Comment on Paglialunga et al, page 3148
Discovery of glucose 6-phosphate dehydrogenase (G6PD) deficiency has provided a foundation for modern hematology, and it has been a shining example of how discovery of the molecular basis of human diseases revolutionizes our understanding of biology and eventually changes how we practice medicine.
The discovery of G6PD deficiency not only led to elucidation of X-chromosome inactivation by Beutler et al,1 and independently by Lyon,2 but was the first description of epigenetics. The concept of X-chromosome inactivation paved the way for studies of tumor clonality, elucidated the hierarchy of hematopoiesis, and provided an example of the heritability of a phenotype by mechanisms other than nucleotide sequence change. Yet, among the large number of mutants of G6PD, associated with either mild deficiency of the enzyme (causing acute intermittent hemolysis upon oxidant exposure) or more severe deficiency (causing a rare chronic hemolytic anemia), none is a null mutant. This has suggested that the absence of enzyme activity may not be compatible with life.
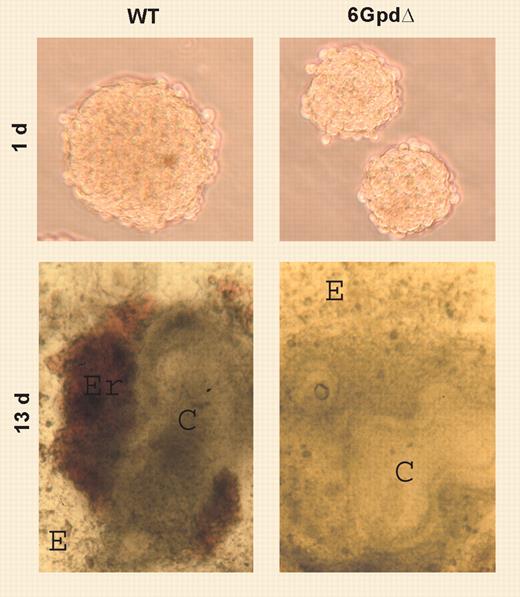
In this issue of Blood, Paglialunga and colleagues examined the effect of the G6PD enzyme on development. They deleted the G6PD gene in mouse totipotent embryonic stem (ES) cells, and while this mutation was embryonically lethal, the ES cells were viable. When these mutated ES cells were induced to differentiation using an ingenious “hanging-drop culture method,” cells of multiple lineages were produced, including cardiac cells, hepatocytes, mesodermal cells, and primitive erythroid cells, albeit with lower efficiency than cells with normal G6PD activity. However, primitive embryonic erythropoiesis seemed unable to undergo the essential developmental switch to definite erythropoiesis (see figure).FIG1
WT and G6pdΔ EB morphology. See the complete figure in the article beginning on page 3148.
WT and G6pdΔ EB morphology. See the complete figure in the article beginning on page 3148.
Erythroid cells are subject to developmental control that is different in the yolk sac (embryonic erythropoiesis) than in fetal liver or adult bone marrow (definite or fetal/adult erythropoiesis). There are substantial developmental differences characterizing each stage, but the factors controlling the developmental switch have not been defined. For example, knock out of erythropoietin or erythropoietin receptor genes in mice allows the generation of erythroid cells in the yolk sac, but precludes the development of mature cells in the embryo leading to the fetal lethality.3 As shown by Paglialungaetal'spaper,after the switch of embryonic to definite (fetal/adult)erythropoiesis, the erythroid progenitors undergo apoptosis that can be abrogated, atleast in part, by reducing agents. This work demonstrates that there are more lessons to be learned from the study of G6PD, and the paper in this issue may provide an unexpected opening for better understanding of the developmental control of erythropoiesis. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal