Abstract
High-dose therapy is an effective standard treatment for multiple myeloma patients. Evidence that intermediate-dose therapy improves survival is limited. At diagnosis, about 70% of patients are older than 65. Intermediate-dose regimen is very well tolerated in older patients. In a multicenter study, 194 patients were randomized to receive at diagnosis either conventional chemotherapy (6 courses of oral melphalan and prednisone [MP]) or intermediate-dose therapy (2 courses of melphalan at 100 mg/m2 [MEL100]) with stem cell support. Response rate was higher after MEL100. Near-complete remission (nCR) was 6% after MP and 25% after MEL100 (P = .0002). At 3 years, MEL100 increased event-free survival (EFS) from 16% to 37% and overall survival (OS) from 62% to 77% (P < .001). Similar results were observed in patients aged 65 to 70: nCR was 8% after MP and 25% after MEL100 (P = .05); at 3 years, MEL100 improved EFS from 18% to 31% (P = .01) and OS from 58% to 73% (P = .01). Patients aged 65 to 70 had a median OS of 37.2 months (MP) versus 58 months (MEL100). Intermediate-dose melphalan improves response rate, EFS, and OS in myeloma patients, specifically in those aged 65 to 70. It constitutes a more effective first-line regimen than standard treatment for elderly patients.
Introduction
Conventional chemotherapy has been the treatment of choice for multiple myeloma (MM) since 1960. Several randomized studies comparing different drug combinations failed to show any major improvement from the original combination of oral melphalan and prednisone (MP).1-3 High-dose therapy followed by stem cell rescue has been shown to increase the response rate and improve remission duration and survival. It represents the standard form of management.4-7 About 33% of myeloma patients at diagnosis are younger than 65; 29% are 65 to 74; and 37% are older than 75.8 High-dose therapy may be too toxic for patients older than 70.9 Alternative approaches that can be safely administered to elderly patients and confer the survival advantage of high-dose therapy have thus been sought. One such approach is to halve the standard conditioning dose of melphalan 200 mg/m2 (MEL200). In a previous experience, melphalan 100 mg/m2 (MEL100) proved to be safe and effective in patients aged 55 to 75.10 We present the results of a multicenter randomized trial of the efficacy of tandem MEL100 compared to MP in MM patients.
Patients, materials, and methods
Patients
The Italian Multiple Myeloma Study Group M97G Trial was conducted from October 1997 to December 2000 in 18 centers in Italy. All patients were untreated and aged 50 to 70. The Southwest Oncology Group (SWOG) diagnostic criteria11 and Durie and Salmon staging system were used.12 Exclusion criteria included prior treatment for myeloma, abnormal cardiac function (systolic ejection fraction less than 50%), respiratory disease (vital capacity or carbon monoxide diffusion less than 50% of normal), abnormal liver function (serum bilirubin level higher than 2 mg/dL or serum aminotransferase value higher than 300% of normal), abnormal renal function (serum creatinine level higher than 3 mg/dL); hepatitis B virus (HBV), hepatitis C virus (HCV), or HIV positivity; concomitant cancer; or psychiatric disease. Approval was obtained from the Divisione di Ematologia dell′Università di Torino, Azienda Ospedaliera S. Giovanni Battista, Torino Institutional Review Board for these studies. Informed consent was provided according to the Declaration of Helsinki.
Patients were randomized at diagnosis according to a sequence determined by the coordinating center in Turin, which issued each treatment assignment by telephone or fax.
Oral MP regimen
Patients received melphalan at 6 mg/m2 and prednisone at 60 mg/m2 on days 1 through 7 of each course, which was repeated every 4 weeks for a total of 6 courses. At the end of therapy, all responding patients were maintained with 3 million IUs interferon alfa 3 times a week and 40 mg dexamethasone on days 1 to 4 every 2 months until the occurrence of any relapse.
MEL100 regimen
All patients received 2 DAV debulking courses (dexamethasone-doxorubicin-vincristine; dexamethasone 40 mg days 1, 2, 3, 4; doxorubicin 50 mg/m2 day 1; vincristine 1 mg day 1; each course repeated every 28 days). Peripheral blood stem cells were mobilized by administration of 3 g/m2 cyclophosphamide in 2 doses with subsequent 4 g/m2 MESNA (sodium 2-mercaptoethane sulfonate) in 5 divided doses. Granulocyte colony-stimulating factor (G-CSF) was administered at 10 μg/kg on day 5 through the last day of leukapheresis initiated upon recovery of leukocytes to 2 × 109/L. The percentage of circulating CD34 cells was evaluated as previously described.13 Three harvest procedures were performed. A Fresenius Cell Separator AS 104 (MTS, Schweinfurt, Germany) was used. Stem cell harvest was split into aliquots when multiples of 3 × 106 CD34/kg were collected. The minimum number of CD34 cells required to deliver melphalan at the dose of 100 mg/m2 was 2 × 106/kg. The dose of melphalan was reduced to 75 mg/m2 if CD34 cells were 1 to 2 × 106/kg and to 50 mg/m2 if they were 0.5 to 1 × 106/kg. Intermediate-dose melphalan was given at the dose of 100 mg/m2, followed by the reinfusion of stem cells 24 hours later. G-CSF was administered at 5 μg/kg until the neutrophil count was higher than 500/μL in 2 consecutive tests. MEL100 was repeated once after 2 months. Maintenance management was as described for the MP group.
Response criteria
Response to treatment was assessed in monthly serum and urine studies and on bone marrow aspirates. Partial remission (PR) was defined as at least 50% reduction of serum myeloma protein, 90% decrease of Bence Jones proteinuria, and 50% reduction of bone marrow infiltration. Near-complete remission (nCR) required disappearance of serum or urine myeloma protein analyzed by standard electrophoresis and marrow plasmacytosis less than 5% for at least 2 months. All other results were regarded as failures or no response. Disease progression was defined as 25% increase in serum or urine myeloma protein. Relapse was defined as an increase in serum or urine myeloma protein of more than 50%. Relapse following nCR was defined as reappearance of the paraprotein (evaluated every month) or recurrence of bone marrow infiltration (every 6 months).
Statistical analysis
A sample size of 240 subjects (120 for each group) was required to detect as statistically significant (alpha, 2-sided = 0.05; beta = 0.10) a 20% difference in event-free survival at 2 years (assuming 25% disease-free patients in the MP group). This number of subjects was estimated to be reached within 2.5 years (from 1997). In December 2000, the study was closed with 200 patients enrolled due to a slowing rate of accrual.
For each response category (and toxicity) the groups were compared with the χ2 test or Fisher exact test.
Event free survival (EFS) and overall survival (OS) were calculated with the Kaplan Meier method and differences between groups evaluated by the χ2 log-rank test. The duration of EFS was calculated from the beginning of treatment until the time of relapse, progression of disease, or death, or the date the patient was last known to be in remission. The duration of OS was calculated from the beginning of therapy until the time of death or the last date known to be alive.
The Cox proportional hazard regression model was used to estimate the hazard ratios (HRs) and the 95% confidence intervals (95% CI) for the treatment, taking into account some prognostic factors defined a priori: age, gender, isotype, stage, and β2-microglobulin.
One of the aims of the study was to evaluate the efficacy and tolerability of MEL100 in older patients. A stratified analysis by age group (younger than 65; 65 and older) was performed for this purpose.
All patients who started the treatment assigned, independently from completion, were included in the analyses.
Results
Patient characteristics and treatments
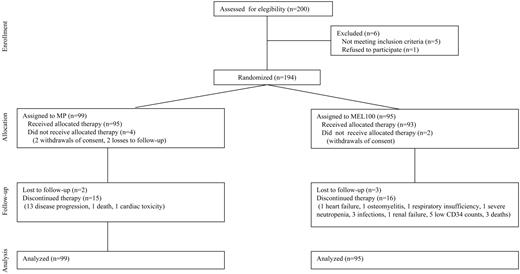
Of 200 patients enrolled, 6 patients were excluded, 1 for being older than 70, 1 for being younger than 50, 2 for HCV positivity, 1 for renal insufficiency, and 1 for withdrawal of consent. A total of 194 patients were randomly assigned to receive MP (n = 99) or MEL100 (n = 95) (Figure 1). The baseline characteristics of patients are summarized in Table 1. No significant differences were found between the 2 treatment groups.
Patient characteristics
Characteristics . | MEL100 . | MP . |
|---|---|---|
| No. | 95 | 99 |
| Age | ||
| Median age, y | 65 | 63 |
| Range, y | 51-70 | 52-70 |
| 65 or older, % | 46.3 | 36.4 |
| Gender | ||
| Male, no. (%) | 51 (54) | 54 (55) |
| Female, no. (%) | 44 (46) | 45 (45) |
| Stage | ||
| IIA, no. (%) | 34 (36) | 32 (32) |
| IIB, no. (%) | 3 (3) | 5 (5) |
| IIIA, no. (%) | 53 (56) | 58 (58) |
| IIIB, no. (%) | 5 (5) | 4 (4) |
| Median β2-microglobulin, nM/L (mg/L) | 246.5 (2.9) | 246.5 (2.9) |
| β2-microglobulin | ||
| Less than 255 nM/L, no. (%) | 37 (39) | 40 (40) |
| At least 255 nM/L, no. (%) | 46 (48) | 37 (37) |
| Data missing, no. (%) | 12 (13) | 22 (22) |
| M-protein class | ||
| IgG, no. (%) | 64 (67) | 59 (60) |
| IgA, no. (%) | 22 (23) | 27 (27) |
| Bence Jones protein, no. (%) | 8 (9) | 11 (11) |
| Others, no. (%) | 1 (1) | 2 (2) |
Characteristics . | MEL100 . | MP . |
|---|---|---|
| No. | 95 | 99 |
| Age | ||
| Median age, y | 65 | 63 |
| Range, y | 51-70 | 52-70 |
| 65 or older, % | 46.3 | 36.4 |
| Gender | ||
| Male, no. (%) | 51 (54) | 54 (55) |
| Female, no. (%) | 44 (46) | 45 (45) |
| Stage | ||
| IIA, no. (%) | 34 (36) | 32 (32) |
| IIB, no. (%) | 3 (3) | 5 (5) |
| IIIA, no. (%) | 53 (56) | 58 (58) |
| IIIB, no. (%) | 5 (5) | 4 (4) |
| Median β2-microglobulin, nM/L (mg/L) | 246.5 (2.9) | 246.5 (2.9) |
| β2-microglobulin | ||
| Less than 255 nM/L, no. (%) | 37 (39) | 40 (40) |
| At least 255 nM/L, no. (%) | 46 (48) | 37 (37) |
| Data missing, no. (%) | 12 (13) | 22 (22) |
| M-protein class | ||
| IgG, no. (%) | 64 (67) | 59 (60) |
| IgA, no. (%) | 22 (23) | 27 (27) |
| Bence Jones protein, no. (%) | 8 (9) | 11 (11) |
| Others, no. (%) | 1 (1) | 2 (2) |
In the oral MP group, 21 patients (21%) did not complete the 6 courses because of 13 episodes of early disease progression, 1 death, 4 lost to follow-up, 2 withdrawals of consent, and 1 severe cardiac toxicity. Among these, 4 had received more than 3 courses, and 17 had received fewer than 3 courses. The median time from diagnosis to start of MP was 1.2 months. The median interval between the first and sixth course of MP was 6.7 months.
In the entire MEL100 group, 21 patients (22%) did not complete both MEL100. Eight patients did not receive cyclophosphamide, 1 because of heart failure, 1 because of osteomyelitis, 2 because of deaths due to infection and myocardial infarction, 2 because of withdrawals of consent, and 2 because of loss to follow-up. Eight patients did not receive the first MEL100, 1 because of acute respiratory insufficiency, 5 because of low CD34 counts (< 0.5 × 106 CD34/kg), 1 because of death due to disease progression, and 1 because of loss to follow-up. Five patients did not receive the second MEL100 dose because of persistent severe neutropenia, sepsis, gastroenteritis, fungal infection, or renal failure. Sixteen percent of patients younger than 65 did not complete the entire program, compared with 29% older than 65 (P = .1). In the subgroup aged 65 to 70, the reasons for stopping treatment were 3 low CD34 counts, 2 patients lost to follow-up, 1 withdrawal of consent, 1 death due to disease progression, and 6 severe toxicities (sepsis, gastroenteritis, fungal infection, renal failure, respiratory insufficiency, or heart failure). The median time from diagnosis to the first MEL100 was 4.3 months. The median time between the first and the second MEL100 was 3.1 months.
Among the responding patients, 94 received interferon alpha and dexamethasone as maintenance therapy (35 in the MP arm and 59 in the MEL100 arm). Sixty-four patients (30 in MP group and 34 in MEL100 group) discontinued this treatment, 17 because of intolerance or adverse events (fever, fatigue, thrombocytopenia, diabetes, or high blood pressure) and 47 because of disease progression.
Mobilization regimen and toxicity
Cyclophosphamide induced a mild toxicity. The median duration of severe neutropenia was 2 days, and only 4% of patients required platelet transfusions and 7% required red cell transfusions. After 1, 2, or 3 leukaphereses, the median of CD34 cells harvested was 9.5 × 106/kg (range, 0-64.7 × 106/kg). Five patients harvested less than 0.5 × 106 CD34/kg and did not receive transplants. Three patients received both first and second melphalan at 75 mg/m2; another 3 patients received only the second dose of melphalan, reduced to 75 mg/m2. There was no relationship between age and number of CD34 collected. The median of CD34 cells harvested was 8 × 106/kg (range, 0-64.7 × 106/kg) for patients older than 65.
Treatment-related toxicity is illustrated in Table 2. Severe hematologic toxicity was significantly shorter in the MP group. After MEL100, the median duration of severe neutropenia and thrombocytopenia was 5 and 3 days, respectively. No differences were observed between the first and the second MEL100. Similarly, transfusion requirements were lower after MP. The incidence of fever of unknown origin (31%) and mucositis (23%) was significantly higher after MEL100. The incidence of at least 1 organ toxicity was higher after MEL100 (30%) than after MP (8%). Five early deaths occurred in the MEL100 group (3 from disease progression after DAV; 1 from acute renal failure; and 1 myocardial infarction) and 1 in the MP group (disease progression). In the patient subgroup aged 65 to 70 the incidence of hematological and extrahematological toxicity was similar to the entire population.
Hematologic and extrahematologic toxicity
. | MEL 100 of patients ages 65-70* . | MEL 100 of patients ages 50-70* . | MP of patients ages 50-70* . |
|---|---|---|---|
| No. | 43 | 93 | 92 |
| Duration of neutropenia†, d, range (median) | 1-13 (5) | 0-13 (5) | — |
| Duration of thrombocytopenia†, d, range (median) | 0-18 (3) | 0-18 (3) | — |
| Patients who required platelet transfusion, no. (%) | 18 (41) | 50 (54) | 2 (2) |
| Patients who required red blood cell transfusion, no. (%) | 21 (48) | 50 (54) | 3 (3) |
| Unknown origin fever, no. (%) | 9 (20) | 29 (31) | 0 (0) |
| Mucositis, no. (%)‡ | 14 (32) | 21 (23) | 0 (0) |
| Viral infections, no. (%)‡ | 2 (4) | 6 (6) | 0 (0) |
| Pneumonia, no. (%) | 4 (9) | 6 (7) | 0 (0) |
| Cardiac toxicity, no. (%)‡ | 0 (0) | 2 (2) | 2 (2) |
| Pulmonary toxicity, no. (%)‡ | 0 (0) | 1 (1) | 0 (0) |
| Renal toxicity grade, no. (%)‡ | 1 (2) | 2 (2) | 0 (0) |
| Gastrointestinal toxicity, no. (%)‡ | 3 (7) | 8 (9) | 0 (0) |
| Neurologic toxicity, no. (%)‡ | 0 (0) | 0 (0) | 1 (1) |
| Sepsis, no (%) | 2 (4) | 2 (2) | 1 (1) |
| Central venous catheter infection, no. (%) | 0 (0) | 2 (2) | — |
| Thromboembolism, no. (%) | 0 (0) | 1 (1) | 3 (3) |
| At least one organ toxicity, no. (%) | 10 (23) | 28 (30) | 7 (8) |
. | MEL 100 of patients ages 65-70* . | MEL 100 of patients ages 50-70* . | MP of patients ages 50-70* . |
|---|---|---|---|
| No. | 43 | 93 | 92 |
| Duration of neutropenia†, d, range (median) | 1-13 (5) | 0-13 (5) | — |
| Duration of thrombocytopenia†, d, range (median) | 0-18 (3) | 0-18 (3) | — |
| Patients who required platelet transfusion, no. (%) | 18 (41) | 50 (54) | 2 (2) |
| Patients who required red blood cell transfusion, no. (%) | 21 (48) | 50 (54) | 3 (3) |
| Unknown origin fever, no. (%) | 9 (20) | 29 (31) | 0 (0) |
| Mucositis, no. (%)‡ | 14 (32) | 21 (23) | 0 (0) |
| Viral infections, no. (%)‡ | 2 (4) | 6 (6) | 0 (0) |
| Pneumonia, no. (%) | 4 (9) | 6 (7) | 0 (0) |
| Cardiac toxicity, no. (%)‡ | 0 (0) | 2 (2) | 2 (2) |
| Pulmonary toxicity, no. (%)‡ | 0 (0) | 1 (1) | 0 (0) |
| Renal toxicity grade, no. (%)‡ | 1 (2) | 2 (2) | 0 (0) |
| Gastrointestinal toxicity, no. (%)‡ | 3 (7) | 8 (9) | 0 (0) |
| Neurologic toxicity, no. (%)‡ | 0 (0) | 0 (0) | 1 (1) |
| Sepsis, no (%) | 2 (4) | 2 (2) | 1 (1) |
| Central venous catheter infection, no. (%) | 0 (0) | 2 (2) | — |
| Thromboembolism, no. (%) | 0 (0) | 1 (1) | 3 (3) |
| At least one organ toxicity, no. (%) | 10 (23) | 28 (30) | 7 (8) |
— indicates not applicable.
Toxicity is related to number of evaluable patients.
Defined as having a neutrophil count lower than .500 × 109/L and platelet count lower than 25 000 × 109/L.
Toxicity grades 3-4 according to the World Health Organization.
The median days of hospitalization were 3 (range, 0-19 days) after cyclophosphamide, 11 (range, 0-40 days) after the first MEL100, and 12 (range, 0-22 days) after the second. In 6 centers, patients were discharged before neutropenia occurred. The median days of hospitalization were 5 days (range, 0-6 days) after the first MEL100 and 4 days (range, 0-7 days) after the second. In 12 centers patients were hospitalized throughout the neutropenic period. The median days of hospitalization were 13 days (range, 7-40 days) after the first MEL100 and 14 days (range, 8-22 days) after the second.
Response rate
The frequencies of PR (nCR) were 23% (3%) after 3 courses of MP and 36% (6%) after 6 courses. In the MEL100 group, these frequencies were 27% (5%) after DAV, 39% (5%) after cyclophosphamide, 60% (10%) after the first MEL100, and 47% (25%) after the second. For patients who attained PR after cyclophosphamide, the incidence of nCR after MEL100 was 60%. In comparison with MP, MEL100 significantly improved the nCR (P = .0002). No response and progression were 55% after MP and 26% after MEL100 (Table 3). Patients aged 65 to 70 were analyzed as a subgroup for response and clinical outcome. The frequencies of PR (nCR) were 41% (8%) after 6 courses of MP and 42% (25%) after MEL100. MEL100 improved the nCR rate (P = .05) (Table 4).
Clinical response to MEL100 and MP
. | MEL 100, no. (%) . | MP, no. (%) . | P . |
|---|---|---|---|
| No. | 95 (100) | 99 (100) | NA |
| Near complete remission | 24 (25) | 6 (6) | .0002 |
| Partial remission | |||
| 75% to 99% reduction | 24 (25) | 11 (11) | <.0001 |
| 50% to 74% reduction | 21 (22) | 25 (25) | — |
| No response | 21 (22) | 35 (35) | — |
| Progressive disease | 4 (4) | 20 (20) | — |
| Not available | 1 (1) | 2 (2) | — |
| Early deaths | 5 (5) | 1 (1) | .6 |
. | MEL 100, no. (%) . | MP, no. (%) . | P . |
|---|---|---|---|
| No. | 95 (100) | 99 (100) | NA |
| Near complete remission | 24 (25) | 6 (6) | .0002 |
| Partial remission | |||
| 75% to 99% reduction | 24 (25) | 11 (11) | <.0001 |
| 50% to 74% reduction | 21 (22) | 25 (25) | — |
| No response | 21 (22) | 35 (35) | — |
| Progressive disease | 4 (4) | 20 (20) | — |
| Not available | 1 (1) | 2 (2) | — |
| Early deaths | 5 (5) | 1 (1) | .6 |
NA indicates not applicable; —, not available.
Clinical response to MEL100 and MP in patients aged 65 to 70
. | MEL 100, no. (%) . | MP, no. (%) . | P . |
|---|---|---|---|
| No. | 44 (100) | 36 (100) | NA |
| Near complete remission | 11 (25) | 3 (8) | .05 |
| Partial remission | |||
| 75% to 99% reduction | 10 (22) | 3 (8) | .09 |
| 50% to 74% reduction | 9 (20) | 12 (33) | — |
| No response | 12 (27) | 13 (36) | — |
| Progressive disease | 2 (5) | 5 (14) | — |
| Early deaths | 3 (7) | 1 (3) | .29 |
. | MEL 100, no. (%) . | MP, no. (%) . | P . |
|---|---|---|---|
| No. | 44 (100) | 36 (100) | NA |
| Near complete remission | 11 (25) | 3 (8) | .05 |
| Partial remission | |||
| 75% to 99% reduction | 10 (22) | 3 (8) | .09 |
| 50% to 74% reduction | 9 (20) | 12 (33) | — |
| No response | 12 (27) | 13 (36) | — |
| Progressive disease | 2 (5) | 5 (14) | — |
| Early deaths | 3 (7) | 1 (3) | .29 |
NA indicates not applicable; —, not available.
EFS and OS
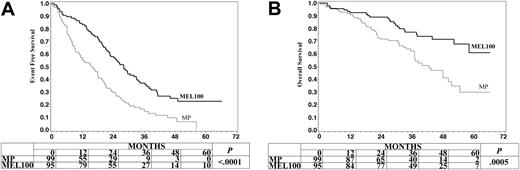
The median follow-up from start of treatment was 39 months (range, 10-65.9 months; standard deviation, 13.5) for MP survivors and 41 months (range, 2.9-64.5 months; standard deviation, 13.8) for MEL100 survivors. The median EFS was 15.6 months after MP and 28 months after MEL100. The probability of EFS at 3 years was 16% after MP and 37% after MEL100 (P < .0001) (Figure 2A). In the univariate analysis, EFS was significantly related to MEL100 administration (HR 0.47, 95% CI 0.34-0.66, P < .0001) and β2-microglobulin level (HR 1.59, 95% CI 1.11-2.28, P < .01). In the multivariate analysis, EFS was related only to the administration of MEL100 (HR 0.43, 95% CI 0.26-0.7, P < .0001) and serum β2-microglobulin level (HR 1.71, 95% CI 1.17-2.49, P < .01).
Event-free survival and overall survival of myeloma patients aged 50 to 70 treated with melphalan at 100 mg/m2 (MEL100) or oral melphalan and prednisone (MP).
Event-free survival and overall survival of myeloma patients aged 50 to 70 treated with melphalan at 100 mg/m2 (MEL100) or oral melphalan and prednisone (MP).
The median OS was 42.5 months for MP and has not been reached (58+ months) for MEL100. The probability of OS for 3 years was 62% after MP and 77% after MEL100 (P = .0005) (Figure 2B). Twenty-nine percent of patients were alive in remission, 35% were alive after relapse or with progressive disease, and 36% had died. In the univariate analysis, OS was related to MEL100 administration (HR 0.40, 95% CI 0.24-0.67, P < .001) and β2-microglobulin level (HR 1.88, 95% CI 1.1-3.24, P = .02). In the multivariate analysis, OS was influenced by the administration of MEL100 (HR 0.37, 95% CI 0.21-0.67, P = .0005) and β2-microglobulin level (HR 2.12, 95% CI 1.18-3.71, P = .015).
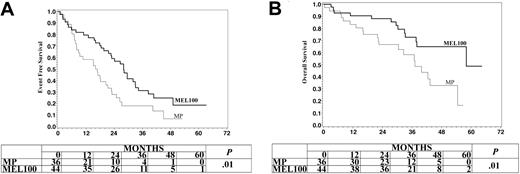
EFS and OS were analyzed after stratification by age (Table 5). In older patients (65 or older), the median EFS was 16.4 months after MP and 28 after MEL100. The probability of EFS for 3 years was 18% after MP and 31% after MEL100 (P = .01) (Figure 3A). The median OS was 37.2 months after MP and 58 after MEL100. The probability of OS for 3 years was 58% after MP and 73% after MEL100 (P = .01) (Figure 3B). In the entire population MEL100 improved both EFS (HR 0.48, 95% CI 0.34-0.66) and OS (HR 0.40, 95% CI 0.24-0.67). The magnitude of these improvements was similar in patients older than 65 for both EFS (HR 0.55, 95% CI 0.33-0.92) and OS (HR 0.46, 95% CI 0.22-0.98) (Table 5).
Survival in patients aged 50 to 70 and those aged 65 to 70
. | MEL100 . | . | . | MP . | . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient ages and survival status . | No. . | No. failed (%) . | Median (mos) . | No. . | No. failed (%) . | Median (mos.) . | HR* . | CI . | P . | ||||
| 50 to 70 years | |||||||||||||
| EFS | 95 | 58 (60) | 28.0 | 99 | 81 (82) | 15.6 | 0.48 | 0.34-0.66 | < .0001 | ||||
| OS | 95 | 19 (20) | 58+ | 99 | 34 (34) | 42.5 | 0.40 | 0.24-0.67 | < .001 | ||||
| 65 to 70 years | |||||||||||||
| EFS | 44 | 29 (66) | 28.0 | 36 | 29 (80.6) | 16.4 | 0.55 | 0.33-0.92 | .023 | ||||
| OS | 44 | 10 (22.7) | 58.0 | 36 | 14 (38.8) | 37.2 | 0.46 | 0.22-0.98 | .04 | ||||
. | MEL100 . | . | . | MP . | . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient ages and survival status . | No. . | No. failed (%) . | Median (mos) . | No. . | No. failed (%) . | Median (mos.) . | HR* . | CI . | P . | ||||
| 50 to 70 years | |||||||||||||
| EFS | 95 | 58 (60) | 28.0 | 99 | 81 (82) | 15.6 | 0.48 | 0.34-0.66 | < .0001 | ||||
| OS | 95 | 19 (20) | 58+ | 99 | 34 (34) | 42.5 | 0.40 | 0.24-0.67 | < .001 | ||||
| 65 to 70 years | |||||||||||||
| EFS | 44 | 29 (66) | 28.0 | 36 | 29 (80.6) | 16.4 | 0.55 | 0.33-0.92 | .023 | ||||
| OS | 44 | 10 (22.7) | 58.0 | 36 | 14 (38.8) | 37.2 | 0.46 | 0.22-0.98 | .04 | ||||
Adjusted for age, gender, isotype, stage, and β2-microglobulin with a Cox proportional hazard model.
Event-free survival and overall survival of myeloma patients aged 65 to 70 treated with melphalan at 100 mg/m2 (MEL100) or oral melphalan and prednisone (MP).
Event-free survival and overall survival of myeloma patients aged 65 to 70 treated with melphalan at 100 mg/m2 (MEL100) or oral melphalan and prednisone (MP).
Salvage therapy
After a median follow-up of 36 months from start of treatment, 133 patients had evidence of disease progression. After MP, 77 (58%) patients relapsed, 18 patients received no treatment (fatal disease progression or withdrawal of consent), 18 received conventional chemotherapy, 4 received thalidomide-based regimens, and 37 were assigned to receive MEL100. Only 21 received both MEL100 (2 disease progressions after DAV and 14 inadequate stem cell harvests). The median follow-up of survivors was 11 months from time of relapse. Median survival for relapsed patients was 22 months.
After MEL100, 56 (42%) patients relapsed, 19 patients received no treatment (fatal disease progression, withdrawal of consent, severe toxicity after MEL100 at diagnosis or no stem cell availability), 12 received conventional chemotherapy, 8 received thalidomide-based regimens, and 17 were assigned to receive MEL100. Two did not complete tandem MEL100 for stem cell shortness. After a median follow-up of 17 months from time of relapse, the median survival was 27 months for both groups.
Discussion
In MM patients, the achievement of a significant proportion of CR prolongs both EFS and OS.7,14 The goal is always to obtain the best response rate with the minimum toxicity, since MM is primarily observed in elderly patients. In the present study we asked if MEL100 is sufficient to improve the clinical outcome in comparison with oral MP, thus expanding the applicability of the intermediate/high-dose procedure to a larger proportion of the MM patients.
Several trials have compared conventional with high-dose therapy. Attal et al4 randomly assigned 200 patients to receive either conventional-dose chemotherapy or combination chemotherapy followed by melphalan 140 mg/m2 plus total body irradiation. In a recent update, median OS was 44 months for patients treated with standard chemotherapy and 57 months for those treated with autologous transplant.15 Fermand et al16 compared conventional chemotherapy with infusion chemotherapy followed by MEL200 or melphalan 140 mg/m2 plus busulfan 16 mg/m2. The median OS was 50.4 months after conventional chemotherapy and 55.3 months after autologous transplant. In this study, patients treated with conventional chemotherapy could cross over to receive a transplant at the physician's discretion. In the study of Child et al,5 407 patients were randomized to receive standard therapy or infusion chemotherapy followed by MEL200 or melphalan 140 mg/m2 plus total body irradiation. Median survival was significantly improved by almost 1 year after transplant, 42 months versus 54 months. MEL100 improved survival by almost 31% at 4 years in comparison with oral MP.
When the odds ratios and 95% CI for the Attal et al,4 Fermand et al,16 and Child et al5 studies were combined, the overall effects of intensified treatments were consistent with a significant survival benefit (odds ratio 0.70, 95% CI 0.53-0.93; P = .01).5 The odd ratios and 95% CI for our study also showed a significant consistent survival benefit of MEL100 (odds ratio 0.36, 95% CI 0.19-0.67).
The Attal et al,4 Fermand et al,16 and Child et al5 trials included only patients younger than 65. No randomized trial has so far compared autologous transplant with conventional chemotherapy in patients aged 65 to 70. In our randomized trial, 40% of patients were older than 65. In the subgroup of patients aged 65 to 70, we first demonstrate that a dose-adjusted autotransplantation is superior to standard treatment.
One question raised by our study is what is the best conditioning regimen for intensified treatments. Melphalan is considered the best. No other drug, such as cyclophosphamide or busulfan, has the same cytoreductive activity.17-19 MEL200 is now considered the standard. It is less toxic and more effective than melphalan 140 mg/m2 plus total body irradiation.20 Our results show that MEL100, too, induces a survival benefit.
A relationship between dose intensity and clinical outcome is evident. The frequency of CR rose from 6% after oral MP to 25% after MEL100. In a case-matched control analysis, tandem MEL100 was compared with tandem MEL200. The CR rate was 35% after MEL100 and 48% after MEL200. Median EFS was 32 months after MEL100 and 42 months after MEL200 (P < .005), but OS was unchanged.21 In a recent study, patients were randomly assigned to receive either tandem melphalan 70 mg/m2 or the same regimen followed by myeloablative therapy. CR increased from 16% to 29%, median time-to-progression was prolonged from 25 to 31 months, but OS was unchanged.21 The tandem approach was particularly useful for MEL100. The second course of MEL100 increased the frequency of CR to 25%, from 10% after the first.
The standard MEL200 conditioning regimen was associated with 16% mortality in patients aged more than 70.9 Dose-adjusted autotransplantation, from MEL200 to MEL100 or melphalan 140 mg/m2, virtually eliminates the high incidence of mucositis or other extramedullary toxicities observed in advanced age.22 After MEL100, the incidence of toxicities was not age related. In a previous study, MEL100 was administered to patients aged 55 to 75, without any increase of toxicity in patients aged 70 to 75.10 This suggests that MEL100 is also suitable for patients older than 70. One third of myeloma patients at presentation are aged 65 to 75. The major adverse events were fever of unknown origin (31%) and mucositis (23%). Eighteen percent of patients did not receive the first MEL100. Most deaths or toxicities occurred before the first MEL100. Only 5% of patients received the first but not the second MEL100. These data suggest that adverse events due to myeloma or concomitant diseases are the major causes of toxicity. In our experience, the first 2 months after diagnosis are the highest risk period for life-threatening adverse events. Similar results were observed by the largest experience on MEL200 transplant, where 16% of patients did not receive the first transplant.14 More effective treatment should be developed for the first 2 months after diagnosis.
In our study, prognostic factors such as β2-microglobulin were well balanced between the MEL100 and the MP arm. The median level of serum β2-microglobulin was 2.9 mg/dL for patients receiving MEL100, compared with more than 3.5 mg/dL in the other randomized studies in the literature. This might explain the best odds ratio observed after MEL100 in our study, despite a significantly higher proportion of elderly patients. Chromosome abnormalities are frequently associated with high β2-microglobulin levels; both are strong adverse prognostic factors.14,23 When this study was started, cytogenetic analysis and, in particular, the fluorescence in situ hybridization (FISH) technique, was not generally available for all the participating groups. This information is present only for a minority of patients.
In conclusion, MEL100 was superior to MP. Halving the standard MEL200 dose still retains and induces a survival benefit. This approach is particularly suitable for elderly patients. The maximum intensification of treatment to achieve the maximum rate of CR should remain the goal of every physician.
Prepublished online as Blood First Edition Paper, July 20, 2004; DOI 10.1182/blood-2004-02-0408.
Supported in part by Associazione Italiana Ricerca Cancro (AIRC), Associazione Italiana Leucemie (AIL), Ministero Università e Ricerca Scientifica e Tecnologica (MURST), Consiglio Nazionale Ricerca (CNR), Compagnia di S. Paolo, and Associazione per lo studio e la cura delle malattie del sangue.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Miss Tiziana Marangon for her technical assistance in the preparation of the manuscript. We thank also the many medical and nursing colleagues who have participated in the treatment of patients.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal