral blood mononuclear cells (PBMCs) also were separated from heparinized venous blood of SLE patients and healthy volunteers by Histo-paque gradient centrifugation as described.25 T cells were isolated by negative selection using magnetic beads (Miltenyi Biotic GmbH, Bergisch, Germany) according to the manufacturer's protocol as described.26 The purity of T cells was more than 95% by FACS scan analysis.
Flow cytometry analysis
To detect expression of TRAIL receptors on PMNs or the expression of TRAIL on T cells, 5 × 105 cells were collected after incubation in various states. Cells were washed with PBS 3 times and then incubated with human serum (pooled sample from healthy volunteer) for 10 minutes and subsequently incubated with biotinated anti–TRAIL receptor antibodies or anti-TRAIL antibody (R&D Systems) for 20 minutes at 4° C. After washing 3 times with PBS, the cells were incubated with streptavidin-fluorescein isothiocyanate (FITC) (PharMingen, San Diego, CA) for 15 minutes at 4° C. Flow cytometry analysis was performed by a FACscan using Cellquest software (PharMingen).
Assay for apoptosis
1 × 106 PMNs were collected after incubation in various states, and exposure of phosphatidylserine was detected by flow cytometry analysis as described in the previous section using Annexin V–FITC Apoptosis Detection kit (PharMingen). In all experiments, apoptotic data were confirmed by TdT-mediated dUTP nick-end labeling (TUNEL) assay using a commercially available kit, according to the manufacturer's instructions (TUNEL Label Mix, Roche Diagnostics).
51Cr release assay
To investigate T-cell–mediated neutrophil apoptosis, we performed 51Cr release assay as previously described.26 Briefly, 10 million PMNs (target cells) were incubated with 100 μCi (3.7 MBq) [51Cr] sodium chromate/106 cells for 1 hour and washed 5 times with PBS. The labeled PMNs (5 × 103 cells/well) were incubated with autologous T cells (effector cells). Preliminarily, we performed co-incubation for various time durations (2, 4, 6, 10, and 14 hours) and found that 10 hours of co-incubation is optimal. After 10 hours of incubation, supernatants of each well were collected and radioactivity was measured with a gamma counter (1480 WIZARTM 3; PerkinElmer, Downers Grove, IL). Specific lysis was calculated by the formula: specific 51Cr-release = [(mean experimental cpm – mean spontaneous cpm)/(mean maximum cpm – mean spontaneous cpm)] × 100%, in which spontaneous release represents counts per minute (cpm) in supernatants from wells containing target cells with medium only, and maximum release represents cpm in supernatants from wells containing target cells in medium with 2% Triton X-100. The data were presented as the mean ± standard deviation from the data of 8 patients.
Northern blotting analysis
T cells from SLE patients were incubated with IFN-γ (50 U/mL), IL-13 (50 ng/mL), and corticosterone (500 nM) for various time durations. 5 × 106 T cells of SLE patients or healthy volunteers were collected and total RNA was extracted by using TRIzol Reagent according to manufacturer's protocol. Northern blot analysis was performed as previously described.27 Human TRAIL cDNA was kindly donated by Dr T. Yoshimura (Laboratory of Molecular Immunoregulation, National Cancer Institute–Frederick, Frederick, MD).
Western blotting analysis
To determine the amount of cFLIP in PMNs of SLE patients and healthy volunteers, 1 × 108 PMNs were collected and lysed on ice for 20 minutes in 1 milliliter of lysis buffer containing 50 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 150 mM NaCl, 1% Triton X-100, 10% glycerol, and a cocktail of protease inhibitors (Roche). The lysates were spun, and the 20-μL supernatants were collected and the same volume (20 μL) of double-strength sample buffer (20% glycerol, 6% sodium dodecyl sulfate (SDS), 10% 2-mercaptoethanol) was added. The samples were boiled for 10 minutes. Proteins were analyzed on 10% polyacrylamide gels by SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) and transferred electrophoretically to nitrocellulose membranes at 150 mA for 1 hour by using a semidry system. The membranes were incubated with rabbit polyclonal anti–human cFLIP antibody or mouse anti–human actin monoclonal antibody, followed by a sheep anti-rabbit or -mouse IgG coupled with horseradish peroxidase. Peroxidase activity was visualized by the Enhanced Chemiluminescence Detection System (Amersham).
Statistical analysis
We used one-way factorial analysis of variance (ANOVA) with the Bonferroni-Dunn test. We also used Student t test and Pearson correlation coefficient test. A P value below .05 was considered significant. Most values were expressed as mean ± standard deviation (SD).
Results
Patients
Fifteen patients had neutropenia and 4 of them had infection (2 had pneumonia, one had urinary tract infection, and one had gastrointestinal tract infection). Infectious agents were Staphylococcus species, Streptococcus species, Pseudomonas aeruginosa, and Haemophilus influenzae. Antibiotic treatment was effective in all patients. All SLE patients were positive for antinuclear antibody, and 5 patients (2 patients with neutropenia) were positive for rheumatoid factor. Six patients were positive for SSB/La antibody, and 5 of them also were positive for SSA/Ro antibody. Among them 4 patients (all patients were positive for both antibodies) were diagnosed as having neutropenia.
TRAIL, G-CSF, and sFc γ IIIR levels in serum
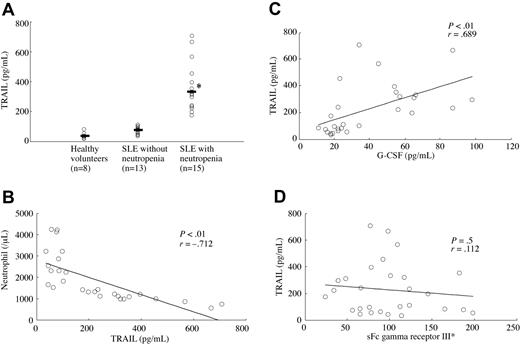
Serum TRAIL levels in SLE patients with neutropenia were significantly higher than in SLE patients without neutropenia and in healthy volunteers (Figure 1A). Serum TRAIL titers of SLE patients showed significant negative correlation with neutrophil counts (Figure 1B). G-CSF and sFc γ RIII are reported to be associated with neutropenia in SLE,28-30 therefore we measured those levels and compared them with serum TRAIL levels. Serum G-CSF levels showed significant positive correlation with serum TRAIL levels (Figure 1C), however there was no significant correlation between serum sFC γ R III levels and serum TRAIL levels (Figure 1D). There was no significant difference of TRAIL levels between SSA/Ro- and/or SSB/La-positive neutropenia patients and negative neutropenia patients (positive patients = 1072.8 ± 246.3 pg/mL, negative patients = 1105.4 ± 253.6 pg/mL).
Serum TRAIL levels and serum G-CSF and sFc γ IIIR levels. (A) Serum TRAIL levels in SLE patients with neutropenia were significantly higher than in SLE patients without neutropenia and healthy volunteers (*P < .01, Bonferroni/Dunn with one-way factorial ANOVA Bars indicate mean value). (B) Serum TRAIL levels showed a significant negative correlation with absolute neutrophil counts (P < .01, r = –0.712, Pearson correlation coefficient test). (C) Serum TRAIL levels showed a significant positive correlation with serum G-CSF levels (P < .01, r = 0.689, Pearson correlation coefficient test). (D) There was no significant correlation between serum TRAIL levels and serum sFc γ IIIR levels. Sloping lines indicate simple regression in each set of data (B-D)
Serum TRAIL levels and serum G-CSF and sFc γ IIIR levels. (A) Serum TRAIL levels in SLE patients with neutropenia were significantly higher than in SLE patients without neutropenia and healthy volunteers (*P < .01, Bonferroni/Dunn with one-way factorial ANOVA Bars indicate mean value). (B) Serum TRAIL levels showed a significant negative correlation with absolute neutrophil counts (P < .01, r = –0.712, Pearson correlation coefficient test). (C) Serum TRAIL levels showed a significant positive correlation with serum G-CSF levels (P < .01, r = 0.689, Pearson correlation coefficient test). (D) There was no significant correlation between serum TRAIL levels and serum sFc γ IIIR levels. Sloping lines indicate simple regression in each set of data (B-D)
Expression of TRAIL receptors
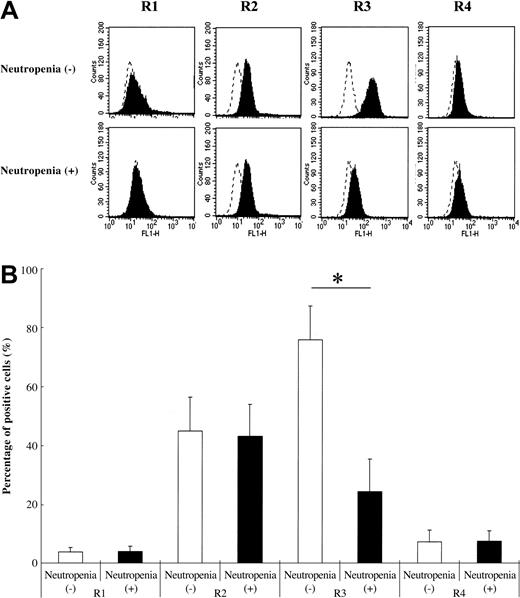
We used PMNs from 8 SLE patients with neutropenia (neutrophil count = 1019.6 ± 285.6/μL) and 8 SLE patients without neutropenia (neutrophil count = 2933.4 ± 902.4/μL). As shown in Figure 2, PMNs from SLE patients expressed all TRAIL receptors on their surface. TRAIL receptor 3–positive cell percentage was significantly lower in SLE patients with neutropenia than in SLE patients without neutropenia (Figure 2B). The expression pattern of TRAIL receptors in PMNs from healthy volunteers was almost the same as that of SLE patients (data not shown).
TRAIL receptor expression on neutrophils of SLE patients. Neutrophils from SLE patients with neutropenia showed significantly lower TRAIL receptor 3 expressions than those from SLE patients without neutropenia (*P < .01, Bonferroni/Dunn with one-way factorial ANOVA). (A) Representative data from 8 individual experiments. Dotted lines show the binding of an isotype control antibody to cells. Dotted line indicates IgG control intensity; solid line indicates TRAIL receptor expression intensity. (B) Data are shown with mean + standard deviations (n = 8 in each group).
TRAIL receptor expression on neutrophils of SLE patients. Neutrophils from SLE patients with neutropenia showed significantly lower TRAIL receptor 3 expressions than those from SLE patients without neutropenia (*P < .01, Bonferroni/Dunn with one-way factorial ANOVA). (A) Representative data from 8 individual experiments. Dotted lines show the binding of an isotype control antibody to cells. Dotted line indicates IgG control intensity; solid line indicates TRAIL receptor expression intensity. (B) Data are shown with mean + standard deviations (n = 8 in each group).
Influence of glucocorticoid therapy on TRAIL receptor expression
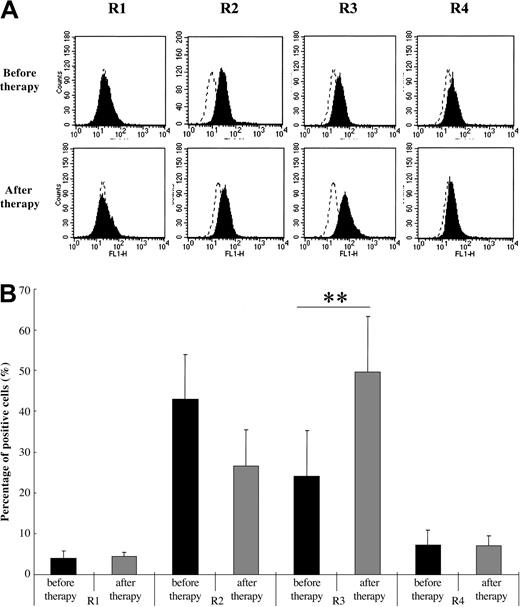
Glucocorticoids are commonly used for the treatment of SLE.31 To evaluate the effect of glucocorticoids on TRAIL receptor expression in PMNs of SLE patients with neutropenia, we compared the expression pattern prior to therapy and after the beginning of therapy. As shown in Figure 3, the expression of TRAIL receptor 3 was significantly increased after therapy (mean therapy period = 6.3 ± 2.1 months, n = 8). Also, PMN counts significantly increased after therapy (before therapy, 2419.3 ± 971.1/μL; after therapy, 6011.5 ± 1567.4/μL, P < .01 Student t test).
Comparison of TRAIL receptor expression on neutrophils of SLE patients with neutropenia before and after therapy. TRAIL receptor 3 expression levels significantly increased after treatment (**P < .05, Bonferroni/Dunn with a one-way factorial ANOVA). (A) Representative data from 8 individual experiments. Dotted line indicates IgG control intensity; solid line indicates TRAIL receptor expression intensity. (B) Data are shown with mean + standard deviations (n = 8 in each group).
Comparison of TRAIL receptor expression on neutrophils of SLE patients with neutropenia before and after therapy. TRAIL receptor 3 expression levels significantly increased after treatment (**P < .05, Bonferroni/Dunn with a one-way factorial ANOVA). (A) Representative data from 8 individual experiments. Dotted line indicates IgG control intensity; solid line indicates TRAIL receptor expression intensity. (B) Data are shown with mean + standard deviations (n = 8 in each group).
Comparison of TRAIL effect on neutrophil apoptosis between SLE and healthy volunteers
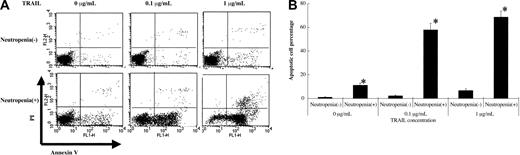
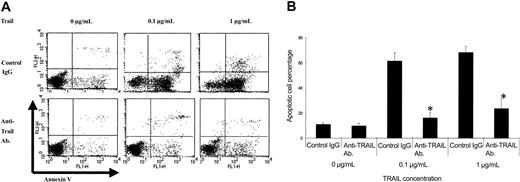
To compare the effect of TRAIL on PMN apoptosis, we incubated PMNs with various amounts of TRAIL for 6 hours and investigated the apoptosis cell percentage. Preliminarily, we performed incubation for various time durations (2, 4, 6, 8, and 10 hours) and found that 6 hours of incubation is optimal. As shown in Figure 4, apoptotic cell percentage induced by TRAIL was significantly higher in SLE patients with neutropenia than in SLE patients without neutropenia. As shown in Figure 5, a TRAIL-neutralizing antibody significantly inhibited the apoptotic effect of TRAIL on PMNs of SLE patients with neutropenia. All apoptotic data were confirmed by TUNEL staining (data not shown).
TRAIL effect on neutrophils from SLE patients. Neutrophils from SLE patients were incubated at various concentrations of TRAIL for 6 hours. TRAIL induced significantly more apoptosis of neutrophils from SLE patients with neutropenia than neutrophils from SLE patients without neutropenia (*P < .01, Bonferroni/Dunn with one-way factorial ANOVA). (A) Representative data from 8 individual experiments. (B) Data are shown with mean + standard deviation (n = 8 in each group).
TRAIL effect on neutrophils from SLE patients. Neutrophils from SLE patients were incubated at various concentrations of TRAIL for 6 hours. TRAIL induced significantly more apoptosis of neutrophils from SLE patients with neutropenia than neutrophils from SLE patients without neutropenia (*P < .01, Bonferroni/Dunn with one-way factorial ANOVA). (A) Representative data from 8 individual experiments. (B) Data are shown with mean + standard deviation (n = 8 in each group).
Effect of a TRAIL-neutralizing antibody against TRAIL-induced neutrophil apoptosis in SLE patients with neutropenia. The TRAIL-neutralizing antibody significantly inhibited TRAIL-induced neutrophil apoptosis in a dose-dependent manner (*P < .001, Bonferroni/Dunn with one-way factorial ANOVA). (A) Representative data from 8 individual experiments. (B) Data were shown with mean + standard deviation (n = 8 in each group).
Effect of a TRAIL-neutralizing antibody against TRAIL-induced neutrophil apoptosis in SLE patients with neutropenia. The TRAIL-neutralizing antibody significantly inhibited TRAIL-induced neutrophil apoptosis in a dose-dependent manner (*P < .001, Bonferroni/Dunn with one-way factorial ANOVA). (A) Representative data from 8 individual experiments. (B) Data were shown with mean + standard deviation (n = 8 in each group).
Expression of TRAIL on lymphocytes and lymphocyte cytotoxicity against PMNs
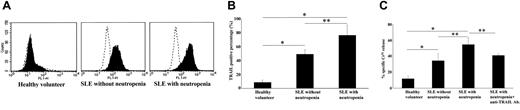
T cells of SLE patients are known to be able to induce apoptosis of monocytes in SLE.20 Therefore, we evaluated the cytotoxicity of T cells against PMNs. T cells obtained from SLE patients expressed surface TRAIL. The expression percentage was significantly higher in SLE patients with neutropenia than in SLE patients without neutropenia. The TRAIL-positive lymphocyte percentage in healthy volunteers was significantly lower than in SLE patients. The result of autologous T-lymphocyte co-incubation with PMNs is shown in Figure 6C. The specific 51Cr release was significantly higher in SLE patients with neutropenia than in SLE patients without neutropenia and healthy volunteers. There was no significant difference in specific 51Cr release between SSA/Ro- and/or SSB/La-positive patients and negative patients (positive patients, n = 4, 58.3% ± 8.1%; negative patients, n = 4, 57.9% ± 9.2%). ATRAIL-neutralizing antibody (1 μg/mL) significantly inhibited the specific 51Cr release. Therefore, we conclude that autologous T cells could kill autologous PMNs in SLE.
TRAIL expression on T cells from SLE patients with neutropenia. (A) Cell surface expression of TRAIL on T cells from SLE patients and healthy volunteers. TRAIL-positive cell percentage was significantly higher in SLE patients with neutropenia than in SLE patients without neutropenia and healthy volunteers. TRAIL-positive cell percentage was significantly higher in SLE patients without neutropenia than in healthy volunteers (*P < .01, **P < .05, Bonferroni/Dunn with one-way factorial ANOVA). (A) Representative data from 8 individual experiments. Dotted line indicates IgG control intensity; solid line indicates TRAIL expression intensity. (B) Data are shown with mean + standard deviation (n = 8 in each group). (C) T-cell cytotoxicity against autologous neutrophils. In SLE patients with neutropenia, T cells killed significantly more autologous neutrophils than in SLE patients without neutropenia and in healthy volunteers (*P < .01, **P < .05, Bonferroni/Dunn with one-way factorial ANOVA. Data were shown with mean + standard deviation, n = 8 in each group).
TRAIL expression on T cells from SLE patients with neutropenia. (A) Cell surface expression of TRAIL on T cells from SLE patients and healthy volunteers. TRAIL-positive cell percentage was significantly higher in SLE patients with neutropenia than in SLE patients without neutropenia and healthy volunteers. TRAIL-positive cell percentage was significantly higher in SLE patients without neutropenia than in healthy volunteers (*P < .01, **P < .05, Bonferroni/Dunn with one-way factorial ANOVA). (A) Representative data from 8 individual experiments. Dotted line indicates IgG control intensity; solid line indicates TRAIL expression intensity. (B) Data are shown with mean + standard deviation (n = 8 in each group). (C) T-cell cytotoxicity against autologous neutrophils. In SLE patients with neutropenia, T cells killed significantly more autologous neutrophils than in SLE patients without neutropenia and in healthy volunteers (*P < .01, **P < .05, Bonferroni/Dunn with one-way factorial ANOVA. Data were shown with mean + standard deviation, n = 8 in each group).
Effect of IFN-γ, IL-13, and glucocorticoid on TRAIL expression
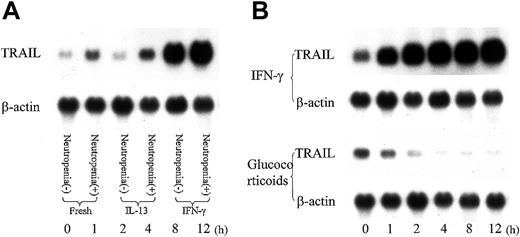
To evaluate the cytokine effect on lymphocyte TRAIL expression of SLE patients, we incubated lymphocytes of SLE patients in the presence or absence of IFN-γ, a representative Th1 cytokine, and IL-13, an important Th2 cytokine, for 8 hours and investigated TRAIL mRNA expression. As shown in Figure 7, IFN-γ upregulated TRAIL mRNA expression in lymphocytes of SLE patients, while IL-13 had no effect. IFN-γ up-regulated TRAIL expression in neutrophils, while glucocorticoid, a common drug to treat SLE, down-regulated TRAIL mRNA expression. IFN-γ also up-regulated TRAIL mRNA expression in lymphocytes of healthy volunteers (data not shown).
Influence of cytokines and glucocorticoid on TRAIL expression in T cells. T cells were incubated with IFN-γ (50 U/mL), IL-13 (50 ng/mL), or corticosterone (500 nM) for various time durations. (A) RNA was extracted from T cells of SLE patient after an 8-hour incubation with IL-13 (50 ng/mL) or IFN-γ (50 U/mL). INF-γ up-regulated TRAIL expression of T cells. Representative data from 4 individual experiments. (B) RNA was extracted from T cells of an SLE patient with neutropenia after incubation with IFN-γ (50 U/mL) or glucocorticoid (500 nM) for various time durations. IFN-γ up-regulated the TRAIL expression level, while glucocorticoid down-regulated the TRAIL expression in T cells. Shown are representative data from 4 individual experiments.
Influence of cytokines and glucocorticoid on TRAIL expression in T cells. T cells were incubated with IFN-γ (50 U/mL), IL-13 (50 ng/mL), or corticosterone (500 nM) for various time durations. (A) RNA was extracted from T cells of SLE patient after an 8-hour incubation with IL-13 (50 ng/mL) or IFN-γ (50 U/mL). INF-γ up-regulated TRAIL expression of T cells. Representative data from 4 individual experiments. (B) RNA was extracted from T cells of an SLE patient with neutropenia after incubation with IFN-γ (50 U/mL) or glucocorticoid (500 nM) for various time durations. IFN-γ up-regulated the TRAIL expression level, while glucocorticoid down-regulated the TRAIL expression in T cells. Shown are representative data from 4 individual experiments.
cFLIP expression in PMNs
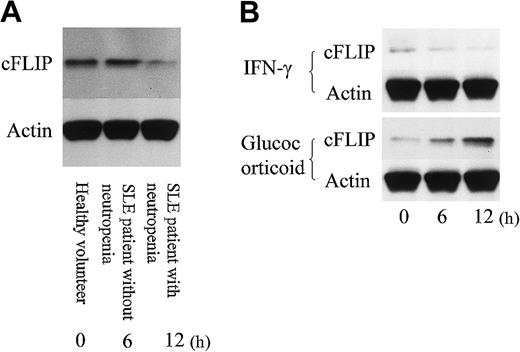
Finally, we evaluated the expression of cFLIP, an inhibitor of death receptor signaling, in PMNs. As shown in Figure 8, the expression amount of cFLIP in PMNs of SLE patients with neutropenia was lower than in SLE patients without neutropenia and in healthy volunteers. Glucocorticoid up-regulated cFLIP expression in PMNs obtained from SLE patients with neutropenia, while IFN-γ slightly down-regulated cFLIP expression.
cFLIP expression in neutrophils. (A) Neutrophils from an SLE patient with neutropenia expressed less cFLIP than from an SLE patient without neutropenia and a healthy volunteer. Representative data from 4 individual experiments. (B) Neutrophils from an SLE patient with neutropenia were incubated with IFN-γ (50 U/mL) or glucocorticoid (500 nM) for various time durations. Glucocorticoid up-regulated cFLIP expression, while IFN-γ slightly down-regulated cFLIP expression in neutrophils. Shown are representative data from 4 individual experiments.
cFLIP expression in neutrophils. (A) Neutrophils from an SLE patient with neutropenia expressed less cFLIP than from an SLE patient without neutropenia and a healthy volunteer. Representative data from 4 individual experiments. (B) Neutrophils from an SLE patient with neutropenia were incubated with IFN-γ (50 U/mL) or glucocorticoid (500 nM) for various time durations. Glucocorticoid up-regulated cFLIP expression, while IFN-γ slightly down-regulated cFLIP expression in neutrophils. Shown are representative data from 4 individual experiments.
Discussion
Although the molecular mechanism of TRAIL-induced apoptosis in tumor cells has been described, the role of TRAIL in health and disease is virtually unknown. Results presented here strongly suggest that TRAIL can accelerate the apoptosis of PMNs in SLE patients. TRAIL recently has been identified as a member of the TNF family that triggers rapid apoptosis in various types of tumor cells.32 TRAIL can interact with 2 DRs, DR4 (TRAIL receptor 1)33 and DR5 (TRAIL receptor 2),34,35 and 2 DcRs, DcR1 (TRAIL receptor 3)34,36,37 and DcR2 (TRAIL receptor 4).38,39 TRAIL receptors 1 and 2 contain an intracellular motif called the “death domain” that subsequently activates caspase-8, and the caspase cascade leads to apoptosis.33,34,40,41 In contrast, TRAIL receptors 3 and 4 have either truncated or missing intracellular domains and are unable to transduce the death signal,34,37,39 suggesting that these receptors may compete for ligand-binding and act as antiapoptotic receptors.42 Previous studies showed expressions of TRAIL receptors 2 and 3 on PMN cell surface and suggested that these receptors contribute to the maintenance of the balance of PMN apoptosis.19 In our study, the expression level of TRAIL receptor 3 was significantly lower in SLE patients with neutropenia. Besides, glucocorticoid up-regulated the expression level of TRAIL receptor 3. To our knowledge, this is the first report showing a difference in TRAIL receptor expression patterns of PMNs in SLE and a change in the TRAIL receptor 3 expression level of PMNs by treatment with glucocorticoid. Furthermore, serum TRAIL levels showed a significant correlation with neutrophil numbers in SLE patients. Also, the level in patients with neutropenia was significantly higher than in patients without neutropenia. Therefore, we believe that TRAIL is involved in the molecular mechanism of neutropenia in SLE. On the other hand, one of the important causes of neutropenia in SLE is an anti-PMN autoantibody.43 An anti-PMN autoantibody can facilitate the opsonization of PMNs surrounded by mononuclear phagocytes.43,44 Although the autoantibody can facilitate apoptosis, it cannot induce apoptosis by itself.45 Furthermore, PMN apoptosis is necessary to induce the production of autoantibody against PMNs.46 It is possible that TRAIL and autoantibodies against PMNs may interdependently contribute to the pathogenesis of neutropenia in SLE, and additional experiments are necessary to clarify their relationship.
In SLE, apoptosis occurs also in the monocyte.20,47 In this process, an autoreactive T-cell subset induces apoptosis of autologous monocytes through TRAIL.20 Although it is reported that PMNs can express TRAIL mRNA, there are no reports that PMNs express functional TRAIL.19 Therefore, we hypothesize that autologous T cells may induce PMN apoptosis in SLE and examined whether autologous T cells could kill PMNs. As a result, we found that T cells expressed TRAIL on their surface, and autologous T cells could kill autologous PMNs. SSA/Ro and SSB/La antibodies are known as anti-neutrophil antibodies, and SLE patients are sometimes found to be positive for those antibodies.43,48 There was no significant difference in TRAIL levels between SSA/Ro- and/or SSB/La-positive neutropenia patients and -negative neutropenia patients. Furthermore, there was no significant difference in specific 51Cr release between patients with an anti-neutrophil antibody and patients without an anti-neutrophil antibody. Therefore, we think that autologous T cells may be responsible for the PMN apoptosis in SLE via TRAIL.
Furthermore, we examined whether the TRAIL expression level in T cells was affected by cytokines or glucocorticoid, and we found that IFN-γ could up-regulate TRAIL expression and glucocorticoid could down-regulate TRAIL mRNA expression in T cells. We also found that glucocorticoid up-regulated cFLIP expression in PMNs, while IFN-γ slightly down-regulated the cFLIP expression in PMNs. IFN-γ is reported as having a capacity to up-regulate the TRAIL expression on T cells49 and enhance TRAIL-induced apoptosis.50 The concentration of IFN-γ is also increased in the serum of lupus patients, particularly those in the active stages of the disease,51 and the expression of IFN-γ transcripts were increased in PBMCs of patients with SLE.52 In contrast, glucocorticoids exert a protective effect on human neutrophil survival by delaying apoptosis.53,54 Glucocorticoid can down-regulate TRAIL mRNA expression in vitro55 and can suppress PMN apoptosis by the continuous stimulation of new protein synthesis,56 while IFN-γ does not affect cFLIP expression at all.57 An important function of cFLIP is to be a negative regulator of gene induction by death receptors such as Fas ligand and TRAIL.58,59 Thus, previous reports support our results, and therefore we encourage the consideration of the TRAIL contribution to neutropenia in SLE. Further studies addressing this point are necessary to clarify the molecular pathogenesis of SLE neutropenia.
It has been reported that TRAIL is associated with the pathogenesis of some autoimmune diseases such as Sjogren syndrome,60 autoimmune encephalomyelitis,61 and thyroid disease.62,63 However, there have been no reports suggesting a TRAIL association in the pathogenesis of SLE neutropenia. Our results suggest that unique mechanisms contribute to increased PMN apoptosis as characterized by patients with SLE. We also propose the possibility that this mechanism can provide a source of potentially antigenic material for the autoimmune response and interfere with normal clearing mechanisms.
Prepublished online as Blood First Edition Paper, March 4, 2004; DOI 10.1182/blood-2003-12-4274.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We appreciate Dr Carole Galligan and Dr Kip Wigmore (Laboratory of Molecular Immunoregulation, National Cancer Institute, Frederick, MD) for their technical help. We offer special thanks to Mrs Rumi Matsuyama and Dr Arlene Ng (Third Department of Internal Medicine, Kagoshima University Faculty of Medicine, Kagoshima, Japan) for their invaluable help with this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal